Abstract
Purpose
Temporal lobe epilepsy (TLE) affects resting state brain networks in adults. This study aims to correlate resting state functional MRI (rsMRI) signal latency in pediatric TLE patients with their laterality.
Methods
From 2006 to 2016, 26 surgical TLE patients (12 left, 14 right) with a mean age of 10.7 years (range 0.9–18) were prospectively studied. Preoperative rsMRI was obtained in patients with concordant lateralizing structural MRI, EEG and PET studies. Standard preprocessing techniques and seed-based rsMRI analyses were performed. Additionally, the latency in rsMRI signal between each 6 mm voxel sampled was examined, compared to the global mean signal, and projected onto standard atlas space for individuals and the cohort.
Results
All but one of the 26 patients improved seizure frequency postoperatively with a mean follow-up of 2.9 years (range 0–7.7), with 21 patients seizure-free. When grouped for epileptogenic laterality, the latency map qualitatively demonstrated that the right TLE patients had a relatively early signal pattern whereas the left TLE patients had a relatively late signal pattern compared to the global mean signal in the right temporal lobe. Quantitatively, the two groups had significantly different signal latency clusters in the bilateral temporal lobes (p<0.001).
Conclusion
There are functional MR signal latency changes in medical refractory pediatric TLE patients. Qualitatively, signal latency in the right temporal lobe precedes the mean signal in right TLE patients and is delayed in left TLE patients. With larger confirmatory studies, preoperative rsMRI latency analysis may offer an inexpensive, noninvasive adjunct modality to lateralize pediatric TLE.
Keywords: default mode network, functional magnetic resonance imaging, pediatric epilepsy, resting state, temporal lobe epilepsy
Introduction
There is an excellent treatment response to temporal lobectomy in medically refractory unilateral pediatric temporal lobe epilepsy, especially in the setting of mesial temporal sclerosis.[1–3] With an incidence of 1/1000[4], there is substantial under-treatment of the disease[5] and the ability to substantially improve the quality of life of this patient population remains in sight. In addition to high rates of seizure control, surgery also offers an opportunity to improve development and reduce cognitive side effects by reducing the need for impairing anticonvulsant medications.[6] However, the identification of under-treated epilepsy is complex and requires patient access to pediatric epilepsy surgery centers, with basic imaging and EEG-video capabilities as well as advanced diagnostic techniques such as positron emission tomography (PET), single photon emission computed tomography (SPECT) or magnetic encephalography (MEG).[5] Concordance of sometimes disparate neuropsychological testing, MRI, EEG and advanced imaging data may result in a recommendation for resective surgery.[7]
Within the realm of advanced functional MR imaging, resting state functional MRI (rsMRI) has proved promising in identifying diseased states of the brain.[8–12] The merits of rsMRI in pre-surgical correlation-based (functional connectivity) mapping have already been demonstrated for language and motor cortex.[10, 11, 13] In addition, specific neurological disorders like juvenile psychopathy[14], Alzheimer’s disease[15], and depression[16] demonstrate aberrant communication between diseased brain regions and the default mode network[17, 18] compared to normal, healthy controls. As a disorder of networks, epilepsy is a model disease to study resting state network architecture in both adults[19–25] and children.[26] Cataldi, et al., recently reviewed[8] such rsMRI functional connectivity analysis done on awake, adult temporal lobe epilepsy patients with similar demonstrably diminished DMN communication with the diseased hippocampus.[23, 24, 27–29] Pediatric epilepsy patients provide a certain challenge due to their need for sedation during MR imaging, though there is some evidence that network correlations persist regardless of sedation paradigm.[26]
However, mesial temporal structures have poor BOLD signal owing to artifact from the cisternal fluid pulsation and adjacent sphenoid sinus and temporal bone.[30] At the group level, recent data-driven analysis of the temporal latency in cross-covariance in the BOLD rsMRI signal demonstrates an excellent and novel ability to examine the resting state and the DMN[31] and has been applied to assess patients with autism.[32] In addition, the clinical value of rsMRI latency has been suggested by several studies in adult stroke patients[33, 34] and awake adult epilepsy patients.[35] This study examines the clinical value of rsMRI in lateralizing the epileptogenic side in pediatric patients with unilateral, surgical TLE. In addition to conventional seed-based functional connectivity analysis, this study employs rsMRI temporal latency analysis to test the hypothesis that latency patterns may be useful in determining laterality in pediatric medically refractory temporal lobe epilepsy.
Methods
Patient Selection
With institutional review board approval and informed consent from the parents, all children with medically refractory temporal lobe epilepsy undergoing surgery had preoperative structural and resting-state functional MRI sequences and were prospectively enrolled in the study between 2006 and 2014. Owing to the young age of some of the participants, intravenous propofol was used to obtain the images, while the more tolerant patients had no sedation. We have previously reported robust signal correlations and resting state network architecture in the rsMRI analysis, despite the sedation protocol heterogeneity in this patient cohort.[26] A total of 26 patients were identified and imaged with useable rsMRI imaging data; 12 had left-sided disease and 14 had right-sided disease based on concordant, lateralizing structural MRI, EEG, and neuropsychological testing, with 12 patients also having 18-fluorodeoxyglucose positron emission tomography (18FDG-PET). Patients had all been discussed in a multidisciplinary epilepsy surgery conference leading to temporal lobectomy. Their charts were reviewed for clinical variables including histopathology and postoperative seizure outcome.
rsMRI Data Acquisition and Preprocessing
As described previously[26, 36], gradient echo, echo planar BOLD contrast-sensitive rsMRI sequences were obtained on a Siemens Trio 3T scanner with a repetition time (TR) of 2.07 s, echo time of 25 ms, flip angle of 90°, with two runs of 7 minutes each, covering the brain in 36 slices (4 mm cubic voxels). These acquisition parameters did not change significantly over the study period, with only a minor variability in TR (2.05s, n=1; 2.07s, n=22; 2.08s n=2; 2.15s, n=1). The BOLD volumes were then registered to high-resolution anatomical T1 and T2 images with slice timing compensation, motion reduction and atlas transformation performed in one step.[37, 38] Nuisance regression was carried out with regressors extracted from the ventricular, white matter as well as the global mean signal.[36, 39] To reduce the impact of motion artifact volume, frame censoring was implemented as previously described[31], excluding from the final analyses frame-to-frame motion exceeding 2.5 mm. To specifically exclude the effect of BOLD signal autocovariance, autocorrelation of the nuisance-regressed rsMRI signal was examined for each patient at 3 lags (1, 2 and 3 TR). In addition, to exclude effects of sedation on the signal covariance magnitude,[26] voxel-wise mean standard deviation maps of the nuisance-regressed rsMRI signal were also examined for each patient. The Washington University Neuroimaging Laboratory 4dfp Suite was used for registration and rsMRI processing (ftp://imaging.wustl.edu/pub/raichlab/4dfp_tools/) along with MATLAB (r2015).
Resting-State Functional Connectivity Analysis
Conventional region of interest (ROI) seed-based functional connectivity analysis was performed on the preprocessed rsMRI data using previously identified 36 seed ROIs representing the “canonical” networks[40] as well as the bilateral amygdala and hippocampus seeds.
Resting-State MRI Lag Analysis
Time series were extracted from 6 mm cubic regions of interest (voxels) spanning all gray matter. Lagged cross-covariance matrices were calculated as previously described.[31] Lags between each voxel and every other voxel were estimated using bicubic interpolation. Latency maps then were evaluated in standard atlas space. The group (n=26), the left TLE cohort (n=12) and the right TLE cohort (n=14) maps as well as the individual maps were compared qualitatively to assess the relative signal latency in the temporal lobe regions. The group difference maps were then quantitatively assessed for clusters of voxels with significantly different latencies, using a t-test with significance set at p<0.001, to minimize group comparison issues at the nominal value (FSL 5.0.8, 2014; Mricron 1.0, 2015).
Results
The cases are described in Table 1 (left temporal lobe epilepsy, LTLE) and Table 2 (right temporal lobe epilepsy, RTLE). The mean patient age was 10.7 years (range 0.9–18) with 12 having left-sided and 14 having right-sided medically refractory temporal lobe epilepsy. In the mean follow-up period of 2.9 years (range 0–7.7), 25 patients had improvement in seizure outcome with the majority of patients (21/26 or 81%) Engel class I or II; 1 patient was lost to follow-up. The majority of patients had a histopathological diagnosis of gliosis, mesial temporal sclerosis, or neuronal loss (18/26 or 69%). Three of the patients had worthwhile improvement but not seizure freedom (Engel class III) at their most recent clinical follow-up, with one of those patients having tuberous sclerosis as the primary histopathological diagnosis, a disease known to have recurrence of intractable epilepsy.
Table 1. Left Temporal Lobe Epilepsy patients.
Table 1 demonstrates the demographics and right temporal lobe signal latency pattern for the 12 patients with left temporal lobe epilepsy (TLE). Note that the majority of patients were Engel Class I and seizure free and they had a right temporal lobe signal sink pattern on rsMRI temporal latency analysis. MTS = Mesial temporal sclerosis; DNET = dysembryoplastic neuroepithelial tumor.
| Case | Age (yrs) | Pathology | F/u (yrs) | Outcome | Right Temporal Lobe Signal Latency |
|---|---|---|---|---|---|
| 1 | 10.0 | Gliosis | 5.7 | Seizure-free; Engel class I | Sink |
| 2 | 9.0 | MTS/Meningoencephalitis | 7.7 | Engel class III | Source |
| 3 | 17.0 | MTS | 5.1 | Seizure-free; Engel class I | Equivocal |
| 4 | 8.4 | MTS | 1.3 | Seizure-free but abdominal aura; Engel class I | Sink |
| 5 | 5.6 | MTS | 5.9 | 3x/week; Engel class III | Source |
| 6 | 15.5 | Gliosis | 2.1 | Seizure-free but rare aura; Engel class I | Sink |
| 7 | 16.4 | DNET | 1.9 | Seizure-free; Engel class I | Sink |
| 8 | 2.3 | Focal Cortical Dysplasia | 2.5 | Seizure-free; Engel class I after second surgery | Sink |
| 9 | 14.8 | MTS | 1.5 | Seizure-free; Engel class I | Sink |
| 10 | 11.2 | DNET | 3.3 | Seizure-free; Engel class I | Source |
| 11 | 4.4 | Focal Cortical Dysplasia and Tuber | 3.5 | Rare Seizures; Engel class II | Sink |
| 12 | 13.8 | Mild reactive changes after grid placement | 2.1 | Seizure-free; Engel class I | Source |
Table 2. Right Temporal Lobe Epilepsy patients.
Table 2 demonstrates the demographics and right temporal lobe signal latency pattern for the 14 patients with right temporal lobe epilepsy (TLE). Note that the majority of patients were Engel Class I and seizure free and they had a right temporal lobe signal source pattern on rsMRI temporal latency analysis. MTS = Mesial temporal sclerosis; DNET = dysembryoplastic neuroepithelial tumor.
| Case | Age (yrs) | Pathology | F/u (yrs) | Outcome | Right Temporal Lobe Signal Latency |
|---|---|---|---|---|---|
| 13 | 8.7 | MTS | 0.4 | Seizure-free; Engel class I | Equivocal |
| 14 | 18.0 | MTS | 2.0 | Seizure-free; Engel class I | Source |
| 15 | 16.1 | MTS | 4.5 | rare complex partial, Engel class II | Source |
| 16 | 12.7 | Hippocampal sclerosis | 2.1 | Seizure-free; Engel class I | Equivocal |
| 17 | 3.4 | Patchy neuronal loss + gliosis | 1.1 | Seizure-free; Engel class I | Source |
| 18 | 12.0 | MTS with radiation effect from previous grade II astrocytoma treatment | 4.6 | Seizure-free; Engel class I until death | Sink |
| 19 | 7.5 | MTS | 4.1 | No clear seizures; Engel class I | Source |
| 20 | 0.9 | Cortical Malformation/Tuber | 4.5 | Recurred seizures with Tuberous sclerosis; Engel Class III | Source |
| 21 | 5.7 | Right patchy neuronal loss + gliosis | 4.5 | Seizure-free; Engel class I | Equivocal |
| 22 | 10.8 | Grade I astrocytoma | 0 | Not available | Source |
| 23 | 17.5 | Mild gliosis, no MTS | 1.8 | Engel Class I | Source |
| 24 | 4.3 | Grade I ganglioglioma | 1.5 | Seizure-free; Engel class I | Equivocal |
| 25 | 16.5 | MTS | 1 | Seizure-free; Engel class I | Source |
| 26 | 16.1 | Encephalomalacia, no MTS or neoplasm | 1 | Seizure-free; Engel class I | Source |
Resting-state Functional connectivity
No significant group differences were found in ROI-based rsMRI functional connectivity analysis in these pediatric patients. Regardless of sedation paradigm, the patients’ standard 36-seed correlation maps retained the familiar network architecture as described by Pizoli et al.[26]
Resting-state Lag Analysis
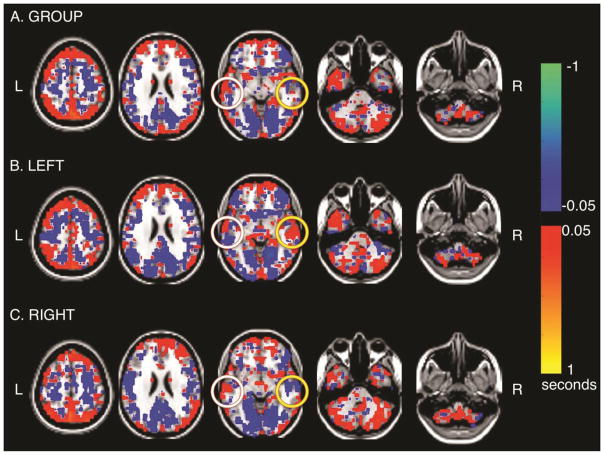
In displays of computed lag values, blue-green hues represent regions in which the BOLD signal is early with respect to the rest of the brain, i.e., signal “source,” and red-orange hues represent relative lateness, i.e., signal “sink”[31]. Figure 1 shows the temporal latency maps of the group average (Figure 1A, n=26), left temporal lobe epilepsy patients (Figure 1B, n=12) and right temporal lobe epilepsy patients (Figure 1C, n=14). The group average demonstrates a substantial signal earliness in the posterior cingulate cortex (PCC) compared to the global mean signal. The PCC is the known driver of the default mode network.[31] The group average also demonstrates substantial signal latency (positive latency values; orange hues) in the cerebellum. Both the PCC signal “source” and the cerebellar signal “sink” patterns are similar to those in the healthy controls described initially with this temporal latency analytical method by Mitra et al.[31] Of note, figures 1B and 1C demonstrate striking lateralized effects: in left temporal lobe epilepsy, the left temporal lobe is a relative signal “source” (blue) and the right temporal lobe is a relative signal “sink” (orange). Conversely, in right temporal lobe epilepsy, the left temporal lobe is a signal “sink” and the right temporal lobe is a signal “source”. Of note, there is little signal in mesial temporal structures in either TLE group or in the combined cohort.
Figure 1. Group resting state MRI Temporal Latency analysis. A.
All 26 patients are included. note the Posterior Cingulate Cortex (PCC) has a blue signal source pattern and the cerebellum has a red signal sink pattern. The left temporal lobe (white circle) demonstrates an equivocal pattern. The right temporal lobe (yellow circle) demonstrates no latency pattern. B. The 12 left temporal lobe epilepsy (TLE) patients are shown in average. Note the PCC is a blue signal source and the cerebellum a red signal sink. The left temporal lobe is a blue relative signal source (white circle) and the right temporal lobe a red relative signal sink (yellow circle). C. The 14 right TLE patients are shown in average. Note the PCC is a blue signal source and the cerebellum a red signal sink. The left temporal lobe is a red signal sink and the right temporal lobe is a blue relative signal source.
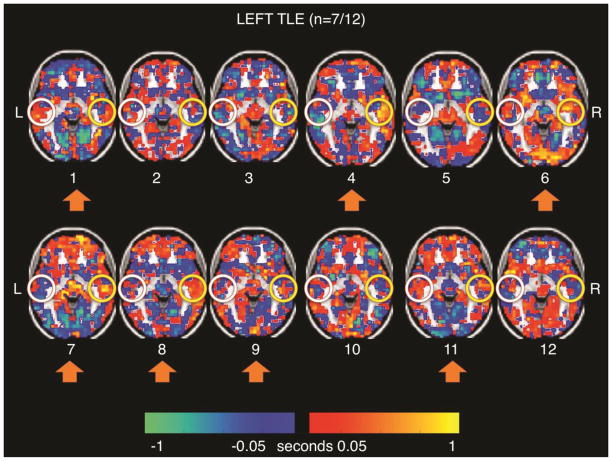
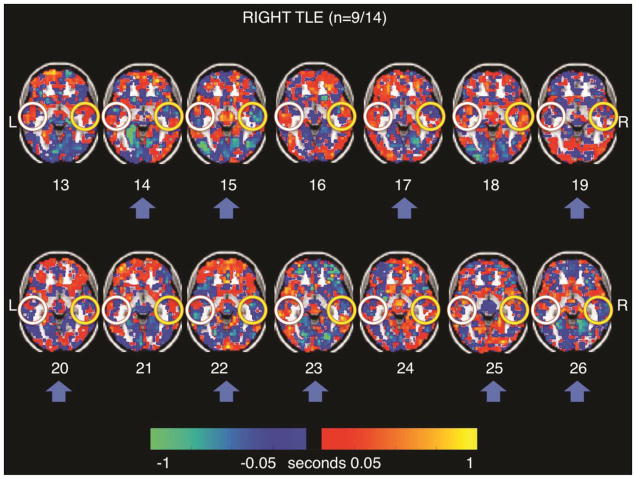
Figure 2 shows the individual signal latency maps in the LTLE patients. Participants 1, 4, 6, 7, 8, 9, and 11 (n=7/12 or 58%) exhibited the right temporal lobe “sink” pattern (orange) seen in the group average latency map (Figure 1B). Figure 3 shows the individual signal latency maps in the RTLE patients. Participants 14, 15, 17, 19, 20, 22, 23, 25, and 26 (n=9/14 or 64%) exhibited the right temporal lobe “source” pattern (blue) seen in the group average latency map in Figure 1C. Mesial structures did not exhibit lags relative to the whole brain in any of the individual patients. A quantitative comparison of the mean rsMRI temporal latency in the right lateral temporal ROI between the two groups of patients seen in Figures 2 and 3 was not significant (p = 0.158).
Figure 2. Left Temporal Lobe Epilepsy resting state MRI Individual Latency Maps.
Note that cases 1, 4, 6, 7, 8, 9, and 11 (n=7/12, 58%) demonstrate the group trend of the right temporal lobe as a red relative signal sink (yellow circles). The left temporal lobe pattern (white circles) does not hold up from the left TLE cohort level analysis (Figure 1B).
Figure 3. Right Temporal Lobe Epilepsy resting state MRI Individual Latency Maps.
Note that cases 14, 15, 17, 19, 20, 22, 23, 25 and 26 (n=9/14, 64%, blue arrows) demonstrate the group trend of the right temporal lobe as a blue relative signal sink (yellow circles). The left temporal lobe pattern (white circles) does not hold up from the right TLE cohort level analysis (Figure 1C).
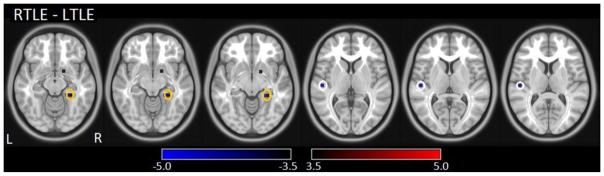
Figure 4 illustrates the latency group difference map (RTLE patients minus LTLE patients). Within the temporal lobes, two clusters with significant latency differences between the two groups (p<0.001) were found bilaterally in the posteromedial aspects; the Montreal Neurological Institute (MNI) coordinates of these significant clusters are listed in Table 3. In addition, the cross-correlation of the averaged time series between the two ROIs in the left and right temporal lobes was r=0.50 +/− 0.20 for the LTLE cohort and r=0.43 +/− 0.21 for the RTLE cohort, indicating weak interhemispheric cross-correlation in these patients. Furthermore, an autocorrelation plot (Supplementary Figure 1) shows that temporal autocorrelation is negligible in this data. Examining rsMRI signal standard deviation maps (Supplementary Figure 2) reveal that the standard deviation maps do not correlate with the temporal latency maps in this cohort and there was no large difference in the standard deviation maps of the covariance across the patients. These results combined suggest that the rsMRI temporal latency differences in this cohort were not due to autocorrelation or covariance amplitude artifact of individual patients.
Figure 4. Latency group difference map (RTLE patients minus LTLE patients).
Clusters with significant group differences in the temporal lobe (P<0.001) are depicted as white circles in the left temporal lobe and orange circles in the right temporal lobe. Blue clusters indicate RTLE group brain regions that had significantly earlier signal than compared to the LTLE group.
Table 3. Significant clusters in the temporal lobe on the temporal latency group difference map.
There are two significant posteromedial temporal lobe clusters in the RTLE-LTLE group difference map as seen in Figure 4. LTLE: left temporal lobe epilepsy; RTLE: right temporal lobe epilepsy; MNI: Montreal Neurological Institute.
| Temporal Lobe Location | Cluster color | Latency in RTLE | Latency in LTLE | x, y, z (MNI) | Cluster size | t- value | p- value |
|---|---|---|---|---|---|---|---|
| 1 Left Posteromedial | Blue | Early | Late | −50, −19, 8 | 8 | −4.0 | <0.001 |
| 2 Right posteromedial | Blue | Early | Late | 24, −37, −11 | 16 | −4.3 | <0.001 |
Discussion
This is the first study to examine resting state BOLD MRI signal temporal latency changes in sedated children with medically refractory temporal lobe epilepsy and to utilize the results to lateralize the epileptogenic side. Current pre-surgical workup paradigms involve high-resolution, structural brain MRI, electroencephalography, neuropsychological workup and usually at least one advanced diagnostic modality, such as magnetoencephalography, PET or SPECT.[5] However, the latter workup and advanced diagnostics require additional patient appointments and costs to the medical system. In this study, the rsMRI BOLD signal is obtained with the initial, clinically necessary structural MRI, so temporal latency analysis could help identify temporal lobectomy candidates without potentially harmful further sedation, radiation or costly appointments. Once obtained, the rsMRI temporal latency data could be centrally processed and children who would benefit from surgical intervention could be referred to an academic epilepsy center. Using a refined method of this technique could potentially identify and benefit untreated young children with unilateral refractory temporal lobe epilepsy by offering a simple screening modality. The earlier epilepsy is treated, the better the overall cognitive outcome[41]; successful epilepsy treatment may also normalize resting state architecture and help preserve normal brain development.[26]
This study demonstrates that LTLE and RTLE patients had both qualitative and quantitative group differences in rsMRI signal latency in the temporal lobes. Quantitatively, table 3 describes significant (p< 0.001) differences of rsMRI temporal latency between the two patient groups in the posteromedial temporal lobes. Such results (Figure 4 and Table 3) may provide guidance for ROI placement to detect group differences in TLE lateralization in a future, larger cohort of these patients. The relative signal earliness in the temporal lobe ipsilateral to the epileptogenic side and the relative lateness in the contralateral temporal lobe in TLE patients may be due to the impact of neuronal activities such as interictal epileptiform discharges on the rsMRI signal. A previous study in adult TLE demonstrated that interictal discharge-triggered brain activation is mainly located in the epileptogenic focus of the mesial temporal lobe.[42] Zhang et al. also found increased amplitude of low-frequency fluctuations in the ipsilateral mesial temporal lobe and correlated these changes with interictal discharges.[43] Further, an EEG-fMRI study demonstrated that generalized spike-and-wave discharges elevated synchronization of intrinsic brain network activity in absence seizures.[44] Interictal discharges may affect the intrinsic network connectivity in a similar way that causes the relative rsMRI signal earliness in the ipsilateral temporal lobe in our TLE cohort. Further studies are needed to better understand the mechanisms of quantitative rsMRI temporal latency changes in TLE patients.
Similar to the quantitative data, the qualitative latency maps in Figure 1B and 1C indicate that the lateral temporal lobe contralateral to the seizure focus exhibited relative lateness with respect to the whole brain mean signal. Thus, in left TLE, a right lateral temporal region was late (Figure 1B) whereas, in right TLE, a left lateral temporal region was late (Figure 1C). At the individual level, the group pattern qualitatively held up in 7/12 (58.3%) left TLE patients (Figure 2) and 9/14 (64.2%) right TLE patients (Figure 3). A quantitative comparison of the mean rsMRI temporal latency in this right lateral temporal region trended towards significance (p = 0.158), suggesting further work in a larger dataset could confirm this right temporal lobe signal latency pattern. In this study, there were only three patients, cases 2, 5 and 20 with Engel class III outcomes, i.e., persistent seizures despite surgery. Interestingly, the two left TLE patients, cases 2 and 5, that had Engel class III outcomes also had opposite right temporal latency patterns, i.e., signal sources, rather than the signal sink seen in the left TLE group (Figure 1B). This discordance between the initial preoperative temporal latency rsMRI pattern and postoperative outcome could mean that temporal latency analysis may be a useful adjunctive tool in preoperative counseling and surgical decision-making. For case 20, the etiology of the temporal lobe epilepsy was tuberous sclerosis, a condition often associated with recurrent, medically-refractory multi-focal epilepsy.[45] Overall, a concordant right temporal lobe temporal latency rsMRI pattern in unilateral TLE patients may be helpful for the identification of surgical candidates; with more studies, a discordant latency pattern may be predictive of persistent postoperative seizures and allow more informed presurgical consent discussions with families.
In contrast to the temporal latency analysis, standard “seed”-based rsMRI functional connectivity analysis did not detect group differences in this study. The weak cross-correlation between the two temporal lobe ROIs in this study is consistent with the physiologic metabolic mismatch typically seen in FDG-PET on the ipsilateral side in unilateral TLE. The insignificant and equivocal (amygdala and hippocampal ROIs) connectivity results were likely due to the aforementioned signal artifact of the sphenoid bone and fluid pulsations of the basal brain cisterns on the mesial temporal brain BOLD signal. In addition, many of the patients had unilateral mesial temporal sclerosis with resulting shrunken mesial temporal structures and the signal region of interest volume would be different between the two sides. Previous studies have reported inconsistent findings, i.e., increased[23] as well as decreased[24, 27] functional connectivity in temporal lobe epilepsy. We did not find obvious qualitative signal latency differences between the groups in the mesial temporal structures. However, the current standard of care in clinical interictal 18FDG-PET relies on qualitative interpretation of brain hypometabolism by physicians for identification of epileptogenic foci, such as lateral temporal lobe structures. Hence, temporal latency analysis similarly focusing on the lateral temporal lobe as described by this study, may also be a way for lateralizing functional abnormalities in temporal lobe epilepsy.
The differences in left versus right temporal lobe epilepsy rsMRI signal latency may reflect a tendency for diseased areas of the brain to reduce communication with the DMN, a major network in the brain’s natural resting state.[46] Loss of functional connectivity is a correlate of pathology.[26] The DMN is an important structure linked to memory consolidation and predictive modeling of the world.[47, 48] Of note, in left TLE at the group level, the left lateral temporal lobe was iso-latent with respect to the PCC (Figure 1B), potentially indicating that the diseased left temporal lobe did not communicate as well with the DMN as the healthy right temporal lobe. The opposite effect was observed in the right TLE patient group (Figure 1C). The lack of strong cross-correlation in rsMRI latency between regions in the two temporal lobes was also indicative of a unilateral temporal lobe dysfunction.
As epilepsy and neurodegenerative diseases can affect the mesial temporal structures bilaterally, concordant data confirming laterality predicates surgical management[49]. Like magnetoencephalography[50] or single photon emission computed tomography[51], lateralized mesial temporal hypometabolism with 18FDG-PET often provides confirmatory data for determining laterality.[52] However, the 18FDG radiotracer, which gauges glucose uptake, yields a temporally static image of pathology, while the BOLD signal, which correlates with cerebral blood flow and cerebral metabolic rate of oxygen provides a dynamic assessment of functional abnormalities. This study suggests that functional abnormalities of signal propagation on a timescale of seconds may represent a sensitive correlate of pathology.
In summary, our study identifies qualitative and quantitative differences in rsMRI temporal latency within the right temporal lobe between a cohort of left and right TLE patients, which may help lateralize unilateral temporal lobe epilepsy in pediatric patients after confirmation with a larger, multi-institutional study. This study is limited by the number of participants in both groups, the absence of age-matched controls and the heterogeneity of underlying cause of epilepsy as well as the sedation protocol. We currently are involved in a multicenter collaboration to increase prospective patient enrollment and standardize the sedation protocol to eventually test and strengthen the conclusion of this hypothesis-generating study, as described and studied by Ioannidis and Trikalinos.[53] Another weakness that comes with studying the temporal structures with low subject numbers is diurnal resting state network variability[40], even using such methods as singular value decomposition[29, 54]. Finally, there may be value in using multi-modal MR-based detection and confirmation of epileptogenic foci[55] such as rsMRI combined with diffusion tractography[21] and myelin mapping.[56]
Conclusions
This study examines temporal latency in the resting state functional BOLD MRI signal in a cohort of children with unilateral surgical temporal lobe epilepsy. Analysis of signal lags (relative to the whole brain mean) in lateral temporal regions revealed lateness (at the group-level) contralateral to the seizure focus. This pattern was detectable in the majority of individual patients and striking, both qualitatively as well as quantitatively. Temporal latency rsMRI analysis may offer a useful adjunct modality for lateralization in pediatric, medically refractory, temporal lobe epilepsy.
Supplementary Material
Acknowledgments
Sources of Support:
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number L30 HD089125 (MNS) as well as U54 HD087011 to the Intellectual and Developmental Disabilities Research Center at Washington University (JSS).
Footnotes
Disclosure:
Conflicts of Interest: On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Informed Consent: Informed consent was obtained from all study participants.
Note: A portion of this work was accepted for oral presentation at the American Society of Pediatric Neurosurgeons Annual Meeting in February 2016.
References
- 1.Gleissner U, Sassen R, Schramm J, Elger CE, Helmstaedter C. Greater functional recovery after temporal lobe epilepsy surgery in children. Brain : a journal of neurology. 2005;128:2822–2829. doi: 10.1093/brain/awh597. [DOI] [PubMed] [Google Scholar]
- 2.Mohamed A, Wyllie E, Ruggieri P, Kotagal P, Babb T, Hilbig A, Wylie C, Ying Z, Staugaitis S, Najm I, Bulacio J, Foldvary N, Luders H, Bingaman W. Temporal lobe epilepsy due to hippocampal sclerosis in pediatric candidates for epilepsy surgery. Neurology. 2001;56:1643–1649. doi: 10.1212/wnl.56.12.1643. [DOI] [PubMed] [Google Scholar]
- 3.Smyth MD, Limbrick DD, Jr, Ojemann JG, Zempel J, Robinson S, O'Brien DF, Saneto RP, Goyal M, Appleton RE, Mangano FT, Park TS. Outcome following surgery for temporal lobe epilepsy with hippocampal involvement in preadolescent children: emphasis on mesial temporal sclerosis. Journal of neurosurgery. 2007;106:205–210. doi: 10.3171/ped.2007.106.3.205. [DOI] [PubMed] [Google Scholar]
- 4.Camfield CS, Camfield PR, Gordon K, Wirrell E, Dooley JM. Incidence of epilepsy in childhood and adolescence: a population-based study in Nova Scotia from 1977 to 1985. Epilepsia. 1996;37:19–23. doi: 10.1111/j.1528-1157.1996.tb00506.x. [DOI] [PubMed] [Google Scholar]
- 5.Cross JH, Jayakar P, Nordli D, Delalande O, Duchowny M, Wieser HG, Guerrini R, Mathern GW International League against Epilepsy SfPES, Commissions of N, Paediatrics. Proposed criteria for referral and evaluation of children for epilepsy surgery: recommendations of the Subcommission for Pediatric Epilepsy Surgery. Epilepsia. 2006;47:952–959. doi: 10.1111/j.1528-1167.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- 6.Boshuisen K, van Schooneveld MM, Uiterwaal CS, Cross JH, Harrison S, Polster T, Daehn M, Djimjadi S, Yalnizoglu D, Turanli G, Sassen R, Hoppe C, Kuczaty S, Barba C, Kahane P, Schubert-Bast S, Reuner G, Bast T, Strobl K, Mayer H, de Saint-Martin A, Seegmuller C, Laurent A, Arzimanoglou A, Braun KP TimeToStop cognitive outcome study g. Intelligence quotient improves after antiepileptic drug withdrawal following pediatric epilepsy surgery. Annals of neurology. 2015;78:104–114. doi: 10.1002/ana.24427. [DOI] [PubMed] [Google Scholar]
- 7.Sugano H, Arai H. Epilepsy surgery for pediatric epilepsy: optimal timing of surgical intervention. Neurol Med Chir (Tokyo) 2015;55:399–406. doi: 10.2176/nmc.ra.2014-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cataldi M, Avoli M, de Villers-Sidani E. Resting state networks in temporal lobe epilepsy. Epilepsia. 2013;54:2048–2059. doi: 10.1111/epi.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Constable RT, Scheinost D, Finn ES, Shen X, Hampson M, Winstanley FS, Spencer DD, Papademetris X. Potential use and challenges of functional connectivity mapping in intractable epilepsy. Frontiers in neurology. 2013;4:39. doi: 10.3389/fneur.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Frontiers in systems neuroscience. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kokkonen SM, Nikkinen J, Remes J, Kantola J, Starck T, Haapea M, Tuominen J, Tervonen O, Kiviniemi V. Preoperative localization of the sensorimotor area using independent component analysis of resting-state fMRI. Magnetic resonance imaging. 2009;27:733–740. doi: 10.1016/j.mri.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Liu H, Buckner RL, Talukdar T, Tanaka N, Madsen JR, Stufflebeam SM. Task-free presurgical mapping using functional magnetic resonance imaging intrinsic activity. Journal of neurosurgery. 2009;111:746–754. doi: 10.3171/2008.10.JNS08846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hacker CD, Laumann TO, Szrama NP, Baldassarre A, Snyder AZ, Leuthardt EC, Corbetta M. Resting state network estimation in individual subjects. NeuroImage. 2013;82:616–633. doi: 10.1016/j.neuroimage.2013.05.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shannon BJ, Raichle ME, Snyder AZ, Fair DA, Mills KL, Zhang D, Bache K, Calhoun VD, Nigg JT, Nagel BJ, Stevens AA, Kiehl KA. Premotor functional connectivity predicts impulsivity in juvenile offenders. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11241–11245. doi: 10.1073/pnas.1108241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder AZ, Raichle ME. A brief history of the resting state: the Washington University perspective. NeuroImage. 2012;62:902–910. doi: 10.1016/j.neuroimage.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broicher SD, Frings L, Huppertz HJ, Grunwald T, Kurthen M, Kramer G, Jokeit H. Alterations in functional connectivity of the amygdala in unilateral mesial temporal lobe epilepsy. Journal of neurology. 2012;259:2546–2554. doi: 10.1007/s00415-012-6533-3. [DOI] [PubMed] [Google Scholar]
- 20.Haneef Z, Lenartowicz A, Yeh HJ, Engel J, Jr, Stern JM. Effect of lateralized temporal lobe epilepsy on the default mode network. Epilepsy & behavior : E&B. 2012;25:350–357. doi: 10.1016/j.yebeh.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, Luo C, Wang Z, Tan Q, Lu G, Chen H. Default mode network abnormalities in mesial temporal lobe epilepsy: a study combining fMRI and DTI. Human brain mapping. 2011;32:883–895. doi: 10.1002/hbm.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo C, Qiu C, Guo Z, Fang J, Li Q, Lei X, Xia Y, Lai Y, Gong Q, Zhou D, Yao D. Disrupted functional brain connectivity in partial epilepsy: a resting-state fMRI study. PloS one. 2011;7:e28196. doi: 10.1371/journal.pone.0028196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stufflebeam SM, Liu H, Sepulcre J, Tanaka N, Buckner RL, Madsen JR. Localization of focal epileptic discharges using functional connectivity magnetic resonance imaging. Journal of neurosurgery. 2011;114:1693–1697. doi: 10.3171/2011.1.JNS10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waites AB, Briellmann RS, Saling MM, Abbott DF, Jackson GD. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Annals of neurology. 2006;59:335–343. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- 25.Luo C, Li Q, Lai Y, Xia Y, Qin Y, Liao W, Li S, Zhou D, Yao D, Gong Q. Altered functional connectivity in default mode network in absence epilepsy: a resting-state fMRI study. Human brain mapping. 2011;32:438–449. doi: 10.1002/hbm.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pizoli CE, Shah MN, Snyder AZ, Shimony JS, Limbrick DD, Raichle ME, Schlaggar BL, Smyth MD. Resting-state activity in development and maintenance of normal brain function. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11638–11643. doi: 10.1073/pnas.1109144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bettus G, Bartolomei F, Confort-Gouny S, Guedj E, Chauvel P, Cozzone PJ, Ranjeva JP, Guye M. Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. Journal of neurology, neurosurgery, and psychiatry. 2010;81:1147–1154. doi: 10.1136/jnnp.2009.191460. [DOI] [PubMed] [Google Scholar]
- 28.Bettus G, Guedj E, Joyeux F, Confort-Gouny S, Soulier E, Laguitton V, Cozzone PJ, Chauvel P, Ranjeva JP, Bartolomei F, Guye M. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Human brain mapping. 2009;30:1580–1591. doi: 10.1002/hbm.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.James GA, Tripathi SP, Ojemann JG, Gross RE, Drane DL. Diminished default mode network recruitment of the hippocampus and parahippocampus in temporal lobe epilepsy. Journal of neurosurgery. 2013;119:288–300. doi: 10.3171/2013.3.JNS121041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu DH, Lewin JS, Duerk JL. Inadequacy of motion correction algorithms in functional MRI: role of susceptibility-induced artifacts. J Magn Reson Imaging. 1997;7:365–370. doi: 10.1002/jmri.1880070219. [DOI] [PubMed] [Google Scholar]
- 31.Mitra A, Snyder AZ, Hacker CD, Raichle ME. Lag structure in resting state fMRI. Journal of neurophysiology. 2014;111:2374–2391. doi: 10.1152/jn.00804.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitra A, Snyder AZ, Constantino JN, Raichle ME. The Lag Structure of Intrinsic Activity is Focally Altered in High Functioning Adults with Autism. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amemiya S, Kunimatsu A, Saito N, Ohtomo K. Cerebral hemodynamic impairment: assessment with resting-state functional MR imaging. Radiology. 2014;270:548–555. doi: 10.1148/radiol.13130982. [DOI] [PubMed] [Google Scholar]
- 34.Lv Y, Margulies DS, Cameron Craddock R, Long X, Winter B, Gierhake D, Endres M, Villringer K, Fiebach J, Villringer A. Identifying the perfusion deficit in acute stroke with resting-state functional magnetic resonance imaging. Annals of neurology. 2013;73:136–140. doi: 10.1002/ana.23763. [DOI] [PubMed] [Google Scholar]
- 35.Xu Q, Zhang Z, Liao W, Xiang L, Yang F, Wang Z, Chen G, Tan Q, Jiao Q, Lu G. Time-shift homotopic connectivity in mesial temporal lobe epilepsy. AJNR Am J Neuroradiol. 2014;35:1746–1752. doi: 10.3174/ajnr.A3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. Journal of neurophysiology. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 38.Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL Brain Development Cooperative G. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shannon BJ, Dosenbach RA, Su Y, Vlassenko AG, Larson-Prior LJ, Nolan TS, Snyder AZ, Raichle ME. Morning-evening variation in human brain metabolism and memory circuits. Journal of neurophysiology. 2013;109:1444–1456. doi: 10.1152/jn.00651.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freitag H, Tuxhorn I. Cognitive function in preschool children after epilepsy surgery: rationale for early intervention. Epilepsia. 2005;46:561–567. doi: 10.1111/j.0013-9580.2005.03504.x. [DOI] [PubMed] [Google Scholar]
- 42.Laufs H, Hamandi K, Salek-Haddadi A, Kleinschmidt AK, Duncan JS, Lemieux L. Temporal lobe interictal epileptic discharges affect cerebral activity in "default mode" brain regions. Human brain mapping. 2007;28:1023–1032. doi: 10.1002/hbm.20323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z, Lu G, Zhong Y, Tan Q, Chen H, Liao W, Tian L, Li Z, Shi J, Liu Y. fMRI study of mesial temporal lobe epilepsy using amplitude of low-frequency fluctuation analysis. Human brain mapping. 2010;31:1851–1861. doi: 10.1002/hbm.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z, Liao W, Wang Z, Xu Q, Yang F, Mantini D, Jiao Q, Tian L, Liu Y, Lu G. Epileptic discharges specifically affect intrinsic connectivity networks during absence seizures. J Neurol Sci. 2014;336:138–145. doi: 10.1016/j.jns.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 45.Arya R, Tenney JR, Horn PS, Greiner HM, Holland KD, Leach JL, Gelfand MJ, Rozhkov L, Fujiwara H, Rose DF, Franz DN, Mangano FT. Long-term outcomes of resective epilepsy surgery after invasive presurgical evaluation in children with tuberous sclerosis complex and bilateral multiple lesions. J Neurosurg Pediatr. 2015;15:26–33. doi: 10.3171/2014.10.PEDS14107. [DOI] [PubMed] [Google Scholar]
- 46.Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neuroscience and biobehavioral reviews. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. NeuroImage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. discussion 1097–1099. [DOI] [PubMed] [Google Scholar]
- 48.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1089. [DOI] [PubMed] [Google Scholar]
- 49.Radhakrishnan K, So EL, Silbert PL, Jack CR, Jr, Cascino GD, Sharbrough FW, O'Brien PC. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy: a multivariate study. Neurology. 1998;51:465–471. doi: 10.1212/wnl.51.2.465. [DOI] [PubMed] [Google Scholar]
- 50.Baumgartner C, Pataraia E, Lindinger G, Deecke L. Neuromagnetic recordings in temporal lobe epilepsy. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2000;17:177–189. doi: 10.1097/00004691-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Ho SS, Berkovic SF, Berlangieri SU, Newton MR, Egan GF, Tochon-Danguy HJ, McKay WJ. Comparison of ictal SPECT and interictal PET in the presurgical evaluation of temporal lobe epilepsy. Annals of neurology. 1995;37:738–745. doi: 10.1002/ana.410370607. [DOI] [PubMed] [Google Scholar]
- 52.Salanova V, Markand O, Worth R, Smith R, Wellman H, Hutchins G, Park H, Ghetti B, Azzarelli B. FDG-PET and MRI in temporal lobe epilepsy: relationship to febrile seizures, hippocampal sclerosis and outcome. Acta neurologica Scandinavica. 1998;97:146–153. doi: 10.1111/j.1600-0404.1998.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 53.Ioannidis JP, Trikalinos TA. Early extreme contradictory estimates may appear in published research: the Proteus phenomenon in molecular genetics research and randomized trials. J Clin Epidemiol. 2005;58:543–549. doi: 10.1016/j.jclinepi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 54.Worsley KJ, Chen JI, Lerch J, Evans AC. Comparing functional connectivity via thresholding correlations and singular value decomposition. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2005;360:913–920. doi: 10.1098/rstb.2005.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osipowicz K, Sperling MR, Sharan AD, Tracy JI. Functional MRI, resting state fMRI, and DTI for predicting verbal fluency outcome following resective surgery for temporal lobe epilepsy. Journal of neurosurgery. 2015:1–9. doi: 10.3171/2014.9.JNS131422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spader HS, Ellermeier A, O'Muircheartaigh J, Dean DC, 3rd, Dirks H, Boxerman JL, Cosgrove GR, Deoni SC. Advances in myelin imaging with potential clinical application to pediatric imaging. Neurosurg Focus. 2013;34:E9. doi: 10.3171/2013.1.FOCUS12426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.