Abstract
Prevalence of cigarette smoking among opioid-dependent individuals is six-fold that of the general US adult population and their quit rates are notoriously poor. One possible reason for the modest cessation outcomes in opioid-dependent smokers may be that they experience more severe tobacco withdrawal upon quitting. In this secondary analysis, we evaluated tobacco withdrawal in opioid-dependent (OD) smokers vs. smokers without co-occurring substance use disorders (SUDs). Participants were 47 methadone- or buprenorphine-maintained smokers and 25 non-SUD smokers who completed one of several two-week studies involving daily visits for biochemical monitoring, delivery of financial incentives contingent on smoking abstinence, and assessment of withdrawal via the Minnesota Nicotine Withdrawal Scale (MNWS). Prior to quitting smoking, OD smokers presented with higher baseline withdrawal scores than non-SUD smokers (1.7±0.2 vs. 0.7±0.2, respectively; F(1,63)=7.31, p<.001). Withdrawal scores in both groups decreased over the subsequent two-week period with no group differences (F(1,910)=0.50, p=.48). A similar pattern was observed on craving (i.e., Desire to Smoke item of the MNWS), though the trajectory of decrease over time on this item was also moderated by gender. Overall, there was no difference in withdrawal during biochemically-verified smoking abstinence between OD and non-SUD smokers, suggesting that elevated withdrawal severity following quitting may not be a major factor contributing to the poor cessation outcomes consistently observed among OD smokers. Further scientific efforts are needed to improve our understanding of the high smoking rates and modest cessation outcomes in this challenging population.
Keywords: Tobacco withdrawal, nicotine, opioid, methadone, buprenorphine
INTRODUCTION
Cigarette smoking is the leading preventable cause of morbidity and mortality in the United States (US) (USDHHS, 2014). While smoking has steadily declined in the general population, it remains entrenched among certain vulnerable populations. Individuals with substance use disorders, in particular, are overrepresented among current cigarette smokers and bear a disproportionate burden of smoking-related disease and premature death. This is especially the case among individuals with opioid use disorder, in whom prevalence of smoking is six-fold that of the general US adult population (84–94% vs. 15%, respectively)(Guydish et al., 2011, 2016; Jamal et al., 2016; Nahvi, Richter, Li, Modali, & Arnsten, 2006; Richter, Gibson, Ahluwalia, & Schmelzle, 2001). Further, their responses to smoking cessation treatments are notoriously poor, with quit rates one-fourth that of smokers without substance use disorders (Miller & Sigmon, 2015; Okoli et al., 2010; Zirakzadeh, Shuman, Stauter, Hays, & Ebbert, 2013).
One potential reason for the modest cessation outcomes in opioid-dependent smokers is that this group may experience more severe tobacco withdrawal upon quitting (Miller & Sigmon, 2015; Story & Stark, 1991). However, while prior studies have found that smokers with current or past alcohol dependence report more severe tobacco withdrawal when they stop smoking (Hughes, 1996), the limited data on tobacco withdrawal in opioid-dependent smokers thus far have been mixed, with one study demonstrating that methadone attenuates tobacco withdrawal (Elkader, Brands, Selby, & Sproule, 2009) and another showing increases in withdrawal during methadone dose increases (Story & Stark, 1991). To our knowledge, no prior studies have examined the effects of buprenorphine on tobacco withdrawal.
In the present secondary analysis, we sought to evaluate tobacco withdrawal in opioid-dependent (OD) smokers vs. smokers without comorbid substance use disorders (SUDs) using data from five outpatient laboratory studies which were conducted at the University of Vermont by our research group and utilized a uniform platform of daily visits involving biochemical monitoring, assessment of withdrawal, and delivery of financial incentives contingent on smoking abstinence (Bradstreet et al., 2014; Dunn, Sigmon, Thomas, Heil, & Higgins, 2008; Dunn et al., 2010; Sigmon et al., 2015; Yoon, Higgins, Bradstreet, Badger, & Thomas, 2009). Also of interest was whether gender was a moderator of nicotine withdrawal given that prior literature in the general population has suggested an association between gender and tobacco withdrawal and/or craving (e.g., Eissenberg, Adams, Riggins, & Likness, 1999; Leventhal et al., 2007; Pang & Leventhal, 2013; Piasecki, Jorenby, Smith, Fiore, & Baker, 2003; Weinberger, Platt, Shuter, & Goodwin, 2016).
MATERIALS AND METHODS
Participants
In the three studies examining the efficacy of voucher-based financial incentives to promote smoking abstinence in OD smokers, participants were methadone- or buprenorphine-maintained adults (Dunn et al., 2008, 2010; Sigmon et al., 2015). Eligible participants had to be 18–65 years old, report smoking ≥10 cigarettes per day for ≥1 year, be on a stable methadone or buprenorphine dose, and have ≤30% samples testing positive for illicit drugs. Participants who were pregnant or presented with active serious mental or physical illness that might interfere with study participation were excluded.
The non-SUD smoker sample included participants from two studies using monetary incentives to experimentally induce smoking abstinence (Bradstreet et al., 2014; Yoon et al., 2009). Eligible participants had to be 18–55 years old, in good health, report smoking ≥10 cigarettes per day for ≥1 year, report no plans to quit smoking in the near future, and have an expired breath carbon monoxide (CO) sample at intake of ≥15 ppm (Bradstreet et al., 2014) or 18 ppm (Yoon et al., 2009). Those who were pregnant, had active serious mental or physical illness, were using psychoactive medications, or had current drug or alcohol dependence other than tobacco dependence were excluded.
Procedures
In the studies with OD smokers, eligible participants were randomly assigned to a Contingent or Noncontingent experimental group and visited the laboratory daily for 14 consecutive days (Dunn et al., 2008, 2010; Sigmon et al., 2015). At each visit, breath CO and urine cotinine samples were collected for biochemical verification of smoking status. Contingent participants received voucher-based incentives for CO levels ≤6 ppm on Study Days 1–5 and cotinine levels ≤80 ng/ml on Days 6–14. Noncontingent participants received vouchers independent of smoking status.
In the studies with non-SUD smokers, eligible participants were randomly assigned to a Contingent or Noncontingent experimental group and visited the laboratory daily for 14 (Yoon et al., 2009) or 15 (Bradstreet et al., 2014) consecutive days. Contingent participants received monetary incentives for CO readings of ≤4 ppm on Study Days 1–7 and cotinine levels of ≤80 ng/ml on Days 8–14. Noncontingent participants received monetary incentives independent of smoking status.
While the present manuscript reports on the results of secondary analyses on deidentified data collapsed across five outpatient laboratory studies, those original studies have been published previously and were approved by the University of Vermont Committee on Human Research in the Medical Sciences. All participants provided written informed consent prior to participating.
Measures
Intake assessments included the Fagerstrom Test for Cigarette Dependence (FTCD; Fagerstrom, 2012) and the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1998) which measure nicotine dependence and tobacco withdrawal, respectively. The MNWS is measured on an ordinal scale: 0=none, 1=slight, 2=mild, 3=moderate, 4=severe. The seven DSM-5 items from the MNWS were averaged for a single severity score (range 0–4; Cronbach’s alpha=0.87), with the Desire to Smoke item analyzed separately as a measure of craving (Hughes & Hatsukami, 1998). At each subsequent visit during the 2-week studies, participants completed the MNWS and provided breath and urine samples.
Data analyses
To prevent ongoing smoking from confounding our evaluation of tobacco withdrawal, analyses were limited to the 72 participants (47 OD and 25 non-SUD smokers) who received the abstinence-contingent interventions and provided >85% of samples indicating smoking abstinence (Dunn et al., 2010). Complete abstinence (i.e., 100% of samples indicating smoking abstinence) was not required as prior research suggests that small amounts of smoking do not substantially influence withdrawal symptoms (Hughes & Hatsukami, 1986).
Baseline characteristics between the OD and non-SUD smoker groups were compared using chi-square tests for categorical variables and t tests for continuous measures. Repeated measures analysis of covariance using maximum likelihood estimation was used to examine mean MNWS and Desire to Smoke scores over time between the two groups. All variables that differed between the two groups at intake were included in the models as covariates (i.e., age, education, number of years smoked regularly, intake breath CO, FTCD score), as were the baseline MNWS and Desire to Smoke scores. As noted earlier, gender was also included as a moderator in subsequent analyses. Change in withdrawal during the first 24 hours of abstinence was examined by comparing the difference in mean scores from the pre-cessation visit to the first day of abstinence (Hughes, 2007a). Time course of withdrawal thereafter was assessed by comparing mean scores between the groups on the remaining 14 days, with time modeled as a continuous variable. Sensitivity analyses were also conducted to examine potential effects of opioid medication type (i.e., methadone, buprenorphine) on withdrawal and craving within the OD smoker group. Regarding missing data, all of the OD and non-SUD smokers included in these analyses completed the 14-day studies, and data was missing for <1% and 1.5% of the non-SUD and OD smokers, respectively. Statistical significance was determined based on α=0.05, with all analyses performed using SAS statistical software version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Participant characteristics
Among OD smokers, 70% and 30% were enrolled in methadone (102±48 mg/day) or buprenorphine (13±5 mg/day) treatment, respectively. Relative to non-SUD smokers, the OD smokers were older (p<.05), had completed fewer years of education (p<.01), and had been smoking regularly for more years (p<.01) (Table 1). While there were no group differences in baseline urinary cotinine levels or self-reported number of cigarettes smoked per day, OD smokers did present with lower CO values and FTCD scores at intake (p’s<.01).
Table 1.
Participant characteristics
| Opioid-Dependent Smokers (N=47) |
Non-SUD Smokers (N=25) |
p-value | |
|---|---|---|---|
| Female, % | 51 | 32 | 0.15 |
| Caucasian, % | 92 | 92 | 0.10 |
| Age, years | 33.4 ± 10.4 | 27.3 ± 9.1 | 0.02 |
| Education completed, years | 12.6 ± 1.5 | 14.1 ± 2.1 | 0.01 |
| Number of cigs/day in past week | 17.6 ± 6.3 | 18.2 ± 6.0 | 0.69 |
| Number of years smoked regularly | 17.7 ± 10.7 | 9.0 ± 9.4 | <0.01 |
| Urinary cotinine at intake, ng/ml | 1189.7 ± 617.7 | 1245.5 ± 618.3 | 0.72 |
| Breath CO at intake, ppm | 9.8 ± 5.4 | 21.0 ± 8.0 | <0.01 |
| FTCDa | 4.7 ± 2.0 | 6.0 ± 1.4 | <0.01 |
Note: Table 1 values represent mean ± SD unless otherwise indicated.
Fagerstrom Test for Cigarette Dependence; possible score range: 0–10
Tobacco withdrawal
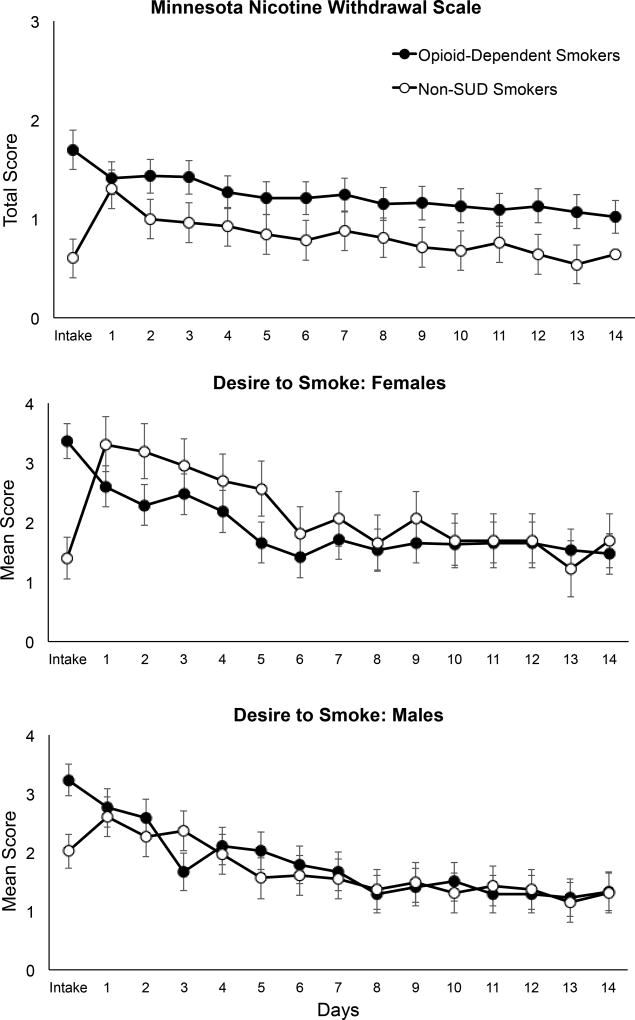
Prior to quitting smoking (i.e., at Study Intake), OD smokers presented with higher MNWS scores than non-SUD smokers (1.7±0.2 vs. 0.7±0.2, respectively; F(1,63)=7.31, p<.001), after adjusting for the co-variates in the model. Changes in withdrawal severity between Intake and the first day of abstinence (i.e., Study Day 1) did not differ by group (F(1,60)=3.02, p=.09) (Figure 1, upper panel). Thereafter, tobacco withdrawal decreased over time in both groups (F(1,911)=105.84, p<.0001) with no difference between groups (F(1,910)=0.50, p=.48). No interactions with gender were observed on MNWS scores. Changes in MNWS scores over time did not vary as a function of opioid medication type within the OD group (F(1,588)=0.99, p=0.32).
Figure 1.
Upper panel: Minnesota Nicotine Withdrawal Scale (MNWS) mean total scores across Study Intake and the 14 consecutive daily visits. Data are presented for OD (closed circles) and non-SUD (open circles) smokers. The y-axis is presented on a smaller scale to permit more detailed inspection of the data (scale range: 0–4). Center and lower panels: Mean craving scores, as measured by the MNWS Desire to Smoke item, across Intake and the 14 daily visits by gender. All error bars represent standard error of the mean (SEM).
With regard to the Desire to Smoke item of the MNWS, OD smokers presented with higher scores prior to quitting smoking (i.e., at Intake) compared to non-SUD smokers (3.2±0.2 vs. 1.7±0.3, respectively; F(1,63)=15.97, p<.001). There was a significant group effect (F(1,60)=9.25, p<0.01) on changes in craving between Intake and the first day of abstinence (i.e., Day 1), with OD smokers experiencing a decrease in craving of −0.61±0.2 (t(60)=2.52, p=0.01) compared to SUD smokers whose craving increased by 0.82±0.3 (t(60)= −2.82, p<0.01). There was also a significant gender × group × time interaction in craving over the remaining study visits (F(1,907)=4.57, p=0.03) (Figure 1, center and lower panels). Among females, there was a significant group × visit interaction in Desire to Smoke scores (F(1,907)=4.41, p=0.04), with females in the non-SUD group reporting a steeper decline in craving from Day 1 through 14 (slope=−0.14±0.02, t(907)= −7.82, p<0.0001) than those in the OD smoker group (−0.08±0.01, t(907)= −7.57, p<0.0001). In contrast, males showed no group × visit differences in Desire to Smoke (F(1,907)=0.52, p=0.47), with similar decreases in craving over time seen in the OD (−0.09±0.01, t(907)=−8.16, p<0.0001) and non-SUD (−0.10±0.01, t(907)=−7.76, p<0.0001) groups. Within the OD smoker group, participants maintained on both medications showed a significant decrease in craving over time, with some evidence of craving decreasing at a faster rate among those maintained on methadone than buprenorphine (F(1,587)=7.47, p=0.01).
DISCUSSION
This study provides the most rigorous evaluation of tobacco withdrawal in opioid-dependent smokers to date, as well as the first comparison with a sample of smokers without comorbid SUDs. Prior to quitting smoking, OD smokers presented with higher baseline tobacco withdrawal scores compared to non-SUD smokers, though the clinical significance of this difference (i.e., approximately one unit on a four-point ordinal scale) is unclear. While the elevated pre-cessation withdrawal observed here is consistent with prior studies among vulnerable smokers with psychiatric or drug abuse problems (Heil, Higgins, Mongeon, Badger, & Bernstein, 2006; Madden et al., 1997; Tidey, Colby, & Xavier, 2014; Tidey, Rohsenow, Kaplan, Swift, & Adolfo, 2008), it runs counter to some prior data suggesting that opioid agonists may attenuate tobacco withdrawal (Elkader et al., 2009; Jackson, Muldoon, De Biasi, & Damaj, 2015). Also important to note is the possibility that methodological differences between studies may have contributed to baseline differences in withdrawal (e.g., timing of withdrawal assessment during the intake process, use of the CO-based inclusion criterion in the non-SUD studies).
Over the two weeks following quitting smoking, OD and non-SUD smokers showed a similar pattern of withdrawal, with scores declining in both groups in a manner that is consistent with that seen in the general population of smokers (Hughes, 2007b). It is worth noting that OD smokers did not show the initial increase in withdrawal upon quitting that was seen in the non-SUD smoker group and is typical in smokers more generally (Hughes, 2007b). Whether this is because their baseline withdrawal was already elevated, their opioid agonist medications attenuated further increases in withdrawal, or other factors is unknown but merits further study.
With regard to tobacco craving, the picture was more mixed with OD smokers evidencing a decrease in craving on the first day of abstinence relative to the increase seen in the non-SUD smoker group. There was also evidence that gender may play a role in craving following quitting smoking, though the effect was modest with group differences only seen in females. This is consistent with prior studies showing generally mixed findings on gender differences in tobacco withdrawal and craving (Leventhal et al., 2007; Pang & Leventhal, 2013; Piasecki et al., 2003; Svikis, Hatsukami, Hughes, Carroll, & Pickens, 1986; Weinberger et al., 2016).
Several strengths of the present study are worth noting. We extended upon the prior findings by Elkader and colleagues (2009) by examining tobacco withdrawal over a longer period of time (i.e., 14 vs. 3 days), using a slightly larger sample size of OD smokers (i.e, 47 vs. 40), and including patients maintained on buprenorphine in addition to methadone. Several limitations also should be mentioned. First, we did not assess withdrawal over several days prior to the quit attempt as has been recommended to avoid confounding due to anticipatory increases in anxiety or withdrawal (Hughes, 2007a; Shiffman, West, & Gilbert, 2004). Additionally, while larger than prior studies, the sample size in the present analyses was still limited and thus could have undermined our ability to detect group differences in nicotine withdrawal. Combining multiple studies together also may have resulted in increased heterogeneity within each group, leading to a reduction in power. Finally, regarding the OD smoker group in particular, we did not control their opioid medication dose or the timing of its administration relative to study procedures. This study also did not permit us to distinguish between the effects of opioid dependence per se versus opioid agonist maintenance in the OD smoker sample.
Conclusions
This secondary analysis offers the most thorough examination of tobacco withdrawal in opioid-dependent smokers to date, using smokers without comorbid SUDs as a comparison sample. Despite presenting with elevated withdrawal prior to quitting smoking, opioid-dependent smokers who successfully abstained from smoking over a two-week period experienced a profile of withdrawal that was remarkably similar to non-SUD smokers. These data suggest that elevated withdrawal severity following quitting may not be a major factor in the poor cessation outcomes consistently observed in this group. Continued investigations are needed to improve our understanding of the high smoking rates and modest cessation outcomes in this challenging smoker population.
Public Significance Statement.
Smoking cessation outcomes among opioid-dependent smokers are notoriously poor, possibly due to elevated tobacco withdrawal upon quitting smoking. We evaluated withdrawal over two weeks in smoking-abstinent smokers with and without opioid dependence. Tobacco withdrawal was generally similar between groups, suggesting that elevated withdrawal severity following quitting may not be a major factor underlying the poor cessation outcomes consistently observed in opioid-dependent smokers.
Acknowledgments
We thank Drs. Matthew Bradstreet, Kelly Dunn, Andrew Meyer, Mollie Miller, and Jin Yoon for assistance in conducting the smoking studies. We also thank Mr. Gary Badger for his statistical consulation on the smoking laboratory studies and Dr. John R. Hughes for his scientific consultation on this manuscript.
Role of Funding Source: The study was funded by NIH grants (R01 DA019550, Sigmon; R01 DA008076, Higgins; P50 DA036114, Higgins). The funding agency had no role in study design, collection, analysis, and interpretation of the data, preparation of the manuscript, or decision to submit the manuscript for publication.
Footnotes
Contributors:
Designing, obtaining funding for, and conducting the study: Sigmon, Higgins
Data analysis: Bunn
Manuscript drafting: Streck, Sigmon
Interpretation of the data and manuscript review and approval: Streck, Heil, Higgins, Bunn, Sigmon
Conflicts of Interest: There are no conflicts of interest to declare.
Preliminary versions of these data were included in oral presentations at two national scientific conferences in October 2017.
References
- Bradstreet MP, Higgins ST, McClernon FJ, Kozink RV, Skelly JM, Washio Y, Parry MA. Examining the effects of initial smoking abstinence on response to smoking-related stimuli and response inhibition in a human laboratory model. Psychopharmacology. 2014;231(10):2145–2158. doi: 10.1007/s00213-013-3360-x. https://doi.org/10.1007/s00213-013-3360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Reimann EF, Badger GJ, Heil SH, Higgins ST. A contingency-management intervention to promote initial smoking cessation among opioid-maintained patients. Experimental and Clinical Psychopharmacology. 2010;18(1):37–50. doi: 10.1037/a0018649. https://doi.org/10.1037/a0018649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Thomas CS, Heil SH, Higgins ST. Voucher-based contingent reinforcement of smoking abstinence among methadone-maintained patients: A pilot study. Journal of Applied Behavior Analysis. 2008;41(4):527–538. doi: 10.1901/jaba.2008.41-527. https://doi.org/10.1901/jaba.2008.41-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg T, Adams C, Riggins EC, Likness M. Smokers’ sex and the effects of tobacco cigarettes: Subject-rated and physiological measures. Nicotine & Tobacco Research. 1999;1(4):317–324. doi: 10.1080/14622299050011441. [DOI] [PubMed] [Google Scholar]
- Elkader AK, Brands B, Selby P, Sproule BA. Methadone-nicotine interactions in methadone maintenance treatment patients. Journal of Clinical Psychopharmacology. 2009;29(3):231–238. doi: 10.1097/JCP.0b013e3181a39113. https://doi.org/10.1097/JCP.0b013e3181a39113. [DOI] [PubMed] [Google Scholar]
- Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine & Tobacco Research. 2012;14(1):75–78. doi: 10.1093/ntr/ntr137. https://doi.org/10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- Guydish J, Passalacqua E, Pagano A, Martínez C, Le T, Chun J, Delucchi K. An international systematic review of smoking prevalence in addiction treatment: Smoking prevalence in addiction treatment. Addiction. 2016;111(2):220–230. doi: 10.1111/add.13099. https://doi.org/10.1111/add.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guydish J, Passalacqua E, Tajima B, Chan M, Chun J, Bostrom A. Smoking prevalence in addiction treatment: A review. Nicotine & Tobacco Research. 2011;13(6):401–411. doi: 10.1093/ntr/ntr048. https://doi.org/10.1093/ntr/ntr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Higgins ST, Mongeon JA, Badger GJ, Bernstein IM. Characterizing nicotine withdrawal in pregnant cigarette smokers. Experimental and Clinical Psychopharmacology. 2006;14(2):165–170. doi: 10.1037/1064-1297.14.2.165. https://doi.org/10.1037/1064-1297.14.2.165. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Treating smokers with current or past alcohol dependence. American Journal of Health Behavior. 1996;20(5):286–290. [Google Scholar]
- Hughes JR. Measurement of the effects of abstinence from tobacco: A qualitative review. Psychology of Addictive Behaviors. 2007a;21(2):127–137. doi: 10.1037/0893-164X.21.2.127. https://doi.org/10.1037/0893-164X.21.2.127. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine & Tobacco Research. 2007b;9(3):315–327. doi: 10.1080/14622200701188919. https://doi.org/10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. https://doi.org/10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami DK. Errors in using tobacco withdrawal scale. Tobacco Control. 1998;7(1):92–93. doi: 10.1136/tc.7.1.92a. https://doi.org/10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Muldoon PP, De Biasi M, Damaj MI. New mechanisms and perspectives in nicotine withdrawal. Neuropharmacology. 2015;96:223–234. doi: 10.1016/j.neuropharm.2014.11.009. https://doi.org/10.1016/j.neuropharm.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults — United States, 2005–2015. MMWR. Morbidity and Mortality Weekly Report. 2016;65:1205–1211. doi: 10.15585/mmwr.mm6544a2. https://doi.org/10.15585/mmwr.mm6544a2. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Boyd S, Moolchan ET, Waters AJ, Lerman C, Pickworth WB. Gender differences in acute tobacco withdrawal: Effects on subjective, cognitive, and physiological measures. Experimental and Clinical Psychopharmacology. 2007;15(1):21–36. doi: 10.1037/1064-1297.15.1.21. https://doi.org/10.1037/1064-1297.15.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden PA, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Heath AC. Nicotine withdrawal in women. Addiction (Abingdon, England) 1997;92(7):889–902. https://doi.org/10.1111/j.1360-0443.1997.tb02957.x. [PubMed] [Google Scholar]
- Miller ME, Sigmon SC. Are pharmacotherapies ineffective in opioid-dependent smokers? Reflections on the scientific literature and future directions. Nicotine & Tobacco Research. 2015;17(8):955–959. doi: 10.1093/ntr/ntv030. https://doi.org/10.1093/ntr/ntv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahvi S, Richter K, Li X, Modali L, Arnsten J. Cigarette smoking and interest in quitting in methadone maintenance patients. Addictive Behaviors. 2006;31(11):2127–2134. doi: 10.1016/j.addbeh.2006.01.006. https://doi.org/10.1016/j.addbeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Okoli CTC, Khara M, Procyshyn RM, Johnson JL, Barr AM, Greaves L. Smoking cessation interventions among individuals in methadone maintenance: A brief review. Journal of Substance Abuse Treatment. 2010;38(2):191–199. doi: 10.1016/j.jsat.2009.10.001. https://doi.org/10.1016/j.jsat.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Pang RD, Leventhal AM. Sex differences in negative affect and lapse behavior during acute tobacco abstinence: A laboratory study. Experimental and Clinical Psychopharmacology. 2013;21(4):269–276. doi: 10.1037/a0033429. https://doi.org/10.1037/a0033429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: III. Correlates of withdrawal heterogeneity. Experimental and Clinical Psychopharmacology. 2003;11(4):276–285. doi: 10.1037/1064-1297.11.4.276. https://doi.org/10.1037/1064-1297.11.4.276. [DOI] [PubMed] [Google Scholar]
- Richter KP, Gibson CA, Ahluwalia JS, Schmelzle KH. Tobacco use and quit attempts among methadone maintenance clients. American Journal of Public Health. 2001;91(2):296–299. doi: 10.2105/ajph.91.2.296. https://doi.org/10.2105/AJPH.91.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, West R, Gilbert D. Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine & Tobacco Research. 2004;6(4):599–614. doi: 10.1080/14622200410001734067. https://doi.org/10.1080/14622200410001734067. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Miller ME, Meyer AC, Saulsgiver K, Badger GJ, Heil SH, Higgins ST. Financial incentives to promote extended smoking abstinence in opioid-maintained patients: A randomized trial. Addiction (Abingdon, England) 2015 doi: 10.1111/add.13264. https://doi.org/10.1111/add.13264. [DOI] [PMC free article] [PubMed]
- Story J, Stark MJ. Treating cigarette smoking in methadone maintenance clients. Journal of Psychoactive Drugs. 1991;23(2):203–215. doi: 10.1080/02791072.1991.10472237. https://doi.org/10.1080/02791072.1991.10472237. [DOI] [PubMed] [Google Scholar]
- Svikis DS, Hatsukami DK, Hughes JR, Carroll KM, Pickens RW. Sex differences in tobacco withdrawal syndrome. Addictive Behaviors. 1986;11(4):459– 462. doi: 10.1016/0306-4603(86)90028-6. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Colby SM, Xavier EMH. Effects of smoking abstinence on cigarette craving, nicotine withdrawal, and nicotine reinforcement in smokers with and without schizophrenia. Nicotine & Tobacco Research. 2014;16(3):326–334. doi: 10.1093/ntr/ntt152. https://doi.org/10.1093/ntr/ntt152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Adolfo AB. Effects of smoking abstinence, smoking cues and nicotine replacement in smokers with schizophrenia and controls. Nicotine & Tobacco Research. 2008;10(6):1047–1056. doi: 10.1080/14622200802097373. https://doi.org/10.1080/14622200802097373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health; Atlanta GA: 2014. [Google Scholar]
- Weinberger AH, Platt J, Shuter J, Goodwin RD. Gender differences in self-reported withdrawal symptoms and reducing or quitting smoking three years later: A prospective, longitudinal examination of U.S. adults. Drug and Alcohol Dependence. 2016;165:253–259. doi: 10.1016/j.drugalcdep.2016.06.013. https://doi.org/10.1016/j.drugalcdep.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Bradstreet MP, Badger GJ, Thomas CS. Changes in the relative reinforcing effects of cigarette smoking as a function of initial abstinence. Psychopharmacology. 2009;205(2):305–318. doi: 10.1007/s00213-009-1541-4. https://doi.org/10.1007/s00213-009-1541-4. [DOI] [PubMed] [Google Scholar]
- Zirakzadeh A, Shuman C, Stauter E, Hays JT, Ebbert JO. Cigarette smoking in methadone maintained patients: An up-to-date review. Current Drug Abuse Reviews. 2013;6(1):77–84. doi: 10.2174/1874473711306010009. https://doi.org/10.2174/1874473711306010009. [DOI] [PubMed] [Google Scholar]