Abstract
Dietary sodium recommendations are expressed as absolute amounts (mg/day) rather than as sodium density (mg/kcal). Our objective was to determine if the strength of the relationship of sodium intake with blood pressure varied with energy intake. The Dietary Approaches to Stop Hypertension-Sodium (DASH) trial was a randomized feeding trial comparing two diets (DASH and control) and three levels of sodium density. Participants with pre- or stage 1 hypertension consumed diets for 30d in random order; energy intake was controlled to maintain body weight. This secondary analysis of 379 non-Hispanic Black and White participants used mixed effects models to assess the association of sodium and energy intakes with blood pressure. The relationships between absolute sodium and both systolic and diastolic blood pressure varied with energy intake. Blood pressure rose more steeply with increasing sodium at lower energy intake than at higher energy intake (p-interaction < 0.001). On the control diet with 2300 mg sodium, both systolic and diastolic blood pressure were higher (3.0 mm Hg, 95% confidence interval 0.2, 5.8; and 2.7 mm Hg, 95% CI 1.0, 4.5, respectively) among those with lower energy intake (higher sodium density) than among those with higher energy intake (lower sodium density). The association of sodium with systolic blood pressure was stronger at lower levels of energy intake in both Blacks and Whites (p<0.001). The association of sodium and diastolic blood pressure varied with energy intake only among Blacks (p=0.001). Sodium density should be considered as a metric for expressing dietary sodium recommendations.
Keywords: Sodium, Dietary, Energy Intake, Blood Pressure, Hypertension, Diet, Body Weight
Introduction
Almost a third of American adults have high blood pressure (BP),1 and only about half of those with high BP have it under control.2 Sodium (Na) reduction is commonly recommended as a means to lower BP. Current clinical and public health recommendations for Na intake are expressed as milligrams of Na per day (mg/d), 2–4 whether these recommendations should be expressed in terms of Na to energy intake (Na density, mg/kcal) has not been explored.
Na density is the ratio of Na to energy in a diet. For example, a 2000 kcal diet that contains 2400 mg Na has a Na density of 1.2 mg/kcal. Because Na and energy intakes are so highly correlated, it is much harder for someone who is larger and/or more active to achieve the current Na recommendations of 2300 or 2400 mg/d than it is for someone who is smaller and/or less active simply because of their differing energy intakes.2,3 Furthermore, smaller or less active persons might not experience the benefits of Na reduction at an intake of 2300 mg/d because that absolute amount of Na results in a high Na density.
The original analysis of the Dietary Approaches to Stop Hypertension (DASH)-Na trial by Sacks et al. is perhaps the most influential evidence underlying current Na recommendations 5,6 A recent policy statement from the American College of Cardiology and the American Heart Association concluded that “Reducing sodium intake to a mean of 2400 mg per day (d), relative to 3300 mg/d, lowers BP by 2/1 mm Hg, and reducing intake to a mean of 1500 mg/d lowers BP by 7/3 mm Hg”,2 based largely on the results of the DASH-Na trial. However, the experimental variable in DASH-Na was not absolute Na (mg/d), but rather Na density (mg/kcal) at three levels.6
The main purpose of this secondary analysis of the DASH-Na trial was to determine if the strength of the relationship between Na intake and BP varied with energy intakes. We also investigated whether this relationship varied with race and obesity status.
Methods
Data are available at the National Heart, Lung and Blood Institute Biologic Specimen and Data Repository (BIOLINCC) and were accessed at (https://biolincc.nhlbi.nih.gov/home/). Age and race of the participants were provided by the DASH-Na trial principal investigator (L. Appel) and were matched at the individual level by BioLINCC staff.
The DASH-Na study was a multicenter, randomized, crossover trial comparing the effects on BP of three levels of Na intake at the same energy intake, that is, Na density, in two types of diets, the DASH diet and a control diet. 6 BP measurements (following screening) were taken by trained, certified observers using a random zero sphygmomanometer and following the protocol that was used in the DASH and Trials of Hypertension Prevention studies. 7–9 Institutional Review Boards (IRB) at each center approved the original study protocol and subsequent analyses. Written informed consent was obtained from each participant. This analysis was approved by the University of Utah, Johns Hopkins University and Wake Forest University IRB.
Participants were adults whose BP exceeded 120/80 mm Hg, including those with stage 1 hypertension (a systolic BP (SBP) of 140 to 159 mm Hg or a diastolic BP (DBP) of 90 to 95 mm Hg). The three Na density levels were defined as high (1.6 mg/kcal, representing a target of 3450 mg/d Na at an energy intake of 2100 kcal and reflecting typical consumption in the United States), intermediate (1.1 mg/kcal, representing a target of 2300 mg/day at 2100 kcal and reflecting Na recommendations),10 and low (0.5 mg/kcal, representing a target of 1150 mg/d at 2100 kcal and reflecting a level was hypothesized to further lower BP).
The DASH-Na trial was a tightly controlled feeding study. Food was provided to participants; in addition, up to three servings of allowed beverages including tea, coffee, some kinds of diet soda and up to 14 alcoholic beverages per week. During a two-week run-in period, participants (n=412) ate the high-Na control diet, which was designed to be similar to what many Americans. Participants were then randomly assigned to the DASH diet or the control diet. Participants ate their assigned diet, provided at three Na density levels, for 30 consecutive days in random order in a crossover design with up to 5 days between study periods. Other than the sodium levels which varied by period, the assigned diets (DASH and control diets) remained similar in all other ways, including their potassium, magnesium, and calcium content.
Each participant’s energy intake was adjusted to ensure that his or her weight remained constant throughout the study, that is, energy intake was equal to energy requirement. Table S1, an online only supplemental table, shows the absolute sodium targets at the three energy density levels by energy intake. Larger or more active persons received more food and, therefore, more Na than smaller or less active persons at each of the three Na levels.11
Inclusion/Exclusion Criteria
The study participants were identified as Black, White, or other Race, and Hispanic or not; the number of participants of a race/ethnicity other than non-Hispanic Black or White was not large enough to be analyzed separately. Therefore, from the original sample of 412 participants, 19 were excluded (Hispanics (n=12) and those of races other than Black and White (n=7)). Data were also excluded from a study period when a measured BP during or at the end of the study period was not available (n=3 participants fully excluded; n=40 periods). The authors defined a valid study day as having a value for energy intake, not more than 2 meals missed, and not more than 4 servings of food that was not allowed according to daily diaries kept by the participants. Also excluded were study periods having <12 valid study days or <5 valid days in the last week of the period (n=11 participants and n=35 study periods), or having an absolute Na intake greater than 6700 mg/d (n=2 study periods), resulting in 379 participants and 1102 study periods included in these analyses.
Energy and Na Intakes
Daily energy intake consumed was computed from the sum of energy from meals served based on the nutrient content of the menus, allowed beverages, and the supplemental nutrition bars that were provided to meet energy targets. The usual energy intake value for each period used in this data analysis was the mean daily energy intake, calculated using all valid diet days. Na intake was calculated in a similar manner. Na density was computed as the mean Na intake for a period divided by the mean energy intake for the period. The mean Na densities (mg/kcal) of the diets consumed by participants, calculated by the authors, for both the DASH and control diets were 0.6 for the low Na density diets, 1.2 for the intermediate Na density diets, and 1.7 for the high Na density diets (Table S2, an online only supplemental table).
Statistical Analysis
Statistical analyses were conducted with SAS version 9.4 (SAS Institute, Inc., Cary, NC). Usual energy, Na intakes, and Na densities were estimated using means, standard deviations, and ranges stratified by Na density and diet arm. Baseline characteristics were described using frequencies for categorical variables and means and standard deviations for continuous variables. For illustration purposes, we present participant characteristics at roughly the lower, middle two, and upper quartiles of energy intake. Differences in participant characteristics by energy intake were tested by chi-square test, Monte Carlo estimate of Fisher’s exact test or ANOVA.
A mixed effects model was used to model SBP and DBP as a function of Na and energy (both as continuous variables) and their interaction, while accounting for age, sex, smoking status (current vs. former or never), race, cohort, carryover (sodium intake in the previous period), and clinical center in all analyses. Diet type (DASH or control) was included in all models; the effect of Na and energy intake on BP was allowed to vary by diet by including interactions of diet type with Na intake and diet type with energy intake. Estimates and corresponding 95% confidence intervals (CI) at Na and energy levels were estimated using linear contrasts. Baseline BP was not included in the models because it is in the causal pathway for BP at the end of each feeding period.
The a priori plan was to determine whether the impact of Na and energy on BP was different in Blacks and Whites. Therefore, we evaluated interactions of race with Na and energy, diet type, and relevant interactions and conducted stratified analysis of the relationship of Na and intake (both as continuous variables) with BP with the same covariates for adjustments, except race. To test whether the slope of the association between Na and BP differed by obesity status, interaction terms for obesity status (BMI</≥30 kg/m2) with Na intake, energy intake, and diet type and relevant interactions were modeled and then the models were fit stratified by obesity status.
Results
Participants with higher energy intakes were more likely to be male, White, and married with a higher BMI and a lower SBP than participants with lower energy intakes (Table 1, and Table S3 online supplement only).
Table 1.
Baseline Characteristics by Energy Intake
| Characteristic | Energy Intake (kcal) | All | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Range, kcal | <2200** | 2200–3000 | ≥3000 | 1630–4069 | |||||
| Median, kcal | 2100 | 2600 | 3200 | 2577 | |||||
| N | 88 | 200 | 91 | 379 | |||||
| n | % | n | % | n | % | n | % | p value | |
| Age (y) | 0.153* | ||||||||
| 21–30 | 1 | 1.1 | 5 | 2.5 | 5 | 5.5 | 11 | 2.9 | |
| 31–50 | 45 | 51.1 | 115 | 57.5 | 59 | 64.8 | 219 | 57.8 | |
| 51–70 | 41 | 46.6 | 75 | 37.5 | 26 | 28.6 | 142 | 37.5 | |
| >70 | 1 | 1.1 | 5 | 2.5 | 1 | 1.1 | 7 | 1.8 | |
| Sex, Female | 87 | 98.9 | 127 | 63.5 | 4 | 4.4 | 218 | 57.5 | <0.001 |
| Black (vs White) | 59 | 67.0 | 122 | 61.0 | 43 | 47.3 | 224 | 59.1 | 0.019 |
| Weight status (BMI kg/m2) | <0.001 | ||||||||
| Underweight (<18.5) | 1 | 1.1 | 0 | 0.0 | 0 | 0.0 | 1 | 0.3 | |
| Normal (18.5–24.9) | 29 | 33.0 | 32 | 16.0 | 7 | 7.7 | 68 | 17.9 | |
| Overweight (25–29.9) | 38 | 43.2 | 88 | 44.0 | 38 | 41.8 | 164 | 43.3 | |
| Obese (30–39.9) | 20 | 22.7 | 80 | 40.0 | 46 | 50.5 | 146 | 38.5 | |
| Assigned Diet | n | % | n | % | N | % | N | % | 0.315 |
| DASH | 48 | 54.5 | 104 | 52.0 | 40 | 44.0 | 192 | 50.7 | |
| Blood pressure status | |||||||||
| Pre-Hypertensive | 44 | 50.0 | 120 | 60.0 | 56 | 61.5 | 220 | 58.1 | .211 |
| Hypertensive, Stage 1 | 44 | 50.0 | 80 | 40.0 | 35 | 38.5 | 159 | 42.0 | |
| Blood pressure | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| SBP mm Hg | 137.9 | 10.5 | 135.8 | 8.4 | 132.7 | 8.1 | 135.5 | 9.0 | <0.001† |
| DBP mm Hg | 86.1 | 4.1 | 85.8 | 3.7 | 86.5 | 4.1 | 86.1 | 3.9 | 0.369† |
Grouped by the lower quartile, the second and third quartiles together and the highest quartile of energy intake. Differences tested by Chi Square test, except:
Monte Carlo estimate of Fisher’s exact test
ANOVA
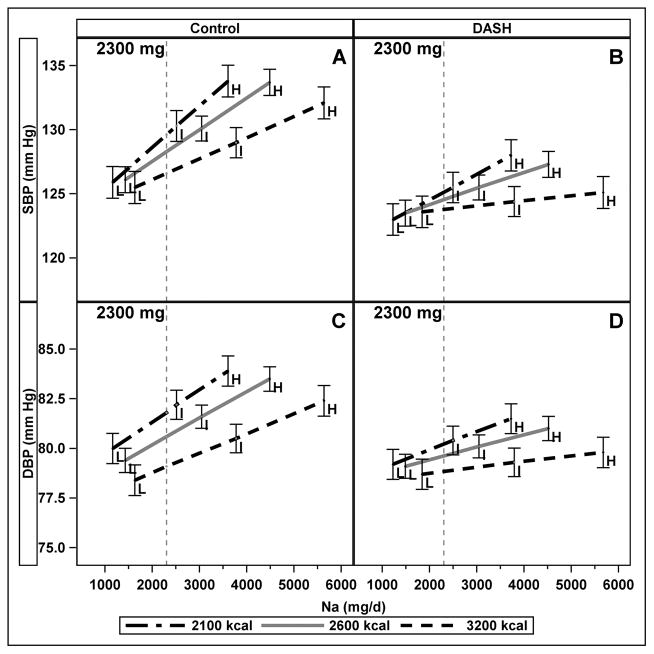
The strength of the relationships between absolute Na and both SBP and DPB varied with energy intake such that BP rose more steeply with increasing Na at lower energy intake than at higher energy intake (interaction of Na and energy p < 0.001, (Figure 1.)) There were no significant differences between men and women in this model. We chose to show comparisons at the midpoint of the low energy group (2100 kcal, higher sodium density) and high energy group (3200 kcal, lower sodium density) at the current recommended upper limit of Na (2300 mg), 3 and to present these estimates by diet type (Figure 1, panels A and C) to illustrate the interaction between Na and energy. At 2300 mg of Na on the control diet, those with usual energy intake of 2100 kcal had an average SBP that was 3.0mm Hg (95% CI: 0.2, 5.8) higher than those with usual energy intake of 3200 kcal; DBP was 2.7 mm Hg (95% CI: 1.0, 4.5) higher. On the DASH diet (Figure 1, panels B and D), the differences between 2100 kcal and 3200 kcal/d intake at 2300 mg were attenuated and not significantly different from zero (SBP: 1.3 mm Hg (95% CI: −1.4, 4.0); DBP: 1.3 mm Hg (95% CI: −0.4, 3.1)). However, on the DASH diet, the differences between 2100 kcal and 3200 kcal/d intake at 3600 mg were significantly different from zero (SBP: 3.4 mm Hg (95% CI: 0.7, 6.1); DBP: 2.1 mm Hg (95% CI: 0.4, 3.9)).
Figure 1.
Interaction of absolute sodium and energy intake on blood pressure (SBP top, DBP bottom) on the Control (panels A and C) and DASH (panels B and D) Diets. The figure illustrates the interaction at three energy levels. The three levels of sodium (Na) density (L, I, H) are labelled and error bars represent 95% confidence intervals. The vertical line draws attention to the contrast at a fixed, 2300 mg Na of absolute sodium across different levels of energy intake. Results were generated from mixed effects models of continuous Na and energy intakes, adjusted for age, sex, race, smoking, cohort, diet type (DASH or control), clinical center, and carryover effects.
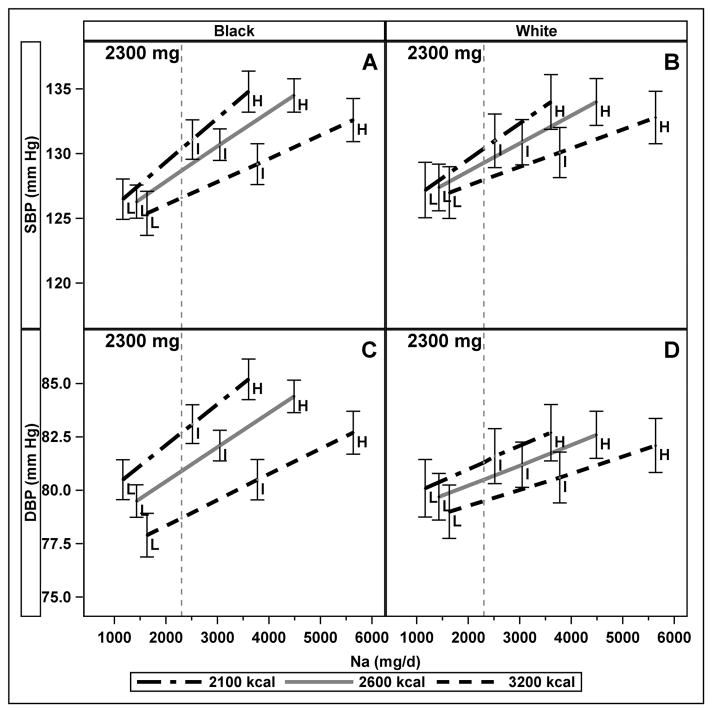
The interaction of Na intake and race in the full model was significant for DBP (p= 0.03), but not for SBP (p=0.07). There was no significant interaction between race and energy, or among race, Na intake, and energy for SBP or DBP. In stratified models, the association of Na with SBP was stronger at lower energy intake (higher sodium density) than higher energy intake (lower sodium density) in both Blacks and Whites (both p<0.001); whereas the association of Na and DBP varied with energy intake among Blacks (p=0.001) but not Whites (p=0.288). On the control diet in Blacks at 2300 mg, the difference in SBP between individuals whose energy intake was 2100 kcal as compared to 3200 kcal was 3.8 mm Hg (95% CI: 0.0, 7.7) whereas in Whites it was not significantly different than zero (2.4 mm Hg (95% CI: −1.6, 6.4)) (Figure 2, Panels A and B). On the control diet in Blacks at 2300 mg Na intake, DBP was 4.0 mm Hg (95% CI: 1.6, 6.3) higher at 2100 kcal compared to 3200 kcal, whereas in Whites it was not significantly higher (1.8 mm Hg (95% CI: −0.8, 4.5)) (Figure 2, Panels C and D). On the DASH diet at 2300 mg Na intake, SBP did not differ at between 2100 kcal and 3200 kcal in Blacks (SBP 2.4 mm Hg (95% CI: −1.3, 6.0)) and Whites (0.3 mm Hg (95% confidence interval (CI) −4.3, 3.7)); differences were also not significant for DBP in Blacks (1.6 mm Hg (95% CI −0.6, 3.9)) or Whites (1.1 mm Hg (95% CI: −1.5, 3.7)) on the DASH diet.
Figure 2.
Interaction of absolute Na intake and energy intake on blood pressure (SBP top, DBP bottom) at three energy levels, indicating three levels of Na density (L, I, and H), among Blacks (panels A and C) and Whites (panels B and D) on the control diet. The vertical line is drawn at 2300 mg absolute Na intake. Error bars represent 95% confidence intervals. Results from mixed effects models of continuous Na and energy intakes, adjusted for age, sex, smoking, cohort, diet type (DASH or control), clinical center, cohort, and carryover effects and stratified by race.
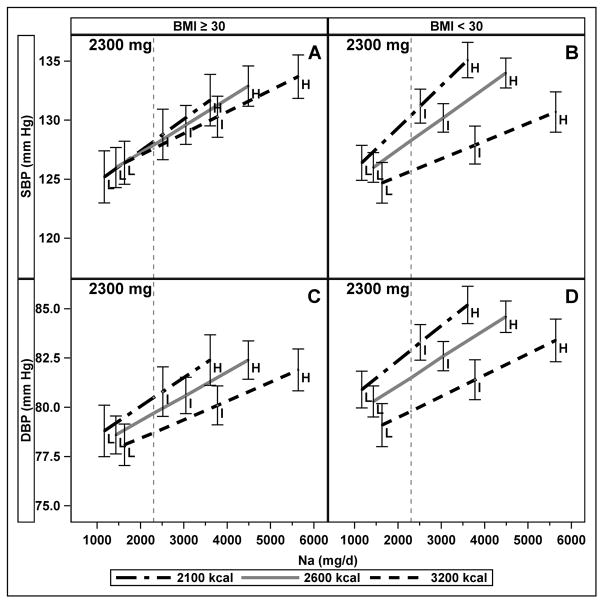
There was a significant three-way interaction between Na intake, energy, and obesity status for SBP (p=0.033); for DBP, the three-way interaction was not significant, but there was a significant interaction between energy intake and obesity (p=0.005). We, therefore, used stratified models to describe the relationship between energy and Na by obesity status (Figure 3). In stratified models, the association of Na with SBP was stronger at lower energy intake (higher sodium density) than higher energy intake (lower sodium density) in both non-obese (interaction of Na with energy, p<0.001) and obese (interaction of Na with energy, p=0.028); the association of Na and DBP by energy intakes were similar (interaction of Na with energy, p=0.005 non-obese; p=0.049 obese). On the control diet at 2300 mg Na intake in the non-obese, SBP was 4.7 mm Hg (95% CI: 1.0, 8.5) higher at 2100 kcal compared to 3200 kcal (Figure 3, panel A), and DBP was 3.1 mm Hg (95% CI: 0.7, 5.5) higher (Panel C). In obese at 2300 mg Na intake on the control diet, there were no differences in SBP (0.6 mm Hg (95%CI: −3.8, 4.9)) (Panel B) or DBP (1.8 mm Hg (95% CI: −1.0, 4.5)) (Panel D) between 2100 and 3200 kcal. On the DASH diet at 2300 mg Na, there were no significant differences in SBP at 2100 vs 3200 kcal regardless of obesity status (2.5 mm Hg (95% CI: −1.0, 6.0) for non-obese and −1.3 mm Hg (95% CI: −6.0, 3.4) for obese). On the DASH diet at 2300 mg Na, DBP was higher at 2100 vs 3200 kcal in the non-obese (2.1 mm Hg (95% CI: 0.1, 4.4)), but not in the obese (−0.3mm Hg (95% CI: −3.2, 2.6)).
Figure 3.
Interaction of absolute Na intake and energy intake on blood pressure at three energy levels, indicating three levels of Na density (L, I, and H), among normal and overweight (BMI < 30 kg/m2, panels A and C) and obese (BMI≥30 kg/m2, panels B and D) participants on the control diet. The vertical line is drawn at 2300 mg absolute Na intake. Error bars represent 95% confidence intervals. Results from mixed effects models of continuous Na and energy, adjusted for age, sex, race, smoking, cohort, diet type (DASH or control), clinical center, and carryover effects stratified by obesity status.
Discussion
This analysis was conducted to determine whether the relationship between Na and BP varies with energy intake. Several key findings emerged. First, the slope of the relationship BP with Na intake varied by energy intake, suggesting that Na density may reflect the relationship with BP better than absolute Na intake does. Second, as previously reported, the effect of sodium reduction was attenuated in the setting of the DASH diet compared to the control diet, suggesting that aspects of diet also influence the BP response to changes in sodium intake12–14. In addition to attenuating the BP response to sodium, the DASH diet also lowered BP, consistent with evidence that aspects of diet, including an increased intake of potassium, independently lower BP.15 Third, the association of Na with energy intake on BP persisted when the analyses were stratified by race, except for DBP among Whites. Fourth, with stratification by obesity status, larger differences in BP by energy intake were observed among those who were non-obese compared to obese.
Although the DASH-Na study controlled Na density, and not absolute Na intake, the original results of this trial were interpreted in terms of Na intake rather than Na density. The lack of reporting results by Na density may reflect the absence of data on energy intake in many studies. Investigations of the relationship of Na density with mortality in observational studies have provided mixed results. The investigation of Na density with mortality in the NHANES II follow-up study and from NHANES III suggested an inverse association of Na and cardiovascular disease mortality, even when considering Na with respect to energy intake. 16,17 In these studies, however, Na and energy intake were estimated using one 24-hour recall, which is not adequate to estimate an individual’s usual dietary intake because of large day-to-day variation in both Na intake and energy intake as well as their ratio. Further, energy intake was implausibly low in both studies. It is possible that the observed relationship between Na intake and cardiovascular disease mortality may be explained by under-reporting bias, i.e., if obese people report lower sodium density and are more likely to die of cardiovascular disease, the observed relationship may reflect the impact of obesity rather than diet. The most recent evidence from a prospective cohort study (n=716, 19-year follow-up) aligns with the results of the current analysis, with reported incidence of cardiovascular events twice as high among those in the highest versus lowest quartile of Na density (22% versus 11%, respectively, p=0.005) according to 7-d food records collected at baseline. 18
Current Na recommendations are expressed in absolute amounts (i.e., 2300 to 2400 mg/d),2–4,19 and this reflects how study findings have been reported; 2,3,20–22 however, when Na recommendations are applied in practice, they are often, but not intentionally, energy adjusted. For example, in the Healthy United States-style dietary pattern, these amounts range from 921 mg Na/d at 1000 calories to 2392 mg Na/d at 3200 calories.23 This is because everyone, is encouraged to choose nutrient-dense, low Na foods. The Healthy Eating Index (HEI), an index that represents adherence of dietary patterns with the Dietary Guidelines for Americans, assesses food and nutrient intakes, including Na, on an energy-adjusted basis, that is, the scoring standards are expressed as densities.24–26 In the HEI-2010 and HEI-2015, a Na density of 1.1 mg/kcal or lower receives the highest (best) score for the Na component of the index. In addition, the Dietary Reference Intakes for Na for children and older adults are lower than for young and middle-aged adults because they, too, were set by energy adjustment.4 Finally, a recent evidence-based guideline for dietitians for the management of hypertension recommends a Na restriction of 1500–2400 mg/d based on several factors, including energy intake.21
Guenther et al.27 demonstrated that it is possible to have a nutritionally adequate diet with a Na intake of 1500 mg/d (Na density 0.4–0.5 mg/kcal) by developing food choice scenarios that encompassed the Na density levels in the DASH-Na trial. Making only the lowest Na choices, such as, unsalted potato chips, unsalted butter or margarine, and bread with a lower Na content, resulted in a Na density similar to the lowest Na density in the DASH-Na trial. Typical food choices resulted in Na density that was similar to the higher Na density in the DASH-Na trial (1.7 mg/kcal) and to the estimated average Na density of diets in the US of 1.7 mg/kcal.23
The authors were interested in understanding whether the association of Na with BP varied in Blacks versus Whites because of the higher prevalence of hypertension among Blacks in the United States.28 The finding of a slightly larger difference in the change in BP in response to Na among Blacks as compared to Whites conflicts with a recent meta-analysis that found no difference in the Blacks and Whites in response to Na reduction 29 but is consistent with original findings from the DASH-Na trial.13 A number of mechanisms may contribute to Black/white differences in hypertension. Chronic stress may be a contributor among Blacks30. Differences in kidney function and sodium retention are hypothesized reasons, as are differences in potassium homeostasis potentially related to differences in aldosterone levels and aldosterone sensitivity 31.
The finding of greater differences in BP by energy intake at the same level of Na intake among participants with normal weight status compared to those who were obese on the control diet is novel, though contrary to what might be expected based on the known increase in hypertension with obesity. Clinical trials show a sustained reduction in BP with Na reduction and weight loss 32 and synergistic influence of Na reduction and weight loss on BP in the elderly.33,34 Increases in energy needs are related to body surface area and exercise, and greater sodium losses will occur with both. Furthermore, salt sensitivity of BP may be inversely related to physical activity. In this study, we do not have data to support whether any of these mechanisms were involved. We can only speculate on whether these findings would apply to the refractory nature of hypertension among individuals with low BMI common in certain ethnic groups. Further studies or secondary analyses of other trials are warranted to explore whether these results hold up among other racial/ethnic groups and body size differences.
Confounding of sex and energy intake is evident in the proportion of women and men in the highest and lowest levels of energy intake. In analyses controlling for energy intake, there we no significant sex differences in the relationship of sodium and BP. Nonetheless, examination of the differences in sodium and energy intake in men and women with respect to BP are warranted in light of the putative mechanisms that might account for differential BP response to diet by sex. Sympathetic nerve activity related to hypertension is different in young women as compared to men and older men and women35–37.
Several strengths of the current analyses stem from the design of the DASH-Na trial. 5 The study documents the effects of the DASH diet and Na reduction, alone and combined, on BP. Because it was a feeding study, Na and energy intakes were controlled and not prone to reporting bias. The crossover study design allowed for control of within-person factors that may impact BP. The study included sufficient numbers of non-Hispanic Whites and Blacks to examine relationships within these races separately. Our analyses also have limitations. An analysis of Hispanics and people of other races was not possible. Second, in this trial, three levels of Na density were tested, but the optimal Na density may or may not have been observed. Third, these analyses extend beyond the randomized design and are, therefore, subject to confounding by factors associated with sodium homeostasis such as sex and energy intake such as physical activity.
Perspectives
Making sodium recommendations based on energy intake instead of making recommendations based on absolute amounts of sodium has practical advantages. Stating Na recommendations on a per calorie basis would reflect the way that dietary guidance for Na is currently applied in practice and would not change the overall message to consumers to reduce Na intake and to the food industry to reduce the Na content of foods. If Na recommendations were expressed as Na density (mg/kcal), it would be much easier for patients and dietitians to plan reduced-Na diets because people with varying energy needs could eat the same foods but in varying amounts. Future work is needed to establish the optimal sodium density. It would be helpful to establish whether the relationship between sodium and BP varies when energy needs vary because of differences in physical activity rather than by differences in body size.
Supplementary Material
Novelty and significance.
What is new
The relationship of sodium intake with BP varied by energy intake; the strength of the relationship was stronger among individuals with lower energy intake than among those with higher energy intake.
What is relevant
Dietary recommendations for sodium recommend one upper limit, regardless of energy needs.
Sodium is present in food naturally so following a low sodium diet is harder for individuals with higher energy needs than for those with lower energy needs.
Summary
The relationship of sodium with BP varies with energy intake.
These results support recommending Na intake as a function of energy, i.e., as Na density (mg/kcal).
Acknowledgments
Sources of Funding
This work was supported by National Heart Lung and Blood Institute, Untangling Sodium, Energy, and Blood Pressure in Whites and African Americans grant number 1R21HL128958. LJA has received a grant from Vital Strategies to provide technical support on sodium reduction in the RESOLVE initiative. MAM, PMG, and LJA designed research; the research reported in this publication was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001067. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Maureen A. Murtaugh, University of Utah, Department of Medicine, Division of Epidemiology
Jeannette M. Beasley, New York University School of Medicine, Department of Medicine
Lawrence J. Appel, Johns Hopkins University, Welch Center for Prevention, Epidemiology and Clinical Research
Patricia M. Guenther, University of Utah, Department of Nutrition and Integrative Physiology
Molly McFadden, University of Utah, Department of Medicine, Division of Epidemiology.
Tom Greene, University of Utah, Department of Medicine, Division of Epidemiology.
Janet A. Tooze, Wake Forest School of Medicine, Department of Biostatistical Sciences
References
- 1.Egan BM, Li J, Hutchison FN, Ferdinand KC. Hypertension in the United States, 1999 to 2012: progress toward Healthy People 2020 goals. Circulation. 2014;130(19):1692–1699. doi: 10.1161/CIRCULATIONAHA.114.010676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76–99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services and US Department of Agriculture. 2015 – 2020 Dietary Guidelines for Americans. (8) 2015 http://health.gov/dietaryguidelines/2015/guidelines/
- 4.Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 5.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. The New England journal of medicine. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 6.Svetkey LP, Sacks FM, Obarzanek E, et al. The DASH Diet, Sodium Intake and Blood Pressure Trial (DASH-sodium): rationale and design. DASH-Sodium Collaborative Research Group. Journal of the American Dietetic Association. 1999;99(8 Suppl):S96–104. doi: 10.1016/s0002-8223(99)00423-x. [DOI] [PubMed] [Google Scholar]
- 7.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. The New England journal of medicine. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 8.The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. Jama. 1992;267(9):1213–1220. doi: 10.1001/jama.1992.03480090061028. [DOI] [PubMed] [Google Scholar]
- 9.Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157(6):657–667. [PubMed] [Google Scholar]
- 10.The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V) Arch Intern Med. 1993;153(2):154–183. [PubMed] [Google Scholar]
- 11.Lin PH, Windhauser MM, Plaisted CS, Hoben KP, McCullough ML, Obarzanek E. The Linear Index Model for establishing nutrient goals in the Dietary Approaches to Stop Hypertension trial. DASH Collaborative Research Group. Journal of the American Dietetic Association. 1999;99(8 Suppl):S40–44. doi: 10.1016/s0002-8223(99)00415-0. [DOI] [PubMed] [Google Scholar]
- 12.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. The New England journal of medicine. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 13.Vollmer WM, Sacks FM, Ard J, et al. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-sodium trial. Ann Intern Med. 2001;135(12):1019–1028. doi: 10.7326/0003-4819-135-12-200112180-00005. [DOI] [PubMed] [Google Scholar]
- 14.Juraschek SP, Miller ER, 3rd, Weaver CM, Appel LJ. Effects of Sodium Reduction and the DASH Diet in Relation to Baseline Blood Pressure. J Am Coll Cardiol. 2017;70(23):2841–2848. doi: 10.1016/j.jacc.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lennon SL, DellaValle DM, Rodder SG, et al. 2015 Evidence Analysis Library Evidence-Based Nutrition Practice Guideline for the Management of Hypertension in Adults. Journal of the Academy of Nutrition and Dietetics. 2017;117(9):1445–1458. e1417. doi: 10.1016/j.jand.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Cohen HW, Hailpern SM, Fang J, Alderman MH. Sodium intake and mortality in the NHANES II follow-up study. Am J Med. 2006;119(3):275 e277–214. doi: 10.1016/j.amjmed.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 17.Cohen HW, Hailpern SM, Alderman MH. Sodium intake and mortality follow-up in the Third National Health and Nutrition Examination Survey (NHANES III) J Gen Int Med. 2008;23(9):1297–1302. doi: 10.1007/s11606-008-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aijala M, Malo E, Santaniemi M, et al. Dietary sodium intake and prediction of cardiovascular events. Eur J Clin Nutr. 2015;69(9):1042–1047. doi: 10.1038/ejcn.2015.40. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 20.Mozaffarian D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation. 2016;133(2):187–225. doi: 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lennon SL, DellaValle DM, Rodder SG, et al. 2015 Evidence Analysis Library Evidence-Based Nutrition Practice Guideline for the Management of Hypertension in Adults. J Acad Nutr Diet. 2017;117(9):1445–1458. doi: 10.1016/j.jand.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 22.MacGregor GA, Markandu ND, Sagnella GA, Singer DR, Cappuccio FP. Double-blind study of three sodium intakes and long-term effects of sodium restriction in essential hypertension. Lancet. 1989;2(8674):1244–1247. doi: 10.1016/s0140-6736(89)91852-7. [DOI] [PubMed] [Google Scholar]
- 23.Center for Nutrition Policy and Promotion, US Department of Agriculture. [Accessed May 17, 2017];Nutrients in Healthy US-Style Food Pattern at Each Calorie Level. https://www.cnpp.usda.gov/sites/default/files/usda_food_patterns/NutrientsInHealthyUS-StyleFoodPattern.pdf.
- 24.Guenther PM, Kirkpatrick SI, Reedy J, et al. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr. 2014;144(3):399–407. doi: 10.3945/jn.113.183079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guenther PM, Casavale KO, Reedy J, et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113(4):569–580. doi: 10.1016/j.jand.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. [Accessed June 20, 2017];Healthy Eating Index. 2015 https://epi.grants.cancer.gov/hei/developing.html.
- 27.Guenther PM, Lyon JM, Appel LJ. Modeling dietary patterns to assess sodium recommendations for nutrient adequacy. Am J Clin Nutr. 2013;97(4):842–847. doi: 10.3945/ajcn.112.047779. [DOI] [PubMed] [Google Scholar]
- 28.Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol. 2012;60(7):599–606. doi: 10.1016/j.jacc.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Graudal N, Jurgens G. The blood pressure sensitivity to changes in sodium intake is similar in Asians, Blacks and Whites. An analysis of 92 randomized controlled trials. Front Physiol. 2015;6:157. doi: 10.3389/fphys.2015.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hicken MT, Lee H, Morenoff J, House JS, Williams DR. Racial/ethnic disparities in hypertension prevalence: reconsidering the role of chronic stress. Am J Public Health. 2014;104(1):117–123. doi: 10.2105/AJPH.2013.301395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu W, Eckert GJ, Hannon TS, et al. Racial differences in sensitivity of blood pressure to aldosterone. Hypertension. 2014;63(6):1212–1218. doi: 10.1161/HYPERTENSIONAHA.113.02989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension. 2000;35(2):544–549. doi: 10.1161/01.hyp.35.2.544. [DOI] [PubMed] [Google Scholar]
- 33.Whelton PK, Appel LJ, Espeland MA, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;279(11):839–846. doi: 10.1001/jama.279.11.839. [DOI] [PubMed] [Google Scholar]
- 34.Rebholz CM, Gu D, Chen J, et al. Physical activity reduces salt sensitivity of blood pressure: the Genetic Epidemiology Network of Salt Sensitivity Study. Am J Epidemiol. 2012;176(Suppl 7):S106–113. doi: 10.1093/aje/kws266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hart EC, Charkoudian N. Sympathetic neural regulation of blood pressure: influences of sex and aging. Physiology (Bethesda) 2014;29(1):8–15. doi: 10.1152/physiol.00031.2013. [DOI] [PubMed] [Google Scholar]
- 36.Kojima S, Murakami K, Kimura G, et al. A gender difference in the association between salt sensitivity and family history of hypertension. Am J Hypertens. 1992;5(1):1–7. [PubMed] [Google Scholar]
- 37.Oh YS, Appel LJ, Galis ZS, et al. National Heart, Lung, and Blood Institute Working Group Report on Salt in Human Health and Sickness: Building on the Current Scientific Evidence. Hypertension. 2016;68(2):281–288. doi: 10.1161/HYPERTENSIONAHA.116.07415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.