Abstract
In addition to the social, communicative and behavioral symptoms that define the disorder, individuals with ASD have difficulty re-orienting attention quickly and accurately. Similarly, fast re-orienting saccadic eye movements are also inaccurate and more variable in both endpoint and timing. Atypical gaze and attention are among the earliest symptoms observed in ASD. Disruption of these foundation skills critically affects development of higher level cognitive and social behavior. We propose that interventions aimed at these early deficits that support social and cognitive skills will be broadly effective. We conducted a pilot clinical trial designed to demonstrate the feasibility and preliminary efficacy of using gaze-contingent video games for low-cost in-home training of attention and eye movement. Eight adolescents with ASD participated in an 8-week training, with pre-, mid- and post-testing of eye movement and attention control. Six of the eight adolescents completed the 8 weeks of training and all six showed improvement in attention (orienting, disengagement) and eye movement control or both. All game systems remained intact for the duration of training and all participants could use the system independently.
Conclusion
We delivered a robust, low-cost, gaze-contingent game system for home use that, in our pilot training sample, improved the attention orienting and eye movement performance of adolescent participants in 8 weeks of training. We are currently conducting a clinical trial to replicate these results and to examine what, if any, aspects of training transfer to more real-world tasks.
Introduction
The major clinical symptoms in autism are difficulties with language and social interaction, and these skills are targets of the most common of behavioral interventions. However, in typical function, these complex higher level social, language and communication skills are not present early in life, but develop over the first few years and depend upon the critical building blocks of sensory-motor, perceptual and attention abilities. Similarly, in autism higher level problems are not the first to appear, but they too develop over the first two post-natal years. Studies of siblings at risk for autism have found that at six months of age, delayed motor development and abnormalities of visual attention predict ASD. In 2005 Zwaigenbaum and colleagues reported early signs observable in baby siblings who go on to develop autism (Zwaigenbaum, Bryson et al. 2005). Deficits were observed in orienting behavior, visual tracking, and in the timing and disengagement of fixation. Others have similarly found impairments of motor development and visual attention as early signs of autism (Landa and Garrett-Mayer 2006; Rogers 2009; Ozonoff, Iosif et al. 2010). Longitudinal studies of infants at risk for and later diagnosed with ASD provide strong evidence for early impairment of covert and overt visual attention in ASD (Elsabbagh, Fernandes et al. 2013; Sacrey, Bryson et al. 2013) and a recent review reports that deficits in attention disengagement and orienting in ASD are evident in the first year of life and persist into adulthood (Sacrey, Armstrong et al. 2014). Our own work has identified deficits in visual attention including: orienting, capture, shifting, disengaging attention and a restricted distribution of visual attention in space (Townsend and Courchesne 1994; Townsend, Courchesne et al. 1996; Townsend, Courchesne et al. 1999; Haist, Adamo et al. 2005; Keehn, Lincoln et al. 2010; Townsend, Keehn et al. 2012; Keehn, Nair et al. 2016; Keehn, Westerfield et al. In Press).These deficits may affect early social development particularly by disrupting joint attention and regulation of social-emotional arousal (Keehn, Muller et al. 2013; Sacrey, Armstrong et al. 2014). For example, slow disengagement, orienting and visual tracking early in life may result in missed opportunities for observing the fast dynamic interactions inherent to any social exchange required for joint attention. In addition, difficulties with attention and gaze fixation disengagement may establish an early bias to not fixate eyes and faces as they could lead to uncomfortable levels of physiological arousal that would typically be remedied by swiftly averting gaze.
Disruptions of eye movement in ASD parallel those of attention (review Johnson, Lum et al. 2016). The majority of studies of gaze in ASD have focused on the social aspect of looking behavior (see for example Klin, Jones et al. 2002; McPartland, Webb et al. 2011) but have neglected details regarding saccade metrics and timing. Our own research has found that ASD children and teens are slow to initiate saccades and are less accurate and require more saccades to reach a target (Keehn, Westerfield et al. 2010; Miller, Chukoskie et al. 2014). A few studies that explicitly examined eye movement metrics during standard visually-guided target tasks have found increased variability in trial-to-trial amplitude in the saccades of individuals with ASD (Takarae, Minshew et al. 2004; Luna, Doll et al. 2007; Johnson, Rinehart et al. 2012). Although this literature is mixed, many studies find a hypometria in the primary saccade that is often compensated by secondary or “corrective” saccades (Takarae, Minshew et al. 2004; Johnson, Rinehart et al. 2012). Since the refractory period is at least 100 milliseconds, this inaccuracy is problematic in terms of gathering information from dynamic scenes. An eye-tracking study of a magician’s performance suggests that these differences may hamper an ASD individual’s ability to successfully collect information from a dynamic environment (Kuhn, Kourkoulou et al. 2010). Over the course of development, the accumulation of missed information can lead to deficits in social and communicative behaviors, and importantly, can also hamper tasks that require integration of multisensory information for fast responses.
Although covert spatial attention and gaze shift processes can be manipulated independently, they use overlapping brain systems (Posner 1980; Goldberg and Segraves 1987; Corbetta, Miezin et al. 1993) (for review, see Goldberg and Colby (1992)). Covert attention shifts may in fact be used to direct a gaze shift (Posner and Cohen 1984; Fischer and Breitmeyer 1987). Single cell recordings in alert monkeys have demonstrated that activity in parietal cortex precedes an intended eye movement to predict the location of expected visual input (Duhamel, Colby et al. 1992). The tight links between the circuitry responsible for planning and launching saccadic eye movements and the redirection of attention suggests that training one might induce benefits of the other (Bisley and Goldberg 2010; Striemer, Chouinard et al. 2015; Basso and May 2017). This link forms the theoretical basis for our current gaze-driven video game training intervention.
Social and language-based therapies may improve the specific behaviors that are targets of the training, but rarely do they generalize to broader function or other clinical symptoms. We propose that interventions aimed instead at the early deficits that support higher-level functions would be more broadly effective. Because attention is a critical scaffold for development of higher cognitive function; disruption of attention is one of the earliest and most persistent symptoms in autism; and because attention is highly subject to improvement with training, it is an important target for intervention. Because attention and eye movement are highly interdependent and there is evidence that eye movement and attention are similarly impaired in ASD, eye movement is an important corollary target for intervention.
Objectives of the study were to: 1) demonstrate the feasibility of using gaze-contingent video games for low-cost in-home training for high functioning adolescents with ASD; 2) demonstrate improvement of spatial attention orienting and eye movement behavior after 8 weeks of at-home play on these gaze-contingent games in a small group of adolescents with ASD.
Methods
Equipment
Our training system used a standard PC with an inexpensive eye-tracker (EyeTribe) with adequate speed and resolution for our purposes. The EyeTribe eye tracker has a manufacturer reported resolution of <0.5 degrees of visual angle under optimal conditions. We compared its accuracy to that of our Eyelink 1000 (remote tracking mode), using the same targets and the same subject. In our tests, the average accuracy (i.e., degrees of error from fixation point) of the EyeTribe tracker was 0.79 and the EyeLink was 0.46. The most important validation of the EyeTribe resolution is that it worked well in our gaze-driven games.
Games
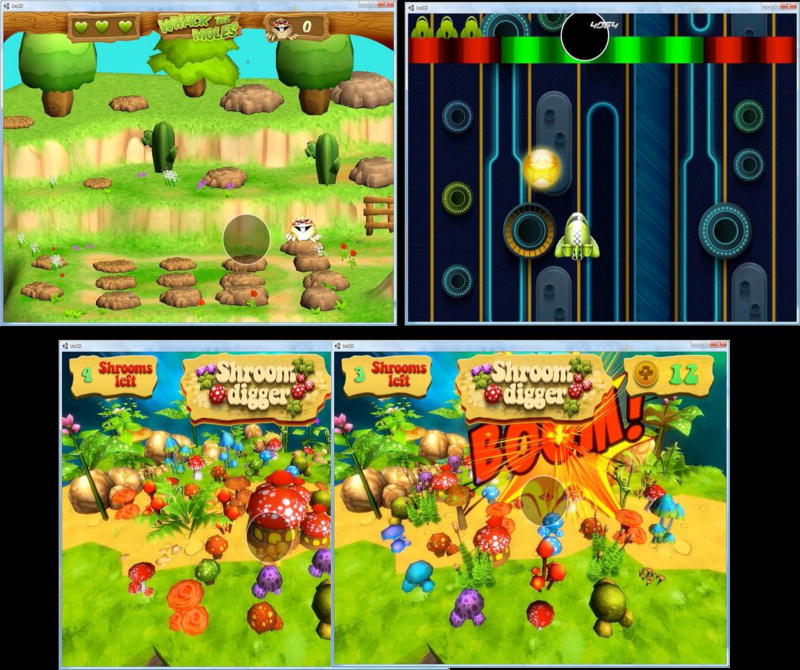
The training games were designed and programmed by professional game developers (Angry Troglodyte). Working with our specifications for training principles, the game developers created three colorful and engaging games with interesting sound effects (See Figure 1a–c.) These games were designed with principles to train fixation, speed and accuracy of eye movements & control of visuo-spatial attention. As the gamers play longer and gain competency, all games increase in difficulty, speed and active field of view (i.e., amount of visual/attentional field used in game).
Figure 1.
Figure 1, Mole Whack (upper left). Cartoon moles emerge from their holes or descend with parachutes from the sky. Some are to be ‘whacked’ by fixed gaze and others are to be avoided. This game trains rapid, accurate shifts and inhibitory control of attention and gaze. Space Race (upper right) requires fixing gaze on green gates to steer the ship through the green gates (which get progressively narrower) and through the stars to gain points. The spaceship moves faster, forcing faster gaze re-orientation as the game progresses. This game trains fast attention and gaze shifts and gaze control. Shroom Digger (lower). The participant fixates mushroom houses to enlarge and eventually explode them. Any failure of steady fixation causes the house to shrink so increases or decreases are dynamically controlled. This game trains gaze fixation and sustained attentional focus.
See (https://www.radlab.ucsd.edu/videos) for sample videos of the three games.
Participants
Eight ASD children/teens began the training and six of those completed the training as required (see Table 1 for demographic and diagnostic data and game play times). One child refused to continue playing after 3 weeks because he was bored and another child became anxious about playing time and withdrew. Two participants played less than the required total time (gamers P3 & P4), but both showed training effects. The remaining participants played the games the required amount of time or more (30 minutes, 5 times per week for 8 weeks—1200 minutes). At their final visit three of the six children who completed the study provided feedback about the games. All found the software and hardware easy to use. All suggested we add additional games (and provided specific feedback for changes to existing games). One considered the games to be fun (P1), another thought they were “sort of” fun (P2) and the third marked them just “OK” (P5).
Table 1.
| Participant | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | Mean (SD) |
|---|---|---|---|---|---|---|---|---|---|
| Age | 10.63 | 12.52 | 14.55 | 15.36 | 16.04 | 16.83 | 14.04 | 11.00 | 13.9 (2) |
| Sex | M | F | M | F | M | F | M | M | 3 F, 5 M |
| V IQ | 104 | 108 | 138 | 95 | 93 | 104 | 130 | 120 | 111.5 (16) |
| P IQ | 87 | 124 | 88 | 101 | 87 | 114 | 118 | 99 | 102.2 (15) |
| SRS Total | 85 | 90 | 76 | 90 | 60 | 76 | 98 | 64 | 79.9 (13) |
| ADOS Soc | 7 | 7 | 8 | 9 | 8 | 11 | 8 | 8.3 (1) | |
| ADOS Comm | 4 | 3 | 2 | 5 | 2 | 4 | 5 | 3.6 (1) | |
| ADOS Rep | 4 | 2 | 2 | 3 | 1 | 3 | 6 | 3.0 (2) | |
| ADI Soc | 24 | 26 | 21 | 21 | 13 | 20 | 20 | 20.7 (4) | |
| ADI Comm | 21 | 23 | 19 | 19 | 6 | 20 | 21 | 18.4 (6) | |
| ADI Rep | 10 | 10 | 6 | 8 | 3 | 7 | 6 | 7.1 (2) | |
| Weeks in Study | 8 | 8 | 8 | 4* | 8 | 8 | 3 | 1 | |
| Total Minutes | 1539 | 1619 | 1455 | 500 | 985 | 1173 | 340 | 215 |
Notes: Participant 4 has a clinical diagnosis of ASD and had a score on the Social Communication Questionnaire (a standardized screen for ASD) of 24, well above the ASD cutoff of 15. The SRS (Social Responsiveness Scale) T-Score cutoff for mild to moderate ASD is 60 and for severe ASD is 76. All ADOS and ADI scores for all participants are above ASD/autism cutoff. Participants 7 and 8 failed to complete training.
Participant 4 began the study after a delay and we reporting her results at 4 weeks. Requested game play time was 8 weeks and a minimum of 1200 minutes of play.
Procedures
Participants came to the lab three times for pre-, mid- and post-training assessment of attention and eye movement. At the first visit following pre-training tests, the participant was trained to use the gaming system. We then installed the gaming system in the participant’s home and assured that the participant could follow calibration procedures and play the games independently. The gaming interface was simple and required only that the child complete a short calibration procedure (looking at bullseye targets in 9 different monitor locations) for which they received feedback (0–5 stars with 3 stars or more required to continue to game play). We were not able to save the calibration data but plan to incorporate this feature in future versions as quality and ease of calibration may affect willingness to play and training effects. Following adequate calibration, the participant would select the game to play and begin.
The participant was asked to play 30 minutes per day, 5 times per week and compliance was monitored over a secure internet connection with software that automatically recorded date, time and duration of play for each game as well as performance data. Participant’s also noted time played on a calendar and wrote notes to us about the games or any problems they were having. Compliance data were checked daily and if a participant’s note required a response, or if the participant was not complying with required training time, we followed-up with e-mail and/or a phone call.
Pre-, Mid- and Post-training Assessment
To test for training efficacy, we used tasks that measure spatial attention and eye movement function. These are established tasks for which we could establish confidence intervals from typical performance to examine whether there was significant training-related change in individual participants.
Spatial Attention Task (E-Task)
This task is patterned after Posner’s classic cued spatial attention tasks (Posner 1980; Posner 1987), but uses target discrimination rather than detection. The task display is a central fixation cross on a computer monitor flanked by boxes on the left and right. Trials begin with either an attention directing cue (one of the boxes brightened) or a null cue (no change or both boxes brightened). Following a short (100 ms) or long (800 ms) delay, a target (letter “E” pointing up, down, left or right) is presented in either the cued (valid) or the opposite uncued (invalid) location. The subject moves a joystick lever to indicate target orientation (up, down, left, right). This task elicits a robust visual attention effect in typical individuals—accuracy is significantly greater at the cued location than at the uncued location or following no cue. This facilitation is maximal early and diminishes over time. The task is controlled for speed of visual perception (the target is masked by a feature mask after 50 ms) and motor response (after the target is masked, participants have up to 2000 ms to respond). Results from studies using this task demonstrate slowed disengagement and orienting of attention in ASD children and adults (Townsend, Courchesne et al. 1996; Townsend, Harris et al. 1996; Townsend, Courchesne et al. 1999).
Analyses
Comparisons for attention measures were based on means and variance from age-matched typical children (Schul, Townsend et al. 2003). We tested: overall attention performance (task accuracy averaged across all conditions), speed of attention orienting (accuracy to correctly cued targets with short attention orienting interval) and attention disengagement (accuracy to correctly vs. incorrectly cued targets).
Gap-Overlap Saccade Task
The Gap-Overlap saccade task is a classic oculomotor paradigm during which a subject’s inclination to “get stuck” on an available fixation target is assessed. In our paradigm, each trial starts with a fixation cross at the center of the screen and a single target drawn randomly from 16 possible peripheral locations appears either simultaneously with the offset of fixation (baseline condition), 400 ms after the offset of fixation (gap condition), or does not disappear at all (overlap condition). We used only the baseline condition to examine training related eye movement change.
Analysis
Measures used from this task were: saccade latency (time to initiate a saccade to a target in the baseline condition), saccade accuracy (degrees error of saccade to the target in the baseline condition), and duration of fixation with error (deviation) less than or equal to [1.5 degrees.]. Confidence intervals to assess change were based on means and variance from age-matched typical children (Miller, Chukoskie et al. 2014).
Results
Note that since this is a very small sample and the purpose of this pilot study was to establish feasibility of methods and procedures, analyses were largely descriptive and confined to testing individual cases against existing typical control samples. Statistically significant improvement was considered to be positive change of 2 or greater SEM (standard error of the mean) in the age-matched control sample.
Post-training Spatial Attention Change
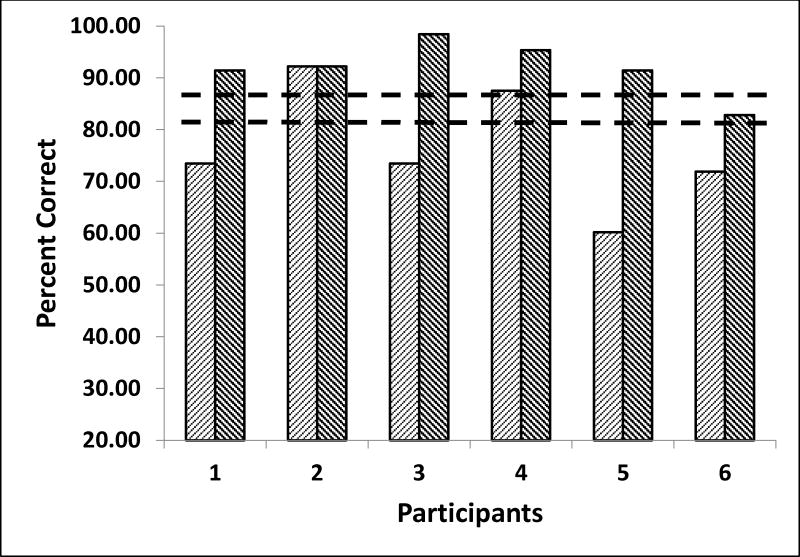
All 6 of the 8 participants who completed the training showed significant improvement on one or more outcome measures. Five of six participants improved significantly in attention performance (percent correct averaged over all conditions; improvement range 0 to 21 sem). The one teen who did not improve was close to ceiling performance at pre-test. On the spatial attention measure that reflects speed of orienting, 4 of 6 improved after 4 weeks training and 5 of 6 improved after 8 weeks (improvement range −0.5 to 20 sem). The one teen that did not improve was close to ceiling performance on this measure at pre-testing. See Figure 2. Four of 6 participants also significantly reduced time to disengage attention after 4 weeks of training (improvement range 1.5 to 11 sem). One of these continued to improve after 8 weeks, the others sustained improvement.
Figure 2.
Figure 2. Spatial Attention Speed of Orienting. Figure shows accuracy (percent correct vertical axis) for each of the six gamers before (light) and after (dark) training. Five of 6 improved more than 2 standard errors after 8 weeks compared to a sample of age-matched typical controls and all improved into the range of typical performance (dashed lines show upper and lower confidence limits for typical performance). Confidence intervals are based on means and variance of typical performance from (Schul, Townsend et al. 2003).
Post-training Eye-Movement Change
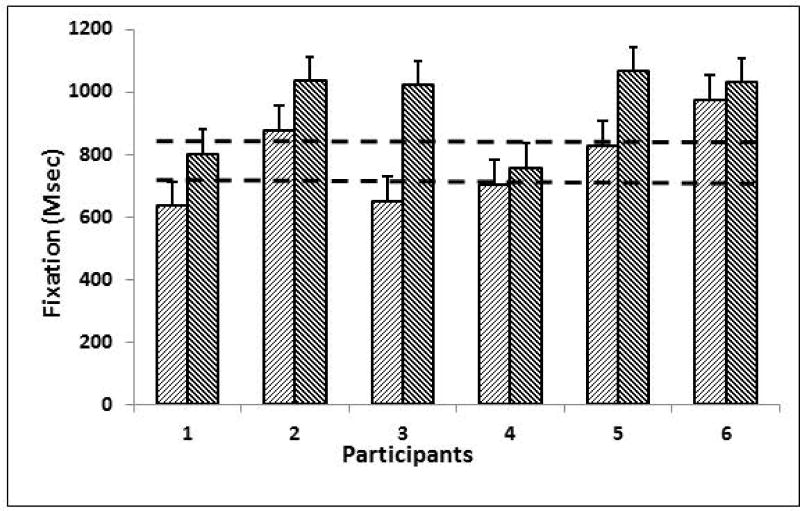
Four of 6 participants significantly improved the duration of gaze fixation. Three of these who had shorter than typical fixation duration improved into the typical range (improvement range 1.7 to 11 sem). See Figure 3. Three of 6 participants improved saccade latency (improvement range −4 to 3.5 sem) and 3 of the 6 participants improved saccade accuracy (improvement range −6 to 15.5 sem).
Figure 3.
Figure 3. Duration of Gaze Fixation. Figure shows average duration of gaze fixation (in milliseconds, vertical axis) for the six gamers (from the Baseline condition of the Gap-Overlap Task) before (light) and after (dark) training. Error bars represent average standard error for pre- and post-training fixation duration. Four of 6 gamers significantly improved the duration of gaze fixation (greater than 2 standard errors compared to a sample of 23 typical children mean age 11). Three of these who had shorter than typical fixation duration improved into the typical range marked by dashed lines representing upper and lower confidence limits of the typical sample from Miller et al (Miller, Chukoskie et al. 2014).
Table 2 summarizes improvement on all 6 attention and eye movement outcome measures.
Table 2.
Summarizes post-training changes in attention and eye movement. Significant change in attention measures were based on means and variance from age-matched typical children (Schul, Townsend et al. 2003). Note that P2 who appears to have less change was at pre- and post-test ceiling on attention measures for overall performance and orienting. Significant change in eye movement measures were based on means and variance from age-matched typical children (Miller, Chukoskie et al. 2014).
| Post-Training Improvement | P1 | P2 | P3 | P4 | P5 | P6 |
|---|---|---|---|---|---|---|
| Spatial Attention | ||||||
| Overall % Correct | *** | *** | *** | *** | *** | |
| Orienting | *** | *** | *** | *** | *** | |
| Disengagement | *** | *** | ** | *** | *** | * |
| Eye Movement | ||||||
| Fixation | *** | *** | *** | * | *** | * |
| Saccade Latency | ** | * | *** | ** | ||
| Saccade Accuracy | *** | ** | ** | *** | * |
Improvement in Standard Errors (SE):
= 1 SE;
= 2 SE;
= 3 or greater SE
Discussion
This project used new technology to implement a novel concept for behavioral intervention to improve basic attention and eye movement skills in ASD. Because these skills form the foundation for good social communication, training these abilities has the potential to improve a broad spectrum of clinical symptoms, and in young children may affect the course of development. In this small trial, we demonstrated feasibility and provided preliminary evidence for efficacy of this approach. Because the primary goal of this pilot study was to demonstrate feasibility of the approach, there was no placebo (control) group. This limits interpretation of efficacy data as it is not possible to rule out the effect on post-training results of practice or increased comfort with testing procedures in the lab. In an on-going small clinical trial we are replicating these pilot results and examining potential transfer to social and cognitive skills. A future large clinical trial will incorporate appropriate controls for practice and placebo effects.
“Brain Training” games have become increasingly common, with companies touting benefits of regular computer-based brain training games for the public. The kinds of cognitive abilities targeted for training typically include attention, memory and processing speed, but have also included interpersonal skills and navigation. Visual attention appears to be readily trained even through commercial video game play (Achtman, Green et al. 2008; Granek, Gorbet et al. 2010; Mishra, Zinni et al. 2011). The games we developed for this intervention used a research-based theoretical framework of structural and functional overlap in spatial attention and eye movement as well as evidence-based selection of specific components of attention and eye movement control as training targets. Results from this pilot sample show that training did improve the intended specific eye movement and spatial attention therapeutic targets. We suggest that efficacy of this training is largely due to the use of gaze to force attention control (a hypothesis we are currently testing).
The feasibility demonstration is equally important to the efficacy. We showed that home-based training with an eye tracker is accessible to families of children with autism, with several teens taking charge of the training by themselves. This is important because other types of training studies echo what we observed in the need for practice several times per week—a burdensome requirement for in-lab training that may lead to high drop-out rates (Vedamurthy, Nahum et al. 2015). Designing our study so participants could train from home increased our focus on developing a robust data collection system early in the study, which took some time. However, doing so opened up the possibility of recruiting a wide range of participants as most could not have committed to lab-based training at the same intensity and duration.
Our pilot data show that this intervention improves attention and eye movement control. Why are these skills important and how do they influence cognitive and social behavior? Delays in the shift of attention and the subsequent launch of a saccade to acquire a target means that information in another’s subtle social signal may be lost. Similarly, delays in visuomotor integration and motor skills can result in a delayed or the appropriate action for the current situation. Deficits in attention and eye movement accuracy can have the same result. Delays and inaccuracies in attention shifts and saccadic eye movement result in more time needed to capture information. In a dynamic environment, the time needed to shift attention and plan additional saccades may be too long to capture a rapidly changing environment. Deficits in such orienting skills are not merely academic, but impact real world performance in tasks such as walking or running on a busy street, bicycling and driving. Not surprisingly, individuals with ASD show slower reaction times to hazards as well as impaired attention shifting in a driving simulator (Sheppard, Ropar et al. 2010).
A major question is how well improvement in gaze or attention orienting will transfer to related real-world tasks and whether changing these basic skills will result in improvement in clinical symptoms. There is some evidence from video game play that suggests training motor behaviors and related processes (such as attention) may result in broader effects, like detection of temporal synchrony of visual and auditory stimuli (Donohue, Woldorff et al. 2010). There are also a few examples that suggest one might see transfer of eye movement training to related tasks. Saccadic adaptation paradigms (a training of sorts) show limited transfer of saccade size changes between different types of saccades (Hopp and Fuchs 2010). Madelain and colleagues (Madelain, Paeye et al. 2011) recently demonstrated the ability to adapt saccade size by using reinforcement similar to that used in a game environment rather than a classic retinal error signal. The optometric vision therapy research reports changes in many patient populations including children with convergence insufficiency (CITTS-Group 2008) and older patients with brain damage who show benefits in visuo-perceptual skills after different types of eye movement practice and wearing glasses with specially adapted lenses and filters (Ciuffreda, Rutner et al. 2008).
Games for assessment and intervention benefit from the psychological and neuroscientific principles of motivation, reinforcement, and scaffolding challenges presented throughout the game. In addition, appropriately designed games generate data that is amenable to computational methods for modeling the behavior and various physiological measures elicited through game play. Although we believe our gaze-contingent intervention games to be a novel home-based therapeutic for autism, we are both aware of and encouraged by the swell of research in game-based research for intervention and assessment in ASD (Pineda, Brang et al. 2008; Whyte, Smyth et al. 2015; Anguera, Brandes-Aitken et al. 2016). We are especially enthusiastic about games that aim to habilitate core skills while doing so in an immersive and, if possible, also social context. Virtual reality (VR) video games have the added advantages of being immersive by design, which engages training in an integrated multi-sensory environment that may help promote transfer of trained skills to real-world scenarios. Although VR has been actively used therapeutically for motor rehabilitation (review: Darekar, McFadyen et al. 2015), its use for cognitive and neurodevelopmental disorders is just beginning. Finally, the integration of wearable sensors into games can adjust the level of play with not only the participant’s behavior, but also physiological arousal (Rani, Sarkar et al. 2006). These developments in game-based technology for hold great promise for creating adaptive assessments and interventions in developmental disorders.
Conclusion
Current therapies in ASD target social behaviors, but due to the high-level nature of these skills any improvement rarely extends beyond the targeted behavior. This project uses new technology and a novel approach to an intervention to improve basic attention and eye movement skills. Because these basic skills form the foundation for good social communication, training these abilities has the potential to improve a broad range of clinical symptoms, and in young children may affect the course of development. In this study we delivered a robust, low-cost, gaze-contingent game system for home use that, in our training sample, improved the attention orienting and eye movement performance of adolescent participants in 8 weeks. We are currently conducting a clinical trial to replicate these results and to examine what, if any, aspects of training transfer to more real-world tasks.
Acknowledgments
Supported by NIH/NIMH R21/R33 MH96967 awarded to JT and NSF SBE-0542013, SMA-1041755 that supported LC
Footnotes
The authors have no conflicts of interest to report.
References
- Achtman RL, Green CS, et al. Video games as a tool to train visual skills. Restor Neurol Neurosci. 2008;26(4–5):435–446. [PMC free article] [PubMed] [Google Scholar]
- Anguera JA, Brandes-Aitken AN, et al. Characterizing cognitive control abilities in children with 16p11.2 deletion using adaptive ‘video game’ technology: a pilot study. Transl Psychiatry. 2016;6(9):e893. doi: 10.1038/tp.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, May PJ. Circuits for Action and Cognition: A View from the Superior Colliculus. Annu Rev Vis Sci. 2017 doi: 10.1146/annurev-vision-102016-061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CITTS-Group. Randomized clinical trial of treatments for symptomatic convergence insufficiency in children. Archives of Opthalmology. 2008;126(10):1336–1349. doi: 10.1001/archopht.126.10.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda KJ, Rutner D, et al. Vision therapy for oculomotor dysfunctions in acquired brain injury: a retrospective analysis. Optometry. 2008;79(1):18–22. doi: 10.1016/j.optm.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, et al. A PET study of visuospatial attention. Journal of Neuroscience. 1993;13(3):1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darekar A, McFadyen BJ, et al. Efficacy of virtual reality-based intervention on balance and mobility disorders post-stroke: a scoping review. J Neuroeng Rehabil. 2015;12:46. doi: 10.1186/s12984-015-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue SE, Woldorff MG, et al. Video game players show more precise multisensory temporal processing abilities. Atten Percept Psychophys. 2010;72(4):1120–1129. doi: 10.3758/APP.72.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, et al. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255:5040. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Fernandes J, et al. Disengagement of visual attention in infancy is associated with emerging autism in toddlerhood. Biol Psychiatry. 2013;74(3):189–194. doi: 10.1016/j.biopsych.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Breitmeyer B. Mechanisms of visual attention revealed by saccadic eye movements. Neuropsychologia. 1987;25(1-A) doi: 10.1016/0028-3932(87)90044-3. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Colby CL. Oculomotor control and spatial processing. Current Opinion in Neurobiology. 1992;2(2):198–202. doi: 10.1016/0959-4388(92)90012-a. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Segraves MA. Visuospatial and motor attention in the monkey. Neuropsychologia. 1987;25(1A):107–118. doi: 10.1016/0028-3932(87)90047-9. [DOI] [PubMed] [Google Scholar]
- Granek JA, Gorbet DJ, et al. Extensive video-game experience alters cortical networks for complex visuomotor transformations. Cortex. 2010;46(9):1165–1177. doi: 10.1016/j.cortex.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Haist F, Adamo M, et al. The functional neuroanatomy of spatial attention in autism spectrum disorder. Developmental Neuropsychology. 2005;27(3):425–458. doi: 10.1207/s15326942dn2703_7. [DOI] [PubMed] [Google Scholar]
- Hopp JJ, Fuchs AF. Identifying sites of saccade amplitude plasticity in humans: transfer of adaptation between different types of saccade. Exp Brain Res. 2010;202(1):129–145. doi: 10.1007/s00221-009-2118-5. [DOI] [PubMed] [Google Scholar]
- Johnson BP, Lum JA, et al. Ocular motor disturbances in autism spectrum disorders: Systematic review and comprehensive meta-analysis. Neurosci Biobehav Rev. 2016;69:260–279. doi: 10.1016/j.neubiorev.2016.08.007. [DOI] [PubMed] [Google Scholar]
- Johnson BP, Rinehart NJ, et al. A closer look at visually guided saccades in autism and Asperger’s disorder. Front Integr Neurosci. 2012;6:99. doi: 10.3389/fnint.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Lincoln AJ, et al. Attentional networks in children and adolescents with autism spectrum disorder. Journal of Child Psychololgy and Psychiatry, and Allied Disciplines. 2010;51(11):1251–1259. doi: 10.1111/j.1469-7610.2010.02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Muller RA, et al. Atypical attentional networks and the emergence of autism. Neurosci Biobehav Rev. 2013;37(2):164–183. doi: 10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Nair A, et al. Under-reactive but easily distracted: An fMRI investigation of attentional capture in autism spectrum disorder. Dev Cogn Neurosci. 2016;17:46–56. doi: 10.1016/j.dcn.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B, Westerfield M, et al. A comparison of overt visuospatial orienting abilities in children with autism, Williams syndrome, specific language impairment, perinatal brain lesions, and typical development. 40th Annual Meeting of the Society for Neuroscience; San Diego, CA. 2010. Society For Neuroscience Abstracts. [Google Scholar]
- Keehn B, Westerfield M, et al. Autism, Attention, and Alpha Oscillations: An Electrophysiological Study of Attentional Capture. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. doi: 10.1016/j.bpsc.2017.06.006. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Jones W, et al. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Kuhn G, Kourkoulou A, et al. How magic changes our expectations about autism. Psychol Sci. 2010;21(10):1487–1493. doi: 10.1177/0956797610383435. [DOI] [PubMed] [Google Scholar]
- Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. J Child Psychol Psychiatry. 2006;47(6):629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Luna B, Doll SK, et al. Maturation of executive function in autism. Biol Psychiatry. 2007;61(4):474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Madelain L, Paeye C, et al. Modification of saccadic gain by reinforcement. J Neurophysiol. 2011;106(1):219–232. doi: 10.1152/jn.01094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JC, Webb SJ, et al. Patterns of visual attention to faces and objects in autism spectrum disorder. J Autism Dev Disord. 2011;41(2):148–157. doi: 10.1007/s10803-010-1033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Chukoskie L, et al. Dyspraxia, motor function and visual-motor integration in autism. Behav Brain Res. 2014;269:95–102. doi: 10.1016/j.bbr.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Zinni M, et al. Neural basis of superior performance of action videogame players in an attention-demanding task. J Neurosci. 2011;31(3):992–998. doi: 10.1523/JNEUROSCI.4834-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49(3):256–266. e251–252. [PMC free article] [PubMed] [Google Scholar]
- Pineda JA, Brang D, et al. Positive behavioral and electrophysiological changes following neurofeedback training in children with autism. Research in Autism Spectrum Disorders. 2008;2:557–581. [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI. Cognitive neuropsychology and the problem of selective attention. Electroencephalography and Clinical Neurophysiology Supplement. 1987;39(1):313–316. [PubMed] [Google Scholar]
- Posner MI, Cohen Y. Components of performance. In: Bouma H, Bouwhuis DG, editors. Attention and performance X. Hillsdale, NJ: Erlbaum; 1984. pp. 531–556. [Google Scholar]
- Rani P, Sarkar N, et al. Maintaining Optimal Challenge in Computer Games Through Real-Time Physiological Feedback. In: Schmorrow DD, editor. Task Specific Information Processing in Operational and Virtual Environments, Foundations of Augmented Cognition. Lawrence Erlbaum Associates; 2006. pp. 184–192. [Google Scholar]
- Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Research. 2009;2(3):125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacrey LA, Armstrong VL, et al. Impairments to visual disengagement in autism spectrum disorder: a review of experimental studies from infancy to adulthood. Neurosci Biobehav Rev. 2014;47:559–577. doi: 10.1016/j.neubiorev.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Sacrey LA, Bryson SE, et al. Prospective examination of visual attention during play in infants at high-risk for autism spectrum disorder: a longitudinal study from 6 to 36 months of age. Behav Brain Res. 2013;256:441–450. doi: 10.1016/j.bbr.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Schul R, Townsend J, et al. Development of attention orienting during the school-age years. Developmental Science. 2003;6(3):262–272. [Google Scholar]
- Sheppard E, Ropar D, et al. Brief report: driving hazard perception in autism. J Autism Dev Disord. 2010;40(4):504–508. doi: 10.1007/s10803-009-0890-5. [DOI] [PubMed] [Google Scholar]
- Striemer CL, Chouinard PA, et al. Overlapping neural circuits for visual attention and eye movements in the human cerebellum. Neuropsychologia. 2015;69:9–21. doi: 10.1016/j.neuropsychologia.2015.01.024. [DOI] [PubMed] [Google Scholar]
- Takarae Y, Minshew NJ, et al. Oculomotor abnormalities parallel cerebellar histopathology in autism. J Neurol Neurosurg Psychiatry. 2004;75(9):1359–1361. doi: 10.1136/jnnp.2003.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Courchesne E. Parietal damage and narrow “spotlight” spatial attention. Journal of Cognitive Neuroscience. 1994;6(3):220–232. doi: 10.1162/jocn.1994.6.3.220. [DOI] [PubMed] [Google Scholar]
- Townsend J, Courchesne E, et al. Spatial attention deficits in patients with acquired or developmental cerebellar abnormality. Journal of Neuroscience. 1999;19(13):5632–5643. doi: 10.1523/JNEUROSCI.19-13-05632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Courchesne E, et al. Slowed orienting of covert visual-spatial attention in autism: Specific deficits associated with cerebellar and parietal abnormality. Development and Psychopathology. 1996;8(3):503–584. [Google Scholar]
- Townsend J, Harris NS, et al. Visual attention abnormalities in autism: delayed orienting to location. Journal of the International Neuropsychological Society. 1996;2(6):541–550. doi: 10.1017/s1355617700001715. [DOI] [PubMed] [Google Scholar]
- Townsend J, Keehn B, et al. Abstraction of Mind: Attention in autism. Cognitive Neuroscience of Attention M Posner New York, Guilford Press. 2012;1:357–373. [Google Scholar]
- Vedamurthy I, Nahum M, et al. A dichoptic custom-made action video game as a treatment for adult amblyopia. Vision Res. 2015;114:173–187. doi: 10.1016/j.visres.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte EM, Smyth JM, et al. Designing Serious Game Interventions for Individuals with Autism. J Autism Dev Disord. 2015;45(12):3820–3831. doi: 10.1007/s10803-014-2333-1. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, et al. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23(2–3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]