Abstract
Background
Trichomonas vaginalis is a STI associated with increased transmission of HIV and significant adverse birth outcomes; culture and PCR are commonly used in diagnosis.
Methods
Consenting HIV-infected pregnant women were recruited from clinics in South Africa and screened for T. vaginalis using PCR. PCR-positive women provided an additional sample for culture. We compared T. vaginalis detection between PCR and culture, and investigated how PCR cycle threshold (Ct) values differ among culture results.
Results
A total of 359 women were enrolled and 76 (20%) tested T. vaginalis PCR-positive. Cultures were obtained from 61 of the PCR-positive women, and 38 (62%) were culture-positive. The median baseline Ct of the PCR-positive/culture-positive group was 22.6 vs. 38.0 among those PCR-positive/culture-negative (p<0.001). Culture positive cases had lower Ct values (higher DNA load); a Ct value < 30 predicted positivity with a sensitivity of 97% and specificity of 96%.
Conclusions
Culture was positive in roughly half of PCR positive cases. The culture-negative cases had significantly higher Ct values, indicating a lower concentration of T. vaginalis DNA. A Ct value of 30 provides a reliable threshold for predicting culture positivity. The clinical significance of culture-negative infections detected by PCR is still unclear.
Keywords: Trichomonas vaginalis, HIV, PCR, culture, cycle threshold
Introduction
Trichomonas vaginalis is one of the most common sexually transmitted infections in the world. The World Health Organization (WHO) estimated that in 2012 there were 143 million new cases of T. vaginalis, more than the incidence of Neisseria gonorrhoeae, Chlamydia trachomatis, or syphilis infections. [1]
The high rates of T. vaginalis infection are particularly troubling in the context of adverse reproductive health and pregnancy outcomes. Women infected with T. vaginalis can experience a range of symptoms, including itching, vaginal discharge, and dysuria, as well as more significant complications such as pelvic inflammatory disease and fallopian tube pathology. [2, 3] T. vaginalis infection is also associated with an increased risk of acquiring HIV, [4] as well as increased vaginal shedding of HIV. [3] A woman infected with T. vaginalis during pregnancy is more likely to experience preterm delivery, premature rupture of membranes, and delivery of low birthweight infants. [5, 6, 7] In addition, T. vaginalis infection during pregnancy has been associated with an increased risk of vertical transmission of HIV. [8]
T. vaginalis is a curable STI, thus prompt detection and treatment are appropriate interventions to mitigate infection-associated complications. Such interventions are particularly important in high-risk populations such as pregnant women and immunocompromised individuals. Estimates of the proportion of asymptomatic T. vaginalis infections are as high as 80% [5], and the US Centers for Disease Control and Prevention (CDC) recommends screening for T. vaginalis among HIV-infected women, especially HIV-infected pregnant women. [9] Three classes of detection methods of increasing sensitivity commonly used to screen for T. vaginalis are: wet-mount microscopy, culture, and nucleic-acid amplification tests (NAATs).
Despite recommendations to routinely screen HIV-infected pregnant women for T. vaginalis, most settings still practice syndromic screening for detection of STIs. [10] The exquisite sensitivity of NAATs makes them great screening tools, but it also raises two important questions: 1) Which PCR-positive cases are clinically significant (i.e. would not have been cleared if left untreated)?; and 2) At what time point does a test-of-cure test accurately detect a persistent infection rather than remnant nucleic acid from an infection that will soon be cleared? The goal of this study was to measure the effectiveness of NAAT screening for T. vaginalis and to attempt to understand the clinical significance of PCR-positivity. To that end, we took advantage of the opportunity to compare culture and PCR for the detection of T. vaginalis as part of an ongoing study of STI detection among HIV-infected pregnant women.
Materials and Methods
Study Participants
Participants were recruited from two clinics in the Soshanguve Township, Tshwane, South Africa from June 2016 through May 2017. All participants were HIV-infected pregnant women, >18 years old and <34 weeks gestation, and presenting for their first antenatal clinic visit for their current pregnancy. Women were assessed for eligibility, consented, and enrolled. Enrolled participants completed a baseline questionnaire, received basic antenatal care (ANC) clinical services from the study nurse per South African national guidelines, [10] and were asked to provide a self-collected vaginal swab for STI testing. The specimens used for this study were collected from a subset of women from a large cohort study assessing the acceptability, feasibility, and impact on birth outcomes of point-of-care PCR diagnosis and treatment of C. trachomatis, N. gonorrhoeae, and T. vaginalis.
Ethics
The study protocol was approved by the institutional review boards of the University of Pretoria (IRB Approval #: 401/2015) and the University of California, Los Angeles (IRB Approval #: 15-001351). All participants provided informed written consent.
PCR Testing
The GeneXpert (Cepheid, Sunnyvale, California) diagnostic platform and Xpert® TV test cartridges were used to screen for T. vaginalis. [11] The Xpert® TV test cartridge includes three internal controls: a sample processing control which detects the presence of PCR reaction inhibitors; a sample adequacy control that confirms the presence of human genes in the specimen; and a probe check control that verifies the integrity of the probe and reagent preparation. The Xpert® TV Assay’s limit of detection is reported by the manufacturer as 5 cells/mL. [12] The cycle threshold (Ct) values of positive PCR runs were recorded. The Ct value represents the number of PCR cycles before a reaction becomes positive, and is inversely related to the initial amount of nucleic acid being amplified; therefore, lower Ct values in an Xpert® TV Assay indicate more T. vaginalis nucleic acid in a sample. Ct values for a positive GeneXpert Assay typically range between 15 – 40. [13] The Xpert® TV Assay stops running after 40 cycles, therefore no amplification by 40 cycles is interpreted as negative.
HIV-infected pregnant women who tested positive for T. vaginalis were provided with a regimen of 400mg of metronidazole, twice a day, for seven days, in accordance with recommendations for similar regimens from the South African [10] and U.S. CDC treatment guidelines. [9] A test-of-cure visit was scheduled for 3 weeks after treatment was initiated. Women who tested positive for T. vaginalis were provided two options for partner treatment: a referral for treatment at the clinic, or a pill packet containing one 2g dose of metronidazole.
T. vaginalis Culture
Women with positive Xpert® TV tests were asked to provide an additional vaginal-swab for culture using a FLOQSwab Dry Swab (Copan Diagnostics, Brescia, Italy). This sample was requested during the same visit as the Xpert® TV testing, right after the test results were available. T. vaginalis culture was performed by inoculating the InPouch TV™ with the vaginal-swab (Biomed, San Jose, CA, USA) per the manufacturer’s instructions. [14] The InPouch TV™ limit of detection has been reported to be 4 organisms per mL [15] and 0.2 organisms per reaction. [16] Inoculated InPouch cultures were incubated at clinic sites at 18° - 37°C, and transported to the University of Pretoria within 36 hours of inoculation.
Upon arrival at the University of Pretoria, cultures were incubated at 37°C and observed by wet-mount microscopy daily for motile trichomonads. An InPouch was declared culture positive if motile trichomonads were observed within the first 7 days of incubation; if no motile trichomonads were observed in this time frame, the InPouch was declared culture negative.
Data Management and Statistics
Study data were collected and managed using REDCap electronic data capture tools hosted at the Foundation for Professional Development (Pretoria, South Africa). [17] GeneXpert Ct values for PCR-positive/culture-positive and PCR-positive/culture-negative samples were compared to assess the parasite burden detected by PCR in the two groups.
Ct values were tested for normality using the Shapiro-Wilk test, and data that were not normally distributed are reported here using median and inter-quartile range (IQR). The non-parametric Mann-Whitney U test was used to compare Ct values between groups. A receiver operating characteristic (ROC) curve was calculated to determine the diagnostic characteristics of the Ct value in relation to culture positivity. The area under the curve was calculated to determine the accuracy of the Ct value as a diagnostic test, and the Youden Index was calculated to determine the optimal Ct threshold to predict culture positivity. A chi-square test was performed to determine if there was a relationship between symptomatology and culture positivity. All statistical testing was two-tailed at a significance level (α) of 0.05. Calculations were performed using IBM SPSS Statistics for Windows (Version 24.0, IBM Corp. in Armonk, NY).
Results
T. vaginalis Screening at Baseline
A total of 359 HIV-infected pregnant women were enrolled, of whom all 359 (mean age: 30.1 years) provided self-collected vaginal swabs for PCR testing of T. vaginalis. Of these, 76 (20%) women tested positive for T. vaginalis (T. vaginalis-positive). Women who tested positive for T.vaginalis had similar demographic characteristics to T. vaginalis-negative women (Table). Of those T. vaginalis-positive women, 19 (25%) reported one or more of the following symptoms: dyspareunia, dysuria, abnormal vaginal discharge, or vaginal bleeding; 25 (33%) were observed to have vaginal discharge and/or bleeding on clinical exam; and 37 (49%) had no symptoms or signs upon examination. Of the 72 (95%) women who chose to inform their partner of their infection with T. vaginalis, 49 (68%) chose to provide their partner with a pill packet, while 23 (32%) chose to provide a clinic referral.
Table.
Demographic characteristics of study participants, stratified by T. vaginalis PCR-status at baseline.
|
T. vaginalis-PCR-Positive (n = 76) |
T. vaginalis-PCR-Negative (n = 283) |
|||
|---|---|---|---|---|
| Age (years) | 29.3 | ± 5.8 | 30.2 | ± 5.3 |
| Est. Gest. Age (weeks) | 19.5 | ± 6.7 | 17.6 | ± 6.9 |
| Prior Pregnancies | 1.8 | ± 1.4 | 1.9 | ± 1.3 |
| On ART – n (%) | 76 | (100) | 280 | (99) |
| Co-infections – n (%) | ||||
| Chlamydia trachomatis | 39 | (51) | 69 | (24) |
| Neisseria gonorrhea | 8 | (11) | 14 | (5) |
| Level of Education – n (%) | ||||
| None | 0 | (0) | 3 | (1) |
| Below Matric | 46 | (61) | 142 | (50) |
| Matric Certificate | 22 | (29) | 121 | (43) |
| Degree/Diploma | 5 | (7) | 12 | (4) |
| No Response | 3 | (4) | 5 | (2) |
| Currently Employed – n (%) | 28 | (36) | 120 | (42) |
| Monthly Income | ||||
| None | 6 | (8) | 28 | (10) |
| < $76 USD per month | 26 | (34) | 78 | (28) |
| $76 USD - $380 USD per month | 33 | (43) | 137 | (48) |
| $381 USD - $760 USD per month | 3 | (4) | 28 | (10) |
| > $760 USD per month | 1 | (1) | 7 | (3) |
| No Response | 7 | (9) | 5 | (2) |
Plus-minus values are means ± standard deviation. Matric is roughly equivalent to high school graduation in South Africa. ART, Antiretroviral therapy; USD, United States Dollars.
A total of 61 InPouch cultures were obtained from T. vaginalis-PCR-positive (PCR-positive) women during the first ANC visit, of which 38 (62%) were culture positive. Women with positive cultures had similar demographic characteristics to culture-negative women (data not shown). The median Ct value of the PCR-positive/culture-positive group was 22.6 (IQR: 21.2 – 27.1), and the median Ct value of the PCR-positive/culture-negative group was 38.0 (IQR: 35.2 – 38.5). The difference in median Ct value between the two groups was 15.4 (p < 0.001; Figure). All culture-positive cases had a Ct value less than 30, with the exception of 1 case (Ct = 37.8). In contrast, all culture-negative cases had a Ct value greater than 30, except for 1 case (Ct = 19.1). The area under the ROC curve was 0.95 (p < 0.001); a Ct value less than 30 correctly predicted culture positivity with a sensitivity of 97% and a specificity of 96% (Youden Index: 0.93).
Figure.
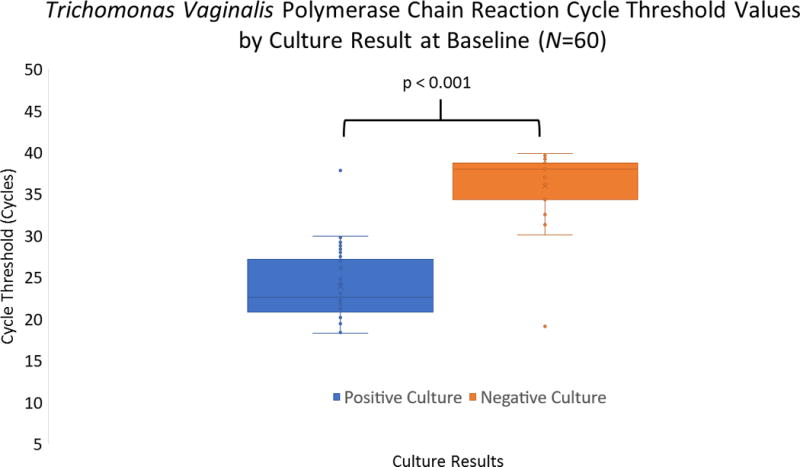
Cycle threshold values for T. vaginalis polymerase chain reaction tests at baseline, by culture result. The nonparametric Mann-Whitney U test was used to compare sample distributions.
Fourteen cultures were not obtained from women who tested positive for T. vaginalis via PCR at baseline. Of those missing cultures, 6 were due to the fact that the PCR testing portion of the study was implemented prior to the availability of cultures at clinic sites, and 8 were not collected due to patient refusal. In addition, 1 culture was inconclusive (negative but was not read according to InPouch TV™ protocol), and so was excluded from the analyses. The demographic characteristics of these participants were not significantly different from the participants from whom cultures were collected (data not shown).
T. vaginalis Positivity and Symptomatology at Baseline
Among the 34 women with self-reported symptoms and/or clinically-observed signs of an STI, the median Ct value was 24.9 (IQR: 21.3 – 30.1), while the median Ct of the 37 with no signs and/or symptoms was 29.1 (IQR: 22.3 – 37.3). The difference in median Ct value between the two groups was 4.3 (p > 0.05).
Women with symptoms and/or signs of an STI were culture-positive in 19 cases and culture-negative in 10 cases. Those without symptoms or signs were also culture-positive in 19 cases and culture-negative in 10 cases. A chi-square analysis revealed no significant difference in the number of positive cultures between those with symptoms and/or signs and those without (χ2 = 0, p > 0.05).
Five women were excluded from the symptomatology analysis because they did not respond to one or more questions on our survey of symptoms. Of those women, two were missing cultures (patient refusal); the other three had negative cultures.
T. vaginalis Testing at Test-of-Cure
Of the 59 PCR-positive women who returned for the 3-week test-of-cure (ToC) visit, 13 (22%) tested PCR positive again. Ten cultures were obtained from PCR-positive women at ToC visits, of which 5 (50%) were culture positive. The median Ct value of the PCR-positive/culture-positive group (N = 5) was 21.3 (IQR: 18.9 – 26.3), and the median Ct value of the PCR-positive/culture-negative group (N = 5) was 37.5 (IQR: 34.3 – 37.8). Four of the five PCR-positive/culture-negative cases had a Ct value greater than 30. Three cultures were not obtained from women who tested positive for T. vaginalis via PCR at ToC visits due to patient refusal.
Of the 13 PCR-positive women at ToC visits, 11 of them provided a response when asked about sexual activity between baseline and the ToC visit. Six (55%) reported having sexual intercourse at least once between the baseline and ToC visits; of these 6, 1 was culture-positive, 3 were culture-negative, and 2 were missing cultures. Among the 5 women who reported abstaining, 2 were culture-negative, 2 were culture-positive, and 1 was missing a culture.
Discussion
We observed a high prevalence of T. vaginalis (20%) in a population of HIV-infected pregnant women seeking antenatal care at public health centers in South Africa. The prevalence in our cohort was similar to the prevalence found in other cohorts of South African women (20%) [18] and HIV-infected pregnant women in the Democratic Republic of Congo (18%) [19] and Nigeria (19%) [20]; however, it was slightly less than other cohorts of HIV-infected women (30%) [21] and non-HIV infected pregnant women (41%) [22] in South Africa. The range of Ct values we observed in PCR-positive cases (18.3 – 39.9) was similar to that observed in another recent study of T. vaginalis prevalence in South African women (17.0 – 37.9). [18] We found that approximately half of the T. vaginalis infections were symptomatic, consistent with current estimates suggesting high proportions of asymptomatic T. vaginalis infection. [5, 18] Given the current policy of syndromic management of STIs in South Africa, and most of the world, only symptomatic women would be treated for T. vaginalis during antenatal care. [10,23]
The CDC recommendation to screen HIV-infected pregnant women for T. vaginalis indicates a need to determine the most effective screening method. The current gold standard in many settings for T. vaginalis diagnosis is culture. Our work shows that culture only detects T. vaginalis in roughly half of cases detected by PCR. Those findings suggest culture may miss many cases of T. vaginalis, possibly due to low organism burden at the time of testing. Peterman and colleagues found evidence suggesting T. vaginalis may be undetectable by culture at one visit but then become detectable at a subsequent visit, without any intervening sexual contact. [24]
While PCR detects many cases that culture misses, the clinical significance of those PCR-positive/culture-negative cases is less certain. The high sensitivity of PCR means that some women who would clear an infection without any intervention may still test positive due to remnant protozoal nucleic acid in injured or phagocytosed cells. [25] Treating such cases means exposing women, and in our population fetuses as well, to unnecessary antibiotics. In addition, overuse of antibiotics increases the risk of developing resistant organisms.
Currently, there is no way to predict which PCR-positive cases will turn into clinically significant infections requiring antibiotic treatment and which will clear without intervention. We observed significantly lower Ct values in the PCR-positive/culture-positive cases, consistent with another study that compared T. vaginalis detection with the InPouch to a laboratory-based PCR method. [16] Our finding of a Ct threshold of less than 30 predicting culture positivity with a sensitivity of 97% and a specificity of 96% could be used to study which PCR-positive cases require treatment. If T. vaginalis infections with a Ct value greater than 30 are found to be clinically insignificant, use of this threshold would allow clinicians to obtain the benefits of increased speed, specificity, and ease-of-use provided by point-of-care PCR platforms, without worrying about the risk of over-treating.
The value of such a clinical study would be twofold. First, there is not strong evidence that culture positivity is a good proxy for clinical significance. In fact, we found no association between culture positivity and symptomatology. Second, given the serious potential complications of a T. vaginalis infection, particularly in pregnant women, a strong argument could be made for treating any infection that is detected; that is, at least until more information is known about which PCR-positive cases truly require treatment. Another potential avenue for predicting clinically significant infections could lie in the use of viability PCR to differentiate between DNA of cells with intact membranes from free-floating DNA. [26]
The high sensitivity of PCR tests can also complicate test-of-cure visits by detecting remnant nucleic acids from non-viable organisms following treatment. Williams and colleagues found 5 out of 52 (10%) women testing positive for T. vaginalis by PCR were still positive at 3-weeks post-treatment, but all 5 later tested negative at 5-weeks post-treatment, without any additional treatment during the intervening period. [27] The temporal relationship between the clearance of pathogen nucleic acid and PCR positivity remains to be elucidated with T. vaginalis testing, which may be one reason why the CDC has no recommendation regarding test-of-cure after treatment for T. vaginalis. The guidelines do advise, however, that NAAT “can be conducted as soon as 2 weeks after treatment.” [9]
In a study investigating how long T. vaginalis nucleic acid remains detectable after metronidazole treatment, Martin and colleagues found that T. vaginalis RNA was no longer detectable by 3 weeks post-treatment in a group of 34 women. Importantly, all of those women reported abstinence during this period and were InPouch culture-positive at baseline and culture-negative at subsequent visits. [27] In contrast to these results, we detected T. vaginalis DNA in 5/10 women who were culture-negative at 3 weeks post-treatment, and 2 of these women reported abstaining from intercourse during those 3 weeks. Although our sample size was small, the low rate of culture positivity at test-of-cure may suggest remnant T. vaginalis DNA persists at least as long as 3 weeks after treatment.
Cycle threshold values also proved informative in our test-of-cure analyses, with the five positive cultures having a significantly smaller median Ct value. In addition, the Ct threshold of less than 30 predicted culture positivity with a sensitivity of 100% and specificity of 80%.
Our study was conducted in a specific population of women who were both infected with HIV and pregnant. Although both of these altered physiological states can cause changes in the vaginal flora, we do not expect that these states would significantly alter PCR or culture results when screening for T. vaginalis. One might expect an increased susceptibility to T. vaginalis infection in HIV-infected women [18]; however, as noted above, we found prevalence rates in line with other studies in different populations. One limitation of this study is that we did not perform wet mount microscopy or collect cultures from women who tested PCR negative for T. vaginalis. Although it is theoretically possible that some infections were missed, the generally accepted higher sensitivity of NAATs compared to both wet mount and culture suggests this is unlikely. [3] Another limitation is that we only collected samples at the 3-week test-of-cure. Multiple consecutive samples in women who did and did not receive treatment could shed more light on the question of clinical significance in PCR-positive/culture-negative cases.
In summary, we found high rates of T. vaginalis infection (symptomatic and asymptomatic) among HIV-infected pregnant women in Soshanguve Township, South Africa. We have shown that culture detects T. vaginalis organisms in only a portion of PCR-positive cases, and demonstrated the potential utility of cycle threshold values in the interpretation of PCR results. We highlight a lingering question in the field, namely what is the clinical significance of T. vaginalis DNA detection among culture-negative cases during STI screening and follow-up? Future work focused on answering this question could significantly improve our understanding of the most effective methods and outcomes of T. vaginalis detection.
Summary.
Among HIV-infected pregnant women in South Africa, culture detected T. vaginalis in only a portion of PCR-positive cases, and cycle threshold values proved valuable in the interpretation of PCR results.
Acknowledgments
We thank Cepheid for the donation of test cartridges and loan of equipment. We would also like to thank our study nurses Chriselda Maginindane and Tshiamo Matlala, and study assistants Mpho Ngubeni and Bertha Theledi for their support and contribution to this study. Erika Morikawa was supported by the David Geffen School of Medicine’s Dean’s Office and the UCLA Center for World Health. We would also like to thank Andrew Biundo for facilitating the delivery of cultures from the clinic to the University of Pretoria. Finally, we would like to thank the patients for their participation in our study.
Funding: This work was supported by the National Institutes of Health [grant number 1-R21-HD084274-01A1]; the U.S. President’s Emergency Plan for AIDS Relief [Cooperative Agreement: AID-674-A-12-00017]; and a travel grant from the UCLA Center for World Health.
Footnotes
Conflict of Interest: The authors state no conflict of interest.
Contributor Information
Collin M Price, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California, United States of America 90095.
Remco PH Peters, Department of Medical Microbiology, Faculty of Health Sciences, University of Pretoria, Pretoria, South Africa and Anova Health Institute, Johannesburg, South Africa 2193.
Janré Steyn, Department of Medical Microbiology, Faculty of Health Sciences, University of Pretoria, Pretoria, South Africa 0002.
Maanda Mudau, Research Unit, Foundation for Professional Development, Pretoria, South Africa 0184.
Dawie Olivier, Research Unit, Foundation for Professional Development, Pretoria, South Africa 0184.
Lindsey De Vos, Research Unit, Foundation for Professional Development, Pretoria, South Africa 0184.
Erika Morikawa, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California, United States of America 90095.
Marleen M Kock, Department of Medical Microbiology, Tshwane Academic Division, National Health Laboratory Service and Department of Medical Microbiology, Faculty of Health Sciences, University of Pretoria, Pretoria, South Africa 0002.
Andrew Medina-Marino, Research Unit, Foundation for Professional Development, Pretoria, South Africa and School of Health Systems and Public Health, University of Pretoria, Pretoria, South Africa 0184.
Jeffrey D Klausner, Division of Infectious Disease, Department of Medicine, University of California, Los Angeles, Los Angeles, California, United States of America 90024.
References
- 1.Newman L, Rowley J, Vander Hoorn S, et al. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLOS ONE. 2015;10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Waaij DJ, Ouburg S, Dubbink JH, et al. Evaluation of Presto plus assay and LightMix kit Trichomonas vaginalis assay for detection of Trichomonas vaginalis in dry vaginal swabs. Journal of Microbiological Methods. 2016;127:102–4. doi: 10.1016/j.mimet.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Kissinger P. Trichomonas vaginalis: a review of epidemiologic, clinical and treatment issues. BMC Infectious Diseases. 2015:15. doi: 10.1186/s12879-015-1055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sexually Transmitted Infections. 2013;89:426–33. doi: 10.1136/sextrans-2012-051005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotch MF, Pastorek JG, Nugent RP, et al. Trichomonas vaginalis Associated With Low Birth Weight and Preterm Delivery. Sexually Transmitted Diseases. 1997;24:353–60. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Mann JR, McDermott S, Gill T. Sexually transmitted infection is associated with increased risk of preterm birth in South Carolina women insured by Medicaid. The Journal of Maternal-Fetal & Neonatal Medicine. 2010;23:563–8. doi: 10.3109/14767050903214574. [DOI] [PubMed] [Google Scholar]
- 7.Silver BJ, Guy RJ, Kaldor JM, et al. Trichomonas vaginalis as a Cause of Perinatal Morbidity. Sexually Transmitted Diseases. 2014;41:369–76. doi: 10.1097/OLQ.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 8.Gumbo FZ, Duri K, Kandawasvika GQ, et al. Risk factors of HIV vertical transmission in a cohort of women under a PMTCT program at three peri-urban clinics in a resource-poor setting. Journal of Perinatology. 2010;30:717–23. doi: 10.1038/jp.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Workowski KA. Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Clinical Infectious Diseases. 2015;61:S759–S62. doi: 10.1093/cid/civ771. [DOI] [PubMed] [Google Scholar]
- 10.South African National Department of Health. Sexually Transmitted Infections: Management Guidelines 2015. 2015 [Google Scholar]
- 11.Van Der Pol B. Clinical and Laboratory Testing for Trichomonas vaginalis Infection. Journal of Clinical Microbiology. 2015;54:7–12. doi: 10.1128/JCM.02025-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xpert® TV package insert. Cepheid; Sunnyvale, CA: 2014. [Google Scholar]
- 13.Xpert® CT/NG package insert. Cepheid; Sunnyvale, CA: 2013. [Google Scholar]
- 14.InPouch™ TV package insert. Biomed Diagnostics, Inc.; White City, OR: 2015. [Google Scholar]
- 15.Borchardt KA, Smith RF. An evaluation of an InPouch TV culture method for diagnosing Trichomonas vaginalis infection. Sexually Transmitted Infections. 1991;67:149–52. doi: 10.1136/sti.67.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caliendo AM, Jordan JA, Green AM, et al. Real-Time PCR Improves Detection ofTrichomonas vaginalisInfection Compared With Culture Using Self-Collected Vaginal swabs. Infectious Diseases in Obstetrics and Gynecology. 2005;13:145–50. doi: 10.1080/10647440500068248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Waaij DJ, Dubbink JH, Ouburg S, et al. Prevalence of trichomonas vaginalis infection and protozoan load in South African women: a cross sectional study. BMJ Open. 2017 doi: 10.1136/bmjopen-2017-016959. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton MY, Sternberg M, Nsuami M, et al. Trichomoniasis in pregnant human immunodeficiency virus–infected and human immunodeficiency virus–uninfected Congolese women: Prevalence, risk factors, and association with low birth weight. American Journal of Obstetrics and Gynecology. 1999;181:656–62. doi: 10.1016/s0002-9378(99)70509-0. [DOI] [PubMed] [Google Scholar]
- 20.Oyeyemi OT, Fadipe O, Oyeyemi IT. Trichomonas vaginalis infection in Nigerian pregnant women and risk factors associated with sexually transmitted infections. International Journal of STD & AIDS. 2015;27:1187–93. doi: 10.1177/0956462415611292. [DOI] [PubMed] [Google Scholar]
- 21.Kock M, Rukasha I, Dijkmans A, et al. P3-S7.07 Detection of Trichomonas vaginalis in HIV positive women in Pretoria, South Africa. Sexually Transmitted Infections. 2011;87:A301–A. [Google Scholar]
- 22.Wilkinson D, Abdool Karim SS, Harrison A, et al. Unrecognized Sexually Transmitted Infections In Rural South African Women. Bulletin of the World Health Organization. 1999;77:22–28. [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Guidelines for the Management of Sexually Transmitted Infections. Reproductive Health and Research. 2004 [Google Scholar]
- 24.Peterman Thomas A, Tian Lin H, Metcalf Carol A, et al. Undetected Trichomonas vaginalis Infections? Clinical Infectious Diseases. 2009;48:259–60. doi: 10.1086/595706. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Wang Z, Zhang Y, et al. Polymerase chain reaction–based assays for the diagnosis of human brucellosis. Annals of Clinical Microbiology and Antimicrobials. 2014:13. doi: 10.1186/s12941-014-0031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janssen KJH, Hoebe CJPA, Dukers-Muijrers NHTM, et al. Viability-PCR Shows That NAAT Detects a High Proportion of DNA from Non-Viable Chlamydia trachomatis. PLOS ONE. 2016;11:e0165920. doi: 10.1371/journal.pone.0165920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams J, Ofner S, Batteiger B, et al. Duration of polymerase chain reaction–detectable DNA after treatment of Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis infections in women. Sexually Transmitted Diseases. 2014;41:215–219. doi: 10.1097/OLQ.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 28.Martin DH, Burnett J, Taylor SN. O10.2 Trichomonas vaginalis nucleic acid clearance following treatment of hiv negative women. Sexually Transmitted Infections. 2015;91:A47.1–A. [Google Scholar]


