We report the case of a 20-year-old male with a rare Epstein Barr virus positive (EBV+) cutaneous T-cell lymphoma status post hematopoietic stem cell transplantation (HCT) who died from complications of right heart failure due to severe pulmonary veno-occlusive disease in the setting of cytomegalovirus (CMV) pneumonia and transplant-associated thrombotic microangiopathy (TA-TMA). This case highlights the anatomic spectrum of post-transplant pulmonary vascular disease and is a cautionary tale regarding the diagnosis and management of patients with pulmonary veno-occlusive disease.
The patient was diagnosed with stage IV EBV+ hydroa vacciniform-like T-cell lymphoma with mediastinal, intramedullary, and CNS involvement. He initially received two cycles of chemotherapy with cyclophosphamide, vincristine, prednisone, doxorubicin (cumulative dose 120 mg/m2), and methotrexate (COPADM), with double intrathecal therapy with cytarabine and hydrocortisone, and achieved a partial response. Following this, he received two cycles of asparaginase, methotrexate, and dexamethasone therapy1 with resolution of cutaneous lesions, FDG-avid lymphadenopathy, and reduction of his marrow disease burden to 0.3% by flow cytometry, but persistent EBV positivity by PCR.
Given concern for possible underlying immunodeficiency with repeatedly low NK cell function, as well as poor outcomes for hydroa vacciniform-like lymphoma,2 he proceeded to HCT approximately 5 months after his initial oncologic diagnosis. His pre-HCT organ function tests were normal, with a left ventricular fractional shortening of 35.3%, left ventricular ejection fraction of 71.5%, pulmonary carbon monoxide diffusing capacity of 89%, glomerular filtration rate of 113.50 mL/min/1.73m2, and Lansky score of 100%. He underwent pre-transplant conditioning with melphalan 140mg/m2, thiotepa 10mg/kg, fludarabine 200mg/m2, and rabbit ATG 3.5mg/kg, and then received a maternal haplocompatible T-cell depleted, CD34+ selected peripheral blood HCT. His immediate post-transplant course was uncomplicated with the exception of multiple low-level herpesvirus reactivations including EBV, CMV, and HHV-6. He was discharged home on day +11 with valganciclovir and 98–100% chimerism in T-cell, B-cell, NK cell, and myeloid lineages. At home, he continued to receive valganciclovir with persistent low-level multiple viremia and also received donor-derived anti-EBV cytotoxic T-cell infusions through clinical trial NCT01956084 on days +54 and +68.
On day +77, he was re-admitted to the hospital for worsened CMV viremia (59,209 copies/mL) requiring intravenous foscarnet as well as upper respiratory symptoms due to RSV-A. Despite an absolute lymphocyte count of 900 cells/nL, the addition of anti-CMV immunoglobulin (Cytogam), and CMV strain genetic testing suggesting susceptibility to ganciclovir and foscarnet, he achieved only partial control of CMV viremia. He remained febrile and developed hypoxemia and fluid overload. An echocardiogram performed on day +81 to evaluate myocardial function showed new grade I left ventricular diastolic dysfunction based on mitral valve inflow Doppler and mitral annular tissue Doppler. An electrocardiogram performed on day +89 showed normal sinus rhythm with rightward axis deviation and no evidence of chamber hypertrophy. He received empiric broad-spectrum antibiotics as well as intravenous ribavirin for his RSV-A infection but developed respiratory failure on day +96 requiring endotracheal intubation and mechanical ventilation. At that time, bronchoscopy yielded 55,000 copies of CMV and 423 copies of EBV per mL of bronchoalveolar lavage. A plasma B-type natriuretic peptide was 1280 pg/mL (ref <25 pg/mL), increased from <5 pg/mL pre-HCT. An echocardiogram was repeated that day that estimated the right ventricular systolic pressure to be at half systemic from a tricuspid valve regurgitation jet velocity. The inferior vena cava was moderately dilated with normal inspiratory collapse indicating mildly elevated right atrial pressure. The branch pulmonary arteries were mildly dilated in comparison to the pre-HCT echocardiogram. There was no right ventricular dilation or hypertrophy and biventricular systolic function was normal. There was persistent grade I left ventricular diastolic dysfunction.
Despite reduction in his BAL CMV to 431 copies/mL by day +102, his respiratory disease evolved to severe acute respiratory distress syndrome with a maximum oxygenation index of 30. He further received rituximab for persistent EBV viremia, tocilizumab for hypercytokinemia (IL-6 level of 65 pg/mL, ref ≤5) as well as eculizumab and defibrotide for transplant-associated thrombotic microangiopathy (criteria: lactate dehydrogenase 515 U/L [ref 102–199], D-dimer 3704 ng/mL [ref <500], haptoglobin <6 mg/dL [ref 36–195], urine protein/creatinine 5.88 [ref <0.16], 2–5 schistocytes per high-powered field [ref 0–1]). However, his sC5b-9 membrane attack complex level was normal (201 ng/mL, ref ≤244). He developed further signs of right heart failure including hepatomegaly, ascites, and jugular venous distension, and a repeat B-type natriuretic peptide level was 1620 pg/mL, prompting a repeat echocardiogram on day +111. The echocardiogram showed signs of acute right heart failure as evidenced by suprasystemic right ventricular pressure estimation based on septal bowing, mild right atrial and right ventricular dilation, and qualitatively moderately decreased right ventricular systolic function. The IVC dilation was unchanged but there was no collapse with inspiration indicating higher right atrial pressure estimation. A small pericardial effusion had also developed. Pulmonary arterial hypertension was suggested by right and left pulmonary artery dilation in the absence of left atrial dilation, left ventricular systolic dysfunction and only grade I left ventricular diastolic dysfunction.
He received right ventricular heart failure management with inhaled nitric oxide, milrinone, and vasopressin as well as continuous veno-venous hemodiafiltration for renal failure and fluid overload. Despite maximal medical treatment for CMV, EBV, TA-TMA, and maximal supportive care for multi-organ system failure, a repeat echocardiogram on day +116 showed persistent suprasystemic right ventricular pressure based on septal bowing (Figure 1). A contrast enhanced chest CT obtained the next day demonstrated diffuse smooth interlobular septal thickening, ground glass opacities, and ill-defined centrilobular nodules suggestive of congestive pulmonary edema (Figure 2). In the absence of left ventricular dysfunction, the diagnosis of pulmonary veno-occlusive disease (PVOD) was made according to the Nice criteria.3 By day +124, his BAL CMV load had decreased to <78 copies/mL. However, despite ongoing supportive therapy, he eventually developed severe right ventricular dilation and died of right heart failure on day +141.
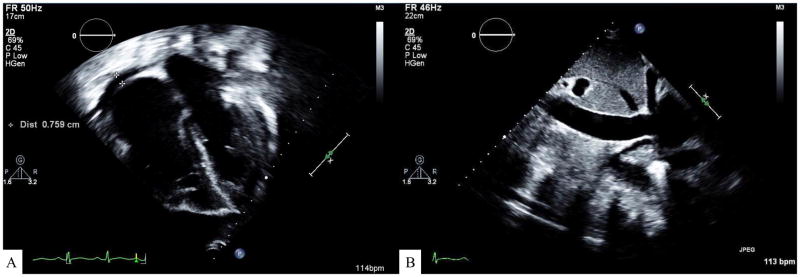
Figure 1. Echocardiogram showing findings typical of pulmonary veno-occlusive disease.
(A) Echocardiographic findings demonstrating right atrial and right ventricular dilation without left atrial dilation. There is also a small pericardial effusion. Tricuspid valve inflow Doppler showed right ventricular systolic pressure estimation of 55 mmHg above the right atrial pressure. Concomitant systemic blood pressure is annotated on the image (100/59 mmHg). (B) Two dimensional echocardiographic image of a moderately dilated inferior vena cava (2 cm). There was no collapse of the IVC with forced inspiration. This is suggestive of a right atrial pressure of 5–10 mmHg.
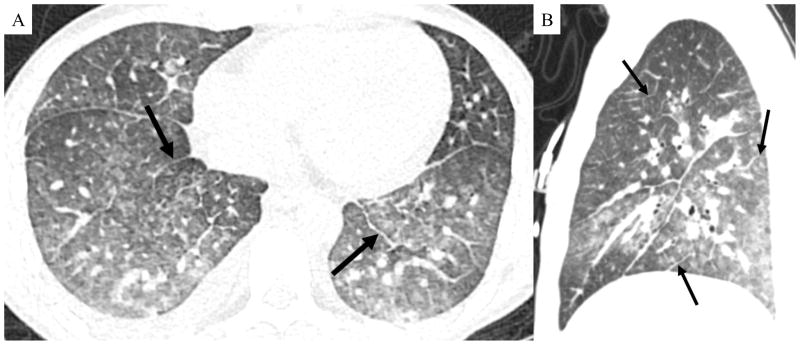
Figure 2. Contrast enhanced chest CT showing findings typical of pulmonary veno-occlusive disease.
Axial (A) and sagittal reformatted (B) CT images demonstrate diffuse interlobular septal thickening (black arrows). Also visible are diffuse ground glass opacities and ill-defined centrilobular nodules.
This case merits discussion for two reasons. First, this report adds to the growing body of evidence for a broad spectrum of post-HCT pulmonary vascular disease. Second, whereas the majority of post-HCT pulmonary vascular disease has been reported as pulmonary arterial hypertension (PAH), this report highlights the diagnostic and treatment challenges of PVOD.
The diagnosis of pulmonary veno-occlusive disease is rare in pediatrics and accounts for fewer than 5% of cases of pediatric pulmonary vascular disease.4 Lantuéjoul et al describe histologic features of 33 patients with PVOD as “inter- or intralobular veins [with] eccentric or concentric narrowing, or occlusion by cellular or collagenous fibrosis, or recanalization.”5 In this report, findings included pulmonary venous intimal fibrosis (100%) with hemosiderosis (79%) and/or interstitial lymphocytic infiltrate (63%), pulmonary capillary congestion (82%) or proliferation (73%), and pulmonary arterial intimal fibrosis and/or medial hypertrophy (88%). Pulmonary interlobular septal thickening was present in 94% of patients. While our patient did not undergo autopsy, PVOD can be differentiated from PAH by characteristic chest CT features. Smooth interlobular septal thickening in the absence of left heart disease is the most suggestive sign on CT.6 Ground glass opacity and ill-defined centrilobular nodules may also been seen, although nodules are more characteristic of capillary hemangiomatosis. Of note, there is significant pathologic and radiographic overlap between PVOD and PCH, thus these may represent a spectrum of the same disease.5 Other CT findings supportive of the diagnosis of PVOD include small pulmonary veins, a normal sized left atrium and symmetric mediastinal/hilar lymphadenopathy. Unfortunately, the visualization of interlobular septal thickening requires intravenous contrast, which is frequently challenging in post-HCT patients with renal insufficiency.6 Differentiating PVOD from PAH is particularly important given that up to 50% of patients with PVOD demonstrate acute worsening with pulmonary arterial vasodilators.7 Our patient was treated with inhaled nitric oxide for two weeks for presumed PAH before the diagnosis of PVOD was made; we speculate that while this may have decreased right ventricular afterload in the short term, it may have ultimately been detrimental by exacerbating pulmonary edema due to increased pulmonary blood flow in the setting of a fixed post-capillary obstruction. The ideal balance of pulmonary vasodilators in patients with combined PAH and PVOD is unknown but must be approached with care on a per-patient basis.8,9
Fewer than 20 reports exist associating PVOD with hematopoietic cellular transplantation, and while some report improvement with methylprednisolone or even spontaneous resolution, the majority of patients die due to progressive right heart failure.10–25 Treatment with alkylating agents such as cyclophosphamide and mitomycin-C has been associated with pulmonary venular injury in animal models and with PVOD in both animal and clinical models.26 While PVOD is a rarely-reported post-HCT endotheliopathy, recent recognition of post-HCT PAH has grown considerably and is frequently associated with the development or progression of TA-TMA.27 One report found that pediatric patients who demonstrated elevated right ventricular pressure on post-HCT day +7 echocardiography were at high-risk for developing TA-TMA, and many of these patients progressed to developed further signs of PAH over time.28 We speculate that a portion of patients with TA-TMA related PAH may have concomitant unrecognized PVOD complicating their response to treatment. The pathogenesis of pulmonary vascular disease in the setting of TA-TMA is believed to be related to deficient nitric oxide scavenging in the setting of severe hemolysis as well as severe post-transplant hypercytokinemia in a background of cardiopulmonary toxicity from prior chemoradiation.27 Therapeutics aimed at promoting fibrinolysis (ie: defibrotide) and pulmonary vasodilation (ie: sildenafil, bosentan) while limiting inflammation (ie: methylprednisolone) and complement-mediated hemolysis (ie: eculizumab) are currently under investigation with limited demonstrated efficacy at this time.13
In conclusion, we report the case of a 20-year-old male HCT recipient who died from complications related to PVOD. This report presents a cautionary tale to clinicians who diagnose post-HCT right heart dysfunction and emphasizes that the spectrum of pulmonary vascular disease may include both PAH and PVOD and requires unique diagnostic and therapeutic strategies. Ongoing investigation into the pathobiology of post-HCT pulmonary vascular disease should include detailed assessment of the anatomy of the lesion in order to further inform the epidemiology of this often fatal complication.
Supplementary Material
Acknowledgments
Funding: NIH NICHD K12HD000850 (Zinter), Pediatric Blood and Marrow Transplant Foundation (Zinter), Amy Strelzer Manasevit Grant (Zinter)
Abbreviation Key
- ATG
Anti-thymocyte globulin
- CMV
Cytomegalovirus
- CNS
Central nervous system
- COPADM
Cyclophosphamide, oncovin (vincristine), prednisone, adriamycin (doxorubicin), and methotrexate
- CT
Computed tomography
- EBV
Epstein Barr virus
- FDG
Fluorodeoxyglucose
- HCT
Hematopoietic cellular transplant
- HHV-6
Human herpesvirus-6
- IVC
Inferior vena cava
- NK
Natural killer cell
- PAH
Pulmonary arterial hypertension
- PCR
Polymerase chain reaction
- PVOD
Pulmonary veno-occlusive disease
- RSV
Respiratory syncytial virus
- TA-TMA
Transplant-associated thrombotic microangiopathy
Footnotes
Conflict of Interest Statement
There are no conflicts of interest.
References
- 1.Jaccard A, Gachard N, Marin B, et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117(6):1834–1839. doi: 10.1182/blood-2010-09-307454. [DOI] [PubMed] [Google Scholar]
- 2.El-Mallawany NK, Geller L, Bollard CM, et al. Long-term remission in a child with refractory EBV(+) hydroa vacciniforme-like T-cell lymphoma through sequential matched EBV(+)-related allogeneic hematopoietic SCT followed by donor-derived EBV-specific cytotoxic T-lymphocyte immunotherapy. Bone Marrow Transplant. 2011;46(5):759–761. doi: 10.1038/bmt.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 4.del Cerro Marin MJ, Sabate Rotes A, Rodriguez Ogando A, et al. Assessing pulmonary hypertensive vascular disease in childhood. data from the spanish registry. Am J Respir Crit Care Med. 2014;190(12):1421–1429. doi: 10.1164/rccm.201406-1052OC. [DOI] [PubMed] [Google Scholar]
- 5.Lantuejoul S, Sheppard MN, Corrin B, Burke MM, Nicholson AG. Pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis: A clinicopathologic study of 35 cases. Am J Surg Pathol. 2006;30(7):850–857. doi: 10.1097/01.pas.0000209834.69972.e5. [DOI] [PubMed] [Google Scholar]
- 6.Resten A, Maitre S, Humbert M, et al. Pulmonary hypertension: CT of the chest in pulmonary venoocclusive disease. AJR Am J Roentgenol. 2004;183(1):65–70. doi: 10.2214/ajr.183.1.1830065. [DOI] [PubMed] [Google Scholar]
- 7.Montani D, Achouh L, Dorfmuller P, et al. Pulmonary veno-occlusive disease: Clinical, functional, radiologic, and hemodynamic characteristics and outcome of 24 cases confirmed by histology. Medicine (Baltimore) 2008;87(4):220–233. doi: 10.1097/MD.0b013e31818193bb. [DOI] [PubMed] [Google Scholar]
- 8.Montani D, Lau EM, Dorfmuller P, et al. Pulmonary veno-occlusive disease. Eur Respir J. 2016;47(5):1518–1534. doi: 10.1183/13993003.00026-2016. [DOI] [PubMed] [Google Scholar]
- 9.Chaisson NF, Dodson MW, Elliott CG. Pulmonary capillary hemangiomatosis and pulmonary veno-occlusive disease. Clin Chest Med. 2016;37(3):523–534. doi: 10.1016/j.ccm.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Mukai M, Kondo M, Bohgaki T, Notoya A, Kohno M. Pulmonary veno-occlusive disease following allogeneic peripheral blood stem cell transplantation for chronic myeloid leukaemia. Br J Haematol. 2003;123(1):1. doi: 10.1046/j.1365-2141.2003.04423.x. [DOI] [PubMed] [Google Scholar]
- 11.Or R, Nagler A, Elad S, Naparstek E, Schechter D. Noncardiogenic pulmonary congestion following bone marrow transplantation. Respiration. 1997;64(2):170–172. doi: 10.1159/000196664. [DOI] [PubMed] [Google Scholar]
- 12.Trobaugh-Lotrario AD, Greffe B, Deterding R, Deutsch G, Quinones R. Pulmonary veno-occlusive disease after autologous bone marrow transplant in a child with stage IV neuroblastoma: Case report and literature review. J Pediatr Hematol Oncol. 2003;25(5):405–409. doi: 10.1097/00043426-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Bunte MC, Patnaik MM, Pritzker MR, Burns LJ. Pulmonary veno-occlusive disease following hematopoietic stem cell transplantation: A rare model of endothelial dysfunction. Bone Marrow Transplant. 2008;41(8):677–686. doi: 10.1038/sj.bmt.1705990. [DOI] [PubMed] [Google Scholar]
- 14.Runo JR, Vnencak-Jones CL, Prince M, et al. Pulmonary veno-occlusive disease caused by an inherited mutation in bone morphogenetic protein receptor II. Am J Respir Crit Care Med. 2003;167(6):889–894. doi: 10.1164/rccm.200208-861OC. [DOI] [PubMed] [Google Scholar]
- 15.Hackman RC, Madtes DK, Petersen FB, Clark JG. Pulmonary venoocclusive disease following bone marrow transplantation. Transplantation. 1989;47(6):989–992. doi: 10.1097/00007890-198906000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Kuga T, Kohda K, Hirayama Y, et al. Pulmonary veno-occlusive disease accompanied by microangiopathic hemolytic anemia 1 year after a second bone marrow transplantation for acute lymphoblastic leukemia. Int J Hematol. 1996;64(2):143–150. doi: 10.1016/0925-5710(96)00467-7. [DOI] [PubMed] [Google Scholar]
- 17.Salzman D, Adkins DR, Craig F, Freytes C, LeMaistre CF. Malignancy-associated pulmonary veno-occlusive disease: Report of a case following autologous bone marrow transplantation and review. Bone Marrow Transplant. 1996;18(4):755–760. [PubMed] [Google Scholar]
- 18.Seguchi M, Hirabayashi N, Fujii Y, et al. Pulmonary hypertension associated with pulmonary occlusive vasculopathy after allogeneic bone marrow transplantation. Transplantation. 2000;69(1):177–179. doi: 10.1097/00007890-200001150-00030. [DOI] [PubMed] [Google Scholar]
- 19.Troussard X, Bernaudin JF, Cordonnier C, et al. Pulmonary veno-occlusive disease after bone marrow transplantation. Thorax. 1984;39(12):956–957. doi: 10.1136/thx.39.12.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams LM, Fussell S, Veith RW, Nelson S, Mason CM. Pulmonary veno-occlusive disease in an adult following bone marrow transplantation. case report and review of the literature. Chest. 1996;109(5):1388–1391. doi: 10.1378/chest.109.5.1388. [DOI] [PubMed] [Google Scholar]
- 21.Hosokawa K, Yamazaki H, Nishitsuji M, et al. Pulmonary veno-occlusive disease following reduced-intensity allogeneic bone marrow transplantation for acute myeloid leukemia. Intern Med. 2012;51(2):195–198. doi: 10.2169/internalmedicine.51.6302. [DOI] [PubMed] [Google Scholar]
- 22.Gutman JA, Allen CT, Madtes DK, Schramm J, Delaney C. Pulmonary veno-occlusive disease following reduced-intensity cord blood transplantation. Bone Marrow Transplant. 2008;42(8):559–561. doi: 10.1038/bmt.2008.210. [DOI] [PubMed] [Google Scholar]
- 23.Bruckmann C, Lindner W, Roos R, et al. Severe pulmonary vascular occlusive disease following bone marrow transplantation in omenn syndrome. Eur J Pediatr. 1991;150(4):242–245. doi: 10.1007/BF01955521. [DOI] [PubMed] [Google Scholar]
- 24.Malhotra P, Varma S, Varma N, et al. Pulmonary veno-occlusive disease as a cause for reversible pulmonary hypertension in a patient with multiple myeloma undergoing peripheral blood stem cell transplantation. Am J Hematol. 2005;80(2):164–165. doi: 10.1002/ajh.20416. [DOI] [PubMed] [Google Scholar]
- 25.Steward CG, Pellier I, Mahajan A, et al. Severe pulmonary hypertension: A frequent complication of stem cell transplantation for malignant infantile osteopetrosis. Br J Haematol. 2004;124(1):63–71. doi: 10.1046/j.1365-2141.2003.04739.x. [DOI] [PubMed] [Google Scholar]
- 26.Ranchoux B, Gunther S, Quarck R, et al. Chemotherapy-induced pulmonary hypertension: Role of alkylating agents. Am J Pathol. 2015;185(2):356–371. doi: 10.1016/j.ajpath.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Jodele S, Hirsch R, Laskin B, Davies S, Witte D, Chima R. Pulmonary arterial hypertension in pediatric patients with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2013;19(2):202–207. doi: 10.1016/j.bbmt.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Dandoy CE, Davies SM, Hirsch R, et al. Abnormal echocardiography 7 days after stem cell transplantation may be an early indicator of thrombotic microangiopathy. Biol Blood Marrow Transplant. 2015;21(1):113–118. doi: 10.1016/j.bbmt.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.