Abstract
Endothelin-1 (ET-1) is implicated in the pathophysiology of preeclampsia (PE). An association between an EDN1 gene polymorphism with high ET-1 and PE was reported in humans, but their cause and effect relationships have not been defined. We examined the pregnancy effects in mice with a modified Edn1 allele that increases mRNA stability and thus ET-1 production. Heterozygous Edn1H/+ females showed no obvious abnormalities before pregnancy, but when mated with wild type (WT) males developed a full spectrum of PE-like phenotypes including increased systolic blood pressure (SBP), proteinuria, glomerular endotheliosis, and intra-uterine fetal growth restriction. At 7.5 days post coitus (dpc), the embryos from Edn1H/+ dams, regardless of their Edn1 genotype, lagged 12h in development compared to embryos from WT dams, had disoriented ectoplacental cones, and retained high E-cadherin expression. In contrast, WT females mated with Edn1H/+ males, which also carried half of the fetuses with Edn1H/+ genotype, showed a mild SBP increase only. These WT dams had 2x higher plasma soluble fms-like tyrosine kinase-1 (sFLT1) than WT dams mated with WT males. In human first trimester trophoblast cells, pharmacological doses of ET-1 increased the cellular sFlt1 transcripts and protein secretion via both type A and B ET-1 receptors. Our data demonstrate that high maternal ET-1 production causes PE-like phenotypes during pregnancy, affecting both initial stage of trophoblast differentiation/invasion and maternal peripheral vasculature during late gestation. High fetal ET-1 production on the other hand could cause increased sFLT1 in the maternal circulation and contribute to BP elevation. (247 words)
Keywords: preeclampsia, endothelin-1, sFLT1, VEGF, ectoplacental cones, mouse model
Introduction
Preeclampsia (PE) is a pregnancy-related disease characterized by the onset of hypertension, proteinuria and end organ damage. It affects approximately 5 to 8% of pregnancies and is one of the leading causes of maternal morbidity and mortality 1. The etiology of PE is largely unknown. Increased sFLT1 (soluble vascular endothelial growth factor receptor -1, sVEGFR-1) in plasma is associated with PE, and evidence has been accumulating that sFLT1production in ischemic/hypoxic placentae appears to play a critical role in PE2, 3. For example, in animal models, sFLT1 administered experimentally either by infusion or via adenoviral expression induces the PE-like phenotype including hypertension, renal problems, and intra-uterine growth restriction (IUGR)3–7.
ET-1, a 21 amino acid proteolytic product of preproET-18 is a potent vasoconstrictor involved in hypertension and kidney damage9–11. We and other investigators have shown that administering exogenous sFLT1 directly or by retroviral delivery increases mRNA levels of Edn1 coding for preproET-1 and Ednra coding for endothelin type A receptor (EDNRA) in the kidneys5, 6. In these models, EDNRA antagonist treatment reverses sFLT1 induced hypertension and proteinuria5, 6, suggesting that up-regulation of ET-1 signaling by sFLT1 plays a key role in PE. Compared to normotensive pregnant women, preeclamptic women have about 2 to 3 times higher circulating ET-1 levels12. Aggarwal et al. have reported an association between EDN1 G5665T polymorphism and elevated plasma ET-1 with PE 13. Women with PE had an increased frequency of T-5665 allele, and both circulating ET-1 levels and maternal EDN1 genotype was correlated with the severity of hypertension in PE13. Taken together, these observations in animal models and human polymorphisms have led investigators to propose that ET-1 is the key link between placental ischemia and hypertension in PE14. However, the cause and effect relationship between ET-1 and PE is still unclear.
PE is a placenta-related disease associated with poor embryo implantation and low placental perfusion, and the maternal symptoms resolve with delivery15–17. The process of implantation and trophoblast invasion is a well-orchestrated epithelial-mesenchymal transition (EMT) event18. E-cadherin mediates specific cell-cell adhesion in a Ca2+ dependent manner19, 20 and plays a critical role in EMT 21, 22. In cancer cells, ET-1 inhibits E-cadherin via up-regulation of E-cadherin transcriptional suppressors such as Snail and Twist 22, 23, transcriptional factors that are also important ET-1 effectors in early embryo development. However, whether ET-1 affects embryonic implantation and plays a causative role in PE has not been investigated.
To further elucidate the relationships between ET-1 and PE, we studied mice with an Edn1 allele modified to increase its mRNA stability leading to high ET-1 expression10. Because homozygosity for the high expressing allele is embryonically lethal10, we used Edn1H/+ heterozygotes carrying one copy of the high-expressing form of the Edn1 gene and one copy of the wild type (WT) gene. Our first objective was to measure the effect of maternal ET-1 overexpression on dam phenotype and trophoblast cell invasion in the early stage of pregnancy. Our second objective was to measure the effects of fetal ET-1 overexpression on dam phenotype and trophoblast invasion. Together, this study aimed to separate the effect of maternal ET-1 overexpression from that of embryonic/fetal ET-1 in a mouse model of PE.
Materials and Methods
The authors declare that all supporting data are available within the article [and its online supplementary files].
Mice
Mice (C57BL/6J) including both sexes and genotypes were housed in standard cages on a 12h light/dark cycle, and were allowed free access to food and water. All experiments were carried out in accordance with the National Institutes of Health guideline for use and care of experimental animals, as approved by the IACUC of the University of North Carolina at Chapel Hill. Genotypes were determined by PCR with primers: p1, 5′-AGACTAACTTAGCAGGAGGC-3′, p2, 5′-AGGTATGGAGCTCAGCTGCA-3′, and p3,5′-AAAACACTGGGGGAGCTCTG-3′. A 520 bp-fragment produced with p1 and p2 detects the targeted locus, and a 450bp-fragment produced with p1 and p3 detects the endogenous locus.
Matings
We compared the dams and embryos/fetuses from 3 different mating strategies. WT females mated with WT males (♀WT x ♂WT) carrying all WT embryos, and Edn1H/+ females mated with WT males (♀Edn1H/+ x ♂WT) carrying both WT and Edn1H/+ embryos, and WT females mated with Edn1H/+ males (♀WT x ♂ Edn1H/+) also carrying both WT and Edn1H/+ embryos. Females were checked for vaginal plugs each morning, and the day of plug detection was designated as 0.5 days post coitus (dpc). To confirm pregnancy, mice were weighed 2x per week. Any females not gaining weight were not included in the study.
Determining developmental stage
For timed matings, males and females were housed together for 2hr and vaginal plugs were checked at the end of the 2 hr. We followed the Theiler staging criteria to determine the stage of development of the embryos for our comparisons24.
BP measurements by telemetry during pregnancy
Female virgin mice of reproductive age were anesthetized with inhaled isoflurane (2–4% isofluorane/oxygen). The catheter (Data Sciences Inc., St. Paul, MN) was inserted into the left carotid artery and secured by ligation, and the transmitter was placed subcutaneously. Buprenorphine (analgesia) was first administered subcutaneously at anesthetic induction and then every 12 hr for the first 48 hrs after surgery at a dose of 0.1mg/kg body weight. One week following implantation, the BP was recorded continuously for 3 to 5 days to establish the baseline6, 7. Following this, male mice were introduced into each cage for breeding and females were checked for vaginal plugs each morning.
Plasma analyses
Plasma ET-1, sFLT1, VEGF and sENG were measured using ELISA kits (R&D Systems, Inc., Minneapolis, MN). The inter- and intra-variability of these assays are less than 10%.
Urinary albumin
Urine was collected by massaging the bladder at one time, and urinary albumin concentration and creatinine were determined using commercially available kits (Exocell Inc., Philadephia, PA) as described previously6, 7, 25.
Immunohistochemistry (IHC)
Rabbit polyclonal antibodies against MMP9 (ab19016, 1:200) and TIMP3 (ab187297, 1:50) were from EMD Millipore (Billerica, MA) and Abcam (Cambridge, MA), respectively, and rabbit monoclonal E-Cadherin (clone 24E10, #3195, 1:400) was from Cell Signaling Technology (Danvers, MA). IHC was performed in the Bond fully-automated slide staining system (Leica Bicrosystems Inc. Vista, CA). Antigen retrieval was performed at 1000C in Bond-epitope retrieval solution 1 pH6.0 (AR9961) for 20 min for E-Cadherin and MMP9 and in solution 2 (pH9.0) for TIMP3.Positive and negative controls (no primary antibody) were included for each antibody. IHC stained sections were digitally imaged (20x objective) in the Aperio ScanScope XT using line-scan camera technology (Leica Biosystems), and were stored within the Aperio eSlide Manager software.
Morphological examination
Pregnant females and embryos were examined both visually and histologically at different time points: 7.5 (also 7.0 for WT because of developmental stage differences), 12.5, 14.5, and 18.5 dpc. Fixed tissues (4% paraformaldehyde) were sectioned (5 μm) and stained with hematoxylin and eosin (H&E) or with Masson Trichrome. For kidneys, periodic acid-Schiff (PAS) staining was also used. Ultra-structural changes were examined by transmission electronic microscope (TEM, Jeol Jem 1230, Peabody, MA)6, 7.
Quantitative RT-PCR
Total RNA from tissues or cells was extracted using Trizol (Life Technologies, St. Paul, MN) following the manufacturer’s instruction. NanoDrop spectrophotometer method and gel electrophoresis was used to check quantity and quality of RNA. mRNA was quantified with TaqMan real-time quantitative RT-PCR (7500 real time PCR system, Applied Biosystems, Foster City, CA) by using one-step RT-PCR Kit (Bio Rad, Hercules, CA) with Hprt as reference genes in each reaction for mouse tissue. For human cell experiments, GAPDH was used as a reference gene. The primer and probe sequences are in Table S3, and 100ng of total RNA was used in each reaction.
Cell culture
The HTR8/SVneo trophoblast cell line was kindly provided by Dr. C.H. Graham, Queen’s University, Kingston, Ontario, Canada26, and maintained in RPMI-1640 medium supplemented with 5% FBS. Cells were starved for 24 hr with 0.5% FBS, then treated with ET-1 at doses of 0.02, 0.1 or 0.5 μM for 18 hr27. Different batches of cells were treated with ET-1 (0.5μM), ET-1 (0.5μM)+BQ123 (a selective EDNRA antagonist, 1μM), ET-1 (0.5μM)+BQ788 (a selective EDNRB antagonist,1μM) 28, 29, and ET-1 (0.5μM)+bosentan (a non-selective EDNR antagonist, 1μM) 30. Another batch of cells was treated with ET-1 (0.5μM) or vehicle as control. After incubation, medium and cells were collected at 4, 8, 12 and 18 hr for analysis. ET-1, BQ123, BQ788 were purchased from Sigma-Aldrich (St.Louis, MO). Bosentan was purchased from Cayman (Ann Arbor, MI).
Statistical analysis
Data are presented as mean ± SEM. Multifactorial ANOVA test was used with the program JMP 12.0 (SAS Institute Inc. Cary, NC). Post hoc analyses were done using the Tukey–Kramer Honest Significant Difference test. Differences were considered to be statistically significant with p values less than 0.05.
Results
Virgin female Edn1H/+ mice with higher ET-1 are normotensive
Consistent with our previously published data on male mice10, 2-month old Edn1H/+ females had four to five times higher tissue Edn1 expression and plasma ET-1 than WT females (1.30 ± 0.26 pg/ml WT vs. 5.4±1.2 pg/ml Edn1H/+, p<0.001), but they had no obvious abnormalities (Table S1). The body weight (BW), daily water and food intake, daily output of urine, plasma cholesterol and glucose were not significantly different between WT and Edn1H/+ mice. In addition, BP and urinary albumin excretion were not different between WT and Edn1H/+ mice (Fig. 1A, Fig. S1). The glomerular structure of the virgin Edn1H/+ female mice was indistinguishable from that of WT mice (Fig. S2).
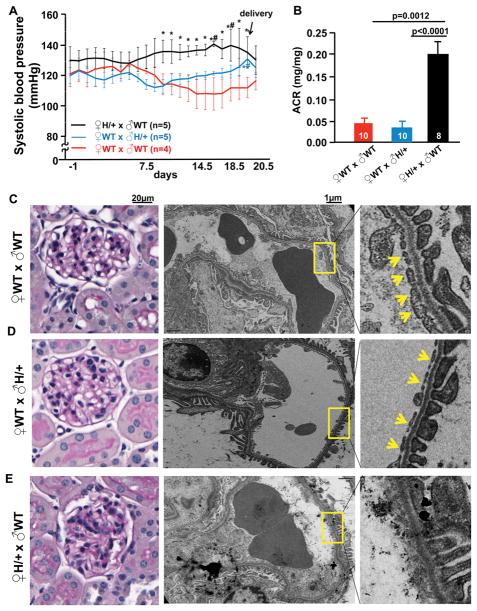
Figure 1. Edn1H/+ dams (x ♂WT) develop hypertension, albuminuria and glomerular endotheliosis during late gestation.
A, Systolic blood pressures of ♀Edn1H/+ (x ♂WT), ♀WT (x ♂Edn1H/+) and ♀WT (x ♂WT) mice during the whole pregnancy. Day -1 corresponds to the day males and females were placed together for mating; the day the plug was detected as 0.5 days post coitus (dpc). *p<0.05 vs. ♀WT (x ♂WT) at the same gestational stage, t test; #p<0.05 vs before pregnancy, paired t test. B, Urinary albumin creatinine ratio (ACR) at 18.5 dpc. Numbers of mice are within the bars. Representative glomeruli from ♀WT (x ♂WT) mice (C); ♀WT (x ♂Edn1H/+) (D); ♀Edn1H/+ (x ♂WT) mice (E) at 18.5 dpc. Left panel: Periodic acid–Schiff stain; Right panel: Electron micrographs of glomeruli. Yellow arrows: endothelial fenestrae, note: the endothelial fenestration is not present in Edn1H/+ dams. Multiple comparisons used the Tukey–Kramer honestly significant difference (HSD) test. Error bars are SEM.
Edn1H/+ female mice develop hypertension during late gestation
During the first week of pregnancy (up to 7.5 dpc) following mating with WT males, systolic blood pressure (SBP) of Edn1H/+ pregnant dams was not significantly different from that of WT dams. However, at the beginning of the second week of pregnancy (8.5 dpc), SBP began to decrease in the WT dams (x ♂WT), falling about 10mmHg during the third week of pregnancy (starting at 14.5 dpc) (red line in Fig. 1A). In contrast, SBP began to rise in Edn1H/+ dams during the second week until delivery, SBP increased about 10 mmHg, and returned to pre-pregnancy levels after delivery (black line in Fig. 1A). Both diastolic blood pressure (DBP) and mean arterial pressures (MAP) of dams showed the same trend as their SBP (Fig. S1A & B). The BP in the Edn1H/+ dams and WT dams (x ♂Edn1H/+) clearly lacked the decrease seen in the WT dams.
In the reciprocal mating (♀WT x ♂Edn1H/+), where half of the pups are WT and half are Edn1H/+ as in the Edn1H/+ dams (x ♂WT), BP of the WT dams did not significantly change during pregnancy (blue line in Fig. 1A, Fig S1). However at the later stage, at around 19.5 dpc, their SBP was 9.5mmHg higher than SBP before pregnancy.
Edn1H/+ dams, but not WT dams, exhibit proteinuria and kidney glomerular endotheliosis
Urinary albumin excretion of the Edn1H/+ dams was not significantly different from that of the WT dams at 7.5 and 14.5 dpc, but was increased about 3 times at 18.5 dpc, and returned to the pre-pregnancy levels when measured at postpartum day 7 (Fig. 1B, Fig. S1). In contrast, the urinary albumin excretion from WT dams (x ♂Edn1H/+) was indistinguishable from WT dams (x ♂WT) at 18.5 dpc (Fig. 1B).
Light microscopy of periodic acid-Schiff stained kidneys at 18.5 dpc showed that the glomeruli of the pregnant Edn1H/+ mice exhibited (Fig 1E) markedly reduced open capillary areas compared to those in WT dams (x ♂WT) or WT dams (x ♂Edn1H/+) (Fig 1C, 1D). Observations using transmission electron microscopy confirmed the loss of fenestrae in the glomerular capillary endothelial cells of Edn1H/+ dams (Fig. 1E). Podocyte foot processes were uniform and no effacement was present. Ultra-structures of kidneys from the WT dams (x ♂Edn1H/+) were indistinguishable from those from WT dams (x ♂WT) (Fig. 1D).
Fetal growth restriction (FGR) and intra-uterine fetal demise occur in the Edn1H/+ dams
WT dams (x ♂Edn1H/+) had similar litter size (Fig. 2A & B) and fetal weights as WT dams (x ♂WT) (Fig. 2C). In contrast, the average number of live pups born from the Edn1H/+ dams (x ♂WT) was about half of that from WT dams (Fig. 2A), but the ratios of Edn1H/+ pups and WT pups as well as sexes (M/F: 0.78/1, p=0.5, Fisher’s exact test) of the pups were not different from the expected 1:1 (Table. S2). At 18.5 dpc, the number of live fetuses from Edn1H/+ dams was already half of that from the WT dams (Fig. 2B), and fetal weight (both WT and Edn1H/+ fetuses) from Edn1H/+ dams was significantly lower than fetal weights from WT dams (Fig. 2C). The placental weight was not different among three groups of dams [0.101± 0.01g WT (x ♂WT), 0.103 ± 0.002g WT (x ♂ Edn1H/+), 0.108 ± 0.01g Edn1H/+ (x ♂WT), p=0.78]. At 14.5 dpc, the total number of fetuses (both alive and dead) was not significantly different between the two different maternal genotypes (Fig. 2D), but the Edn1H/+ dams had a higher percentage (41.6%) of resorption compared to WT dams (Fig. 2D and arrows in Fig. 2E). Furthermore, 2 out of 7 (28.6%) Edn1H/+ dams had poorly developed fetuses at 12.5 dpc, and these abnormal fetuses included both genotypes (Fig. S3C). The placentae of these conceptuses show a strikingly reduced labyrinth zone (LZ), smaller junctional zone (JZ) and larger decidual basalis (DB) compared to placentae of WT dams (Fig. S3E).
Figure 2. Fetal growth restriction and intra-uterine fetal demise occur in pregnant Edn1H/+ dams, but not in WT dams.
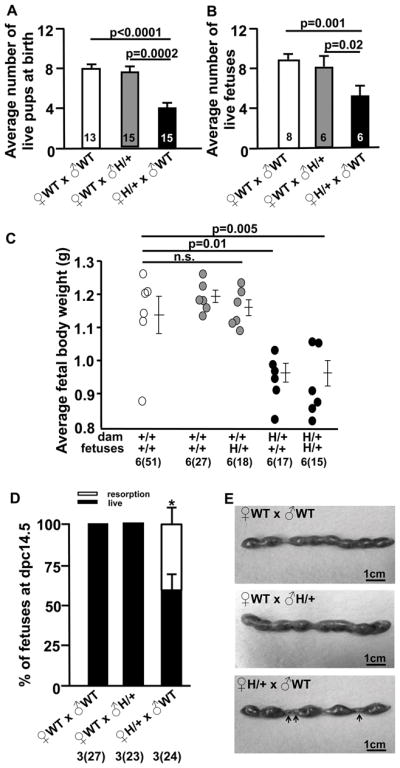
A, Average number of live neonates per pregnancy at the delivery day in ♀WT(x ♂WT), ♀WT (x ♂Edn1H/+) and ♀Edn1H/+ (x ♂WT) mice, numbers of pregnant mice are within the bars. B, Average number of live fetuses per pregnancy at 18.5 dpc in the three groups of mice, numbers of pregnant mice are within the bars. C, Average fetal weight at 18.5 dpc from three groups of mice. Numbers of total fetuses shown in parentheses; numbers of pregnant mice are shown outside of the parentheses. D, Average percentage of fetuses at 14.5 dpc from three groups of mice. Number of total fetuses are shown in parentheses (including both resorption and live ones); number of pregnant mice are shown outside of the parentheses. * p<0.01 vs. ♀WT(x ♂WT), ♀WT (x ♂Edn1H/+). E, Images of uteri harvested at 14.5 dpc. Arrows: resorptions of fetuses. Multiple comparisons used the Tukey–Kramer HSD test. n.s.: not significant. Error bars are SEM.
Implantation sites from Edn1H/+ dams have abnormal ectoplacental cone (EPC) with higher E-cadherin expression
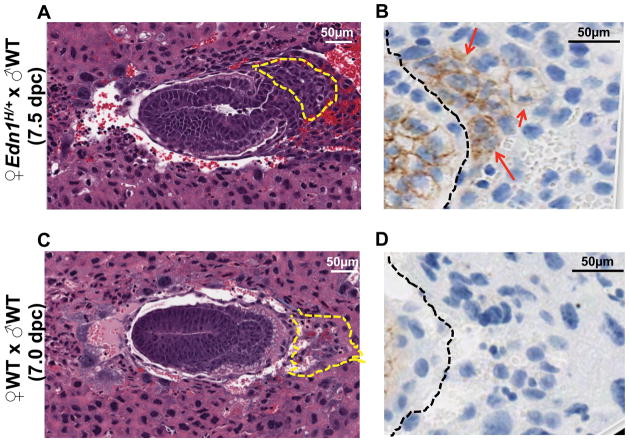
At 7.5 dpc, when trophoblast cell invasion commences, the implantation sites and embryos from Edn1H/+ dams were smaller than those from WT dams that were mated with either WT or Edn1H/+ males. Careful determination of the stage of development of the 7.5 dpc embryos from the mutant dams revealed that development lagged behind by approximately 12 hours, making them comparable to 7.0 dpc embryos of WT dams. All the implantation sites of Edn1H/+ dams at 7.5 dpc also showed a disoriented EPC (Fig. 3A) regardless of the genotype of the embryo in comparison to those from WT dams at 7.0 dpc and 7.5 dpc (Fig. 3C, Fig. S5). EPC regions of the embryos from WT dams (x ♂Edn1H/+) were not different from those of WT dams (x ♂WT) (Fig. S5). As trophoblast cells differentiate and begin to migrate 31, they normally down-regulate E-cadherin expression as we observed at 7.0 – 7.5 dpc in the EPC region of WT dams regardless of whether they were mated with WT or Edn1H/+ males (Fig. 3D, Fig. S5). In contrast, the expression of E-cadherin in the EPC region of Edn1H/+ dams (x ♂WT) at 7.5 dpc retained strong expression of E-cadherin (Fig. 3B). However, IHC showed that the expression of neither matrix metalloproteinase-9 (MMP9), a proteolytic enzyme from the embryo, nor tissue inhibitor of metalloproteinase-3 (TIMP3), an inhibitor from the maternal endometrium, were different in the embryo implantation sites of Edn1H/+ dams compared to WT dams (Fig. S4). Negative isotype control with rabbit IgG staining is shown in Fig. S6.
Figure 3. Implantation sites from pregnant Edn1H/+ mice have abnormal ectoplacental cone (EPC) with higher E-cadherin expression.
Midtransverse sections through implantation sites of ♀Edn1H/+ (x ♂WT) (A&B) and ♀WT (x ♂WT) (C&D). A&C: stained with H&E. Section (A) shows the developmental stage of a 7.5 dpc embryos from ♀Edn1H/+ (x ♂WT) is comparable to a 7.0 dpc embryo from ♀ WT (x ♂WT) (C). EPC region outlined with the yellow dashed lines exhibits the disoriented alignment in the implantation site. B&D: immunohistochemistry staining of E-cadherin in EPC region (right side of the black dashed lines) from A and C, respectively. Red arrows: positive staining.
Plasma ET-1 levels markedly increase in Edn1H/+ dams
The plasma ET-1 levels in WT dams (x ♂WT) did not change during pregnancy (1.29 ± 0.07 pg/ml at 18.5 dpc vs. 1.30 ± 0.26 pg/ml virgin, p=1.0). In contrast, a small but significant increase in the plasma ET-1 of WT dams (x ♂Edn1H/+) was observed during late pregnancy (2.76 ± 0.78 pg/ml at 18.5 dpc, p=0.008, t test) (Fig. 4A). The embryos of these WT dams contributed to the increase, as Edn1 expression was 4-fold higher in the placentae from Edn1H/+ fetuses than in those from WT fetuses (Fig. 4B).
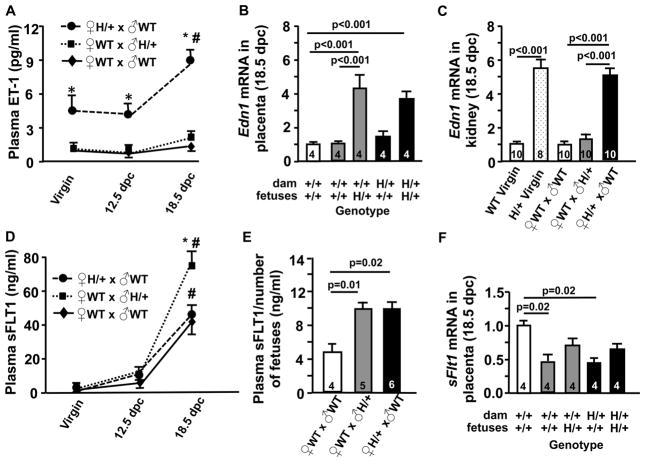
Figure 4. ♀EdnH/+ (x ♂WT) mice have elevated plasma ET-1 levels while ♀WT(x ♂Edn1H/+) mice have elevated plasma sFLT1 levels in late pregnancy.
A, Plasma ET-1 of virgin females and pregnant mice at 12.5 and 18.5 dpc, *p<0.001 vs WT at same gestational stage, #p<0.01 vs. virgin Edn1H/+ mice. N≥5. B, Edn1 mRNA level in placenta from pregnant mice at 18.5 dpc. Numbers of dams are within the bars. C, Edn1 mRNA level in kidneys from virgin females and pregnant mice at 18.5 dpc. Numbers of kidneys are within the bars. D, Plasma sFLT1 of virgin females and pregnant mice at 12.5 and 18.5 dpc, *p<0.01 vs WT at same gestational stage, #p<0.01 vs. virgin Edn1H/+ mice. n≥5. E, Plasma sFLT1 concentration normalized to the number of live fetuses in each dam. Numbers of pregnant mice are within the bars. F, sFlt1 mRNA level in placentae from pregnant mice at 18.5 dpc. Numbers of dams are within the bars. Multiple comparisons used the Tukey–Kramer HSD test. Error bars are SEM.
Plasma ET-1 levels in the Edn1H/+ female mice prior to pregnancy (5.4 ± 1.2 pg/ml) as well as at 12.5 dpc (4.1± 1.0 pg/ml) were ~4x higher than the levels in WT females and markedly increased in late pregnancy to (8.9 ± 0.5 pg/ml) at 18.5 dpc (Fig. 4A). Edn1 mRNA in the placentae of the Edn1H/+ females (x ♂WT) differed according to the genotype of the embryos as it did in the WT females (x ♂Edn1H/+) (Fig. 4B). In the peripheral tissues, the Edn1 mRNA levels in the kidneys of Edn1H/+ females at 18.5 dpc were not different from pre-pregnancy, although they were ~ 5x higher than the levels in the WT females (Fig. 4C). mRNA in the liver also showed a similar result (Fig. S7).
The placentae of Edn1H/+ fetuses contribute to maternal plasma sFLT1 levels
Plasma sFLT1 in the Edn1H/+ virgin females was higher than in WT virgin females (2.03 ± 0.14ng/ml Edn1H/+ vs. 1.35 ± 0.06 ng/ml WT, p=0.003, t test). At 12.5 dpc, the plasma sFLT1 levels were higher in both WT dams (x ♂Edn1H/+) (8.7 ± 0.9 ng/ml) and Edn1H/+ dams (x ♂WT) (8.2 ± 1.1 ng/ml) than in WT dams (x ♂ WT) (6.1 ± 1.0 ng/ml), although the differences did not reach significance (Fig. 4D). At 18.5 dpc, plasma sFLT1 levels in the C57BL/6J WT dams (x ♂ WT) increased to 40.1 ± 5.3 ng/ml, which were higher than in 129S6 WT dams we previously reported7, but was comparable to the value reported by Davisson’s group who also used female mice on a C57BL/6 background 32. In contrast, plasma sFLT1 levels in the WT dams (x ♂Edn1H/+) (77.1 ± 5.8 ng/ml) were significantly higher than in the WT dams (x ♂WT) (p<0.01). Plasma sFLT1 levels in the Edn1H/+ dams were not different from that in the WT dams (x ♂ WT) (47.9 ± 6.3 ng/ml, p=0.78) at 18.5 dpc. However, Edn1H/+dams had on average half the number of live fetuses compared to the WT dams. After normalizing by the number of live fetuses, no difference was detected between WT dams (x ♂Edn1H/+) and Edn1H/+ dams (x ♂WT); both had twice higher plasma sFLT1levels than WT dams (x ♂WT) (Fig. 4E). Intriguingly, sFlt1 mRNA in the placentae from both WT dams (x ♂ Edn1H/+) and Edn1H/+ dams (x ♂WT) was significantly lower than that in placentae from WT dams with all WT pups (Fig. 4F): sFlt1 mRNA levels in the placentae of WT fetuses were approximately 45%, while those in Edn1H/+ fetuses were approximately 65%.
Virgin Edn1H/+ females had significantly higher plasma VEGF levels than virgin WT females (11±3pg/ml WT vs. 42 ± 10pg/ml Edn1H/+, p=0.01, t test). The plasma VEGF levels increased by pregnancy, but at 18.5 dpc, they did not differ among three groups of dams [101± 14 pg/ml WT (x ♂WT), 93 ± 12pg.ml WT (x ♂ Edn1H/+), 98 ± 20 pg/ml Edn1H/+ (x ♂WT), p=0.9). Plasma sENG levels were not different among the three groups of dams (Table. S1).
ET-1 increases sFLT1 expression in human trophoblast cells via EDNRA and EDNRB receptors
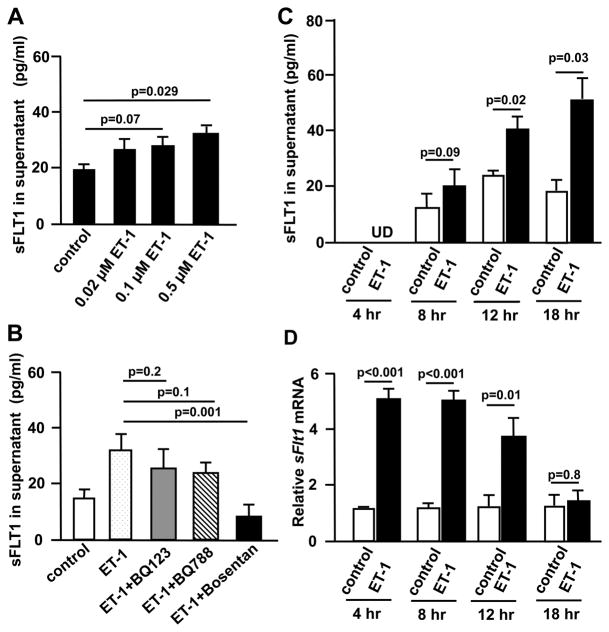
In human first trimester trophoblast cells (HTR8/SVneo), ET-1 treatment increased sFLT1 in the medium in a dose dependent manner, and at 0.5μM differences reached the significant level (Fig. 5A). When HTR8/SVneo cells were treated with ET-1 together with either BQ123 (a selective EDNRA antagonist) or with BQ788 (a selective EDNRB antagonist), sFLT1 concentration in the medium was decreased, although the reductions did not reach significant levels. In contrast, bosentan (a non-selective EDNR antagonist) significantly abolished the induction of sFLT1 by ET-1 (Fig. 5B). ET-1 at 0.5 μM increased sFLT1 concentration in the medium in a time dependent fashion (Fig. 5C). The amount of the major sFlt1 transcript in human placenta, variant e15a33, increased in the cells by 4hr after treatment and returned to the levels of non-stimulated cells by 18hr (Fig. 5D). Similar results were obtained for the amount of another sFlt1 transcript, variant i13 (Fig.S8).
Figure 5. ET-1 induces sFLT1 expression in human trophoblast cells via EDNRA and EDNRB.
After starving with 0.5% FBS medium for 24 hours, human first trimester trophoblast cells (HTR8/SVneo) were exposed to A, ET-1at doses of 0.02, 0.1 or 0.5 μM for 18hr and sFLT1 concentration in culture medium was measured. B, ET-1 (0.5 μM) alone, or with 1μM each of BQ123, BQ788, bosentan for 18 hr and sFLT1 concentration in culture medium were determined. C&D, Cell culture medium and cells were harvested at 4, 8, 12 and 18 hours after treatment with either vehicle or ET-1(0.5 μM). C, sFLT levels in culture medium. UD: undetectable. D, Cellular sFLT1 (variant e15a) mRNA levels (fold change relative to control cells). n=4
Discussion
Our study demonstrates that ET-1 overproduction causes the PE-like phenotype in mice. Maternal but not fetal ET-1 overproduction appears to be the major contributor to delayed placental and fetal development. We further show that fetal ET-1 overproduction alone is not sufficient to induce a full spectrum of PE-like symptoms in dams. Taken together, these data suggest that although both maternal and fetal ET-1 affects the maternal phenotype, the maternal ET-1 plays a major role. In contrast, only maternal ET-1 affects fetal outcome.
Because we mated the Edn1H/+ females with WT males, on average half of the fetuses were Edn1H/+ genotype and produced higher levels of ET-1 compared to the WT fetuses. To separate contributions of maternal and fetal ET-1 to their PE phenotypes, we also studied WT pregnant mice mated with Edn1H/+ males, which carry the same ratios of WT and Edn1H/+ embryos/fetuses as the Edn1H/+ females, and observed mild hypertension during late gestation but no kidney damage or intra-uterine growth restriction in these WT females. Edn1 expression from placentae of Edn1H/+ fetuses led to a two-fold increase in the plasma ET-1 levels in these WT females. Thus an increase of fetal ET-1 may directly affect maternal BP but it is not sufficient to cause kidney damage consistent with PE.
Notably, although plasma ET-1 levels of Edn1H/+ females are 4.5 times higher than those in WT females, their BP is not elevated. This observation is consistent with our previous finding in male mice 10 in which SBP increased slightly in parallel with graded decrease of Edn1 expression of 350%, 100%, 65% and 25% normal. Despite ET-1 being a potent vasoconstrictor, various studies with genetically altered mice have shown that effects of ET-1 on BP are not simple. For example, Kurihara et al. reported that Edn1+/− heterozygotes expressing 50% normal level of ET-1 are hypertensive 34. On the other hand, both endothelial cell specific ET-1-KO mice as well as mice overexpressing EDN1 specifically in endothelial cells have near normal BP 35. Presence of a complex feedback and/or developmental adjustment of the ET-1 system have been postulated and is supported by the finding that animals developed hypertension when endothelial cell specific overproduction of ET-1 was induced in adults36. The homeostatic adjustment could involve the VEGF system since in the current study we also found that plasma VEGF was higher in virgin Edn1H/+ female mice. Increased VEGF may counteract high ET-1 because VEGF is known to activate endothelial nitric oxidase synthase/nitric oxide (eNOS/NO) 37. Maternal physiological adjustments to pregnancy include induction of various vasodilators such as VEGF. When Edn1H/+ dams develop overt hypertension at the later stage of pregnancy, their plasma VEGF was not higher than that of WT dams, raising a possibility that there could be a ceiling to its up-regulation.
We also note that plasma ET-1 in the Edn1H/+ dams increased ~7 times of the WT levels at 18.5 dpc. This marked increase of ET-1 was not accounted for by the Edn1 mRNA levels in the placenta or in peripheral tissues (kidneys and liver), although we did not examine the expression in the lung, which is the major producer of ET-1. The elevated plasma ET-1 in Edn1H/+ dams could result from increased production of this 21 amino acid peptide through cleavage from its 38 amino acid precursor, big ET-1, by endothelin converting enzyme (ECE). An elevated ECE and ET-1 have been reported in pregnant women with PE compared to women with a normal pregnancy38. A disturbance in ET-1 clearance mechanisms could also contribute to its marked increase in the Edn1H/+ dams. Rapid but low capacity clearance of ET-1 takes place in the lung circulation via EDNRB receptor while the liver and kidneys clear ET-1 through non-receptor mediated mechanisms39, 40. In the kidney, the transport of albumin across the glomerular basement membrane is predominantly by diffusion rather than by flow, whereas the transport of water and small molecules is entirely by flow6, 41. Consequently, as the amount of fluid crossing a glomerulus decreases, the concentration of albumin in the glomerular effluent will increase without altering the concentration of small molecules. Thus, a reduced glomerular filtration rate (GFR) and effective renal plasma flow due to high ET-1 and endotheliosis could lead to a reduction of the clearance of small molecules such as ET-1 while increased concentration of albumin could saturate tubular protein reuptake systems, resulting in albuminuria. Future studies of kidney functions in preeclamptic animal models, including GFR in the pregnant Edn1H/+ females, are necessary to shed light on the relationships between circulating ET-1 levels and albuminuria.
Since Karumanchi’s group published their seminal paper in 20033, sFLT1 is perhaps the most studied molecule in PE. However, the precise mechanism(s) of sFLT1 regulation is still unclear. Previously, we and other investigators have shown that hypertension and renal injury induced by excess sFLT1 is through ET-1, since it was ameliorated by the treatment with EDNRA antagonists5, 6. More recently, Amaraoui et al reported that sFLT1 augments vasoconstriction via ET-1, in part by inhibiting VEGF-mediated stimulation of nitric oxide production and in part by enhancing the cyclooxygenase-thromboxane signaling route downstream of ET-142. During pregnancy, the placenta is a major source of sFLT1, which increases throughout the pregnancy. In the current study, we found that the maternal circulation of sFLT1 at 18.5 dpc in WT dams (x ♂Edn1H/+) was twice that of WT dams (x ♂WT). WT dams (x ♂Edn1H/+) had significantly higher plasma sFLT1 levels than Edn1H/+ dams (x ♂WT), at least in part because the number of functional placentae in the WT dams at this late stage of pregnancy was twice as many as those in Edn1H/+ dams (x ♂ WT). The modest increase in SBP of WT dams (x ♂ Edn1H/+) could have resulted from an increase in sFLT1, or other soluble factor(s) that are similarly induced by ET-1 in the placenta. Regardless, placentae of Edn1H/+ fetuses produced more sFLT1 than those of WT fetuses within a single dam, suggesting that ET-1 affects sFLT1 expression and secretion in this model.
The in vitro data in cultured cells provides further evidence to support the theory that ET-1 increases the release of sFLT1. Human first trimester trophoblast cells, HTR8/SVneo, express sFLT143 as well as components of the ET-1 system44. A pharmacological dose of ET-1 (0.5μM) acutely increased sFlt1 mRNA expression, followed by accumulation of its protein in the medium. However, 18 hr after treatment with ET-1, the mRNA level of sFlt1 was no longer elevated, suggesting a potential feedback inhibition. Indeed, in vivo data also suggests this feedback inhibitory effect, as indicated by decreased sFlt1 mRNA levels in placentae from dams carrying mutant fetuses which could have higher sFLT1 protein levels. ET-1 executes its functions through two receptors, EDNRA and EDNRB in HRT8/SVneo cells44, 45. Selective EDNRA or EDNRB receptor antagonists tended to decrease the elevated sFLT1 level mediated by ET-1, and the non-selective EDNR antagonist totally abolished the induction effect of ET-1, suggesting that ET-1 stimulates sFLT1 through both EDNRA and EDNRB receptors.
Many studies suggest that impaired trophoblast cell invasion is the “root” of PE 16, 17, 46. At 7.5 dpc, before the placenta had formed, the embryos from Edn1H/+ dams independent of their genotype showed a 12 hr delay in their developmental state, with disoriented EPC and abnormal E-cadherin expression. This suggests that high maternal ET-1 production impairs trophoblast invasion of embryos. We also found all embryos from dams with high ET-1 exhibit distortion of EPCs at 7.5 dpc. Since all embryos from Edn1H/+ dams had the distorted EPCs regardless of their Edn1 genotype, maternal high ET-1 is more likely to be the cause of this phenomenon. Studies show that both maternal and embryonic factors influence the EPCs and similarly disoriented EPCs have been described in embryos from female mice carrying a dominant negative mutation in Gjpa1 coding for connexin43 (maternal effects)47, and in embryos lacking MMP9 completely (embryonic effects) 17. Transcriptional activation of MMP9 in the differentiated trophoblast giant cells is coincidental with their invasive characteristics, while Gap junction intercellular communication is involved in the development and differentiation process. In all three cases, intrauterine embryonic growth restriction occurs. The Werb group reported that MMP9+/− hetrozygous females develop hypertension, proteinuria and renal injury during late pregnancy, establishing the link between MMP9 and PE17. It is important to note that the graded expression of Edn1 is inversely correlated with the Mmp9 gene expression in the heart10. ET-1, being a strong vasoconstrictor, could restrict blood supply in the maternal circulation, but since it is also expressed in most of the cells in the maternal-fetal interface, it may directly influence the terminal differentiation of the trophoblast giant cells.
In conclusion, our data suggest a chain of events that leads to PE in dams with genetically increased expression of ET-1. First, high ET-1 from maternal tissues negatively influences the differentiation and invasion of trophoblasts in early pregnancy. This is associated with increased sFLT1, which together with high expression of peripheral ET-1 production synergistically enhances vasoconstriction and vessel damage, resulting in the PE phenotype. This underscores the importance of screening maternal EDN1 genotype to identify pregnant women that have higher ET-1 level and thus a higher risk of developing PE.
Perspectives
PE is one of the leading causes of pregnancy-related maternal death, yet, the etiology of PE is still largely unknown. Currently, the only definitive treatment is delivery. Our new mouse model of PE resulting from maternal over production of ET-1 will facilitate answering how maternal ET-1 influences the pathophysiology of PE: the mechanisms of trophoblast differentiation/invasion in embryo implantation stage and maternal vascular dysfunction in late stage of pregnancy. This also should stimulate development of novel blockers of the ET-1 system, which are not teratogenic, to treat PE.
Supplementary Material
Novelty and Significance.
What Is New?
This study of a novel mouse model of preeclampsia demonstrates that maternal ET-1 over-production contributes to PE-like phenotypes including hypertension, proteinuria as well as glomerular endotheliosis and intra-uterine growth restriction of fetuses.
Maternal high ET-1 production impairs trophoblast cell differentiation/invasion in embryos and retention of high E-cadherin expression in ectoplacental cones.
Fetal ET-1 stimulates sFLT1 expression in placenta, which contributes to the increase of maternal circulating sFLT1 and elevated BP.
What Is Relevant?
The causal link between ET-1 and PE underscores the importance of screening maternal EDN1 genotype to identify pregnant women that have higher ET-1 production and thus a higher risk of developing PE. It also encourages the development of safe ET-1 system blockers for treatment of PE.
Summary
PE is the leading cause of maternal morbidity and mortality and there is no real treatment for this disease. The ET-1 system has been implicated for PE as the mediator of sFLT1 effects on PE, including hypertension, and renal damage. However, whether ET-1 is a causative factor of PE is not clear. Here, we show that female mice with high production of ET-1 develop PE-like symptoms, which include elevated blood pressure, urinary albumin excretion, and fetal growth restriction. Maternal high ET-1 production also affects the trophoblast differentiation/invasion, while fetal high ET-1 production leads to increased sFLT1 from placenta. These results encourage the development of ET-1 signaling blockers that are not harmful to babies to treat PE.
Acknowledgments
We thank Drs. Marlon Lawrence and Yukako Kayashima for critical reading of the manuscript. We thank Dr. H-S Kim for performing qRT-PCR, Mr. John Hagaman for performing mouse surgery and Miss. Azraa Ayesha for assisting with cell culture.
Sources of Funding: This work was supported by a grant from the National Institutes of Health (R01HL049277), and a Junior Faculty Development Award from the University of North Carolina at Chapel Hill. The histology core facility at UNC is supported by NIH Grant DK 034987.
Footnotes
Disclosures: None.
References
- 1.American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the american college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 2.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 3.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sflt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, Hankins GD, Saade GR. The effect of over-expression of sflt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol. 2007;196:396e391–397. doi: 10.1016/j.ajog.2006.12.024. discussion 396e397. [DOI] [PubMed] [Google Scholar]
- 5.Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension. 2010;55:394–398. doi: 10.1161/HYPERTENSIONAHA.109.141473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li F, Hagaman JR, Kim HS, Maeda N, Jennette JC, Faber JE, Karumanchi SA, Smithies O, Takahashi N. Enos deficiency acts through endothelin to aggravate sflt-1-induced pre-eclampsia-like phenotype. J Am Soc Nephrol. 2012;23:652–660. doi: 10.1681/ASN.20110403697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li F, Fushima T, Oyanagi G, Townley-Tilson HW, Sato E, Nakada H, Oe Y, Hagaman JR, Wilder J, Li M, Sekimoto A, Saigusa D, Sato H, Ito S, Jennette JC, Maeda N, Karumanchi SA, Smithies O, Takahashi N. Nicotinamide benefits both mothers and pups in two contrasting mouse models of preeclampsia. Proc Natl Acad Sci U S A. 2016;113:13450–13455. doi: 10.1073/pnas.1614947113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khimji AK, Rockey DC. Endothelin--biology and disease. Cell Signal. 2010;22:1615–1625. doi: 10.1016/j.cellsig.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Sandoval YH, Atef ME, Levesque LO, Li Y, Anand-Srivastava MB. Endothelin-1 signaling in vascular physiology and pathophysiology. Curr Vasc Pharmacol. 2014;12:202–214. doi: 10.2174/1570161112666140226122054. [DOI] [PubMed] [Google Scholar]
- 10.Hathaway CK, Grant R, Hagaman JR, Hiller S, Li F, Xu L, Chang AS, Madden VJ, Bagnell CR, Rojas M, Kim HS, Wu B, Zhou B, Smithies O, Kakoki M. Endothelin-1 critically influences cardiac function via superoxide-mmp9 cascade. Proc Natl Acad Sci U S A. 2015;112:5141–5146. doi: 10.1073/pnas.1504557112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hocher B, Thone-Reineke C, Rohmeiss P, Schmager F, Slowinski T, Burst V, Siegmund F, Quertermous T, Bauer C, Neumayer HH, Schleuning WD, Theuring F. Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J Clin Invest. 1997;99:1380–1389. doi: 10.1172/JCI119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain A. Endothelin-1: A key pathological factor in pre-eclampsia? Reprod Biomed Online. 2012;25:443–449. doi: 10.1016/j.rbmo.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal PK, Jain V, Srinivasan R, Jha V. Maternal edn1 g5665t polymorphism influences circulating endothelin-1 levels and plays a role in determination of preeclampsia phenotype. J Hypertens. 2009;27:2044–2050. doi: 10.1097/HJH.0b013e32832f7f3f. [DOI] [PubMed] [Google Scholar]
- 14.George EM, Granger JP. Linking placental ischemia and hypertension in preeclampsia: Role of endothelin 1. Hypertension. 2012;60:507–511. doi: 10.1038/ajh.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conrad KP, Rabaglino MB, Post Uiterweer ED. Emerging role for dysregulated decidualization in the genesis of preeclampsia. Placenta. 2017 doi: 10.1016/j.placenta.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Townley-Tilson WH, Wu Y, Ferguson JE, 3rd, Patterson C. The ubiquitin ligase asb4 promotes trophoblast differentiation through the degradation of id2. PLoS One. 2014;9:e89451. doi: 10.1371/journal.pone.0089451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plaks V, Rinkenberger J, Dai J, Flannery M, Sund M, Kanasaki K, Ni W, Kalluri R, Werb Z. Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proc Natl Acad Sci U S A. 2013;110:11109–11114. doi: 10.1073/pnas.1309561110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokkinos MI, Murthi P, Wafai R, Thompson EW, Newgreen DF. Cadherins in the human placenta--epithelial-mesenchymal transition (emt) and placental development. Placenta. 2010;31:747–755. doi: 10.1016/j.placenta.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 19.van Roy F, Berx G. The cell-cell adhesion molecule e-cadherin. Cell Mol Life Sci. 2008;65:3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshida C, Takeichi M. Teratocarcinoma cell adhesion: Identification of a cell-surface protein involved in calcium-dependent cell aggregation. Cell. 1982;28:217–224. doi: 10.1016/0092-8674(82)90339-7. [DOI] [PubMed] [Google Scholar]
- 21.Lombaerts M, van Wezel T, Philippo K, Dierssen JW, Zimmerman RM, Oosting J, van Eijk R, Eilers PH, van de Water B, Cornelisse CJ, Cleton-Jansen AM. E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer. 2006;94:661–671. doi: 10.1038/sj.bjc.6602996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosano L, Cianfrocca R, Spinella F, Di Castro V, Nicotra MR, Lucidi A, Ferrandina G, Natali PG, Bagnato A. Acquisition of chemoresistance and emt phenotype is linked with activation of the endothelin a receptor pathway in ovarian carcinoma cells. Clin Cancer Res. 2011;17:2350–2360. doi: 10.1158/1078-0432.CCR-10-2325. [DOI] [PubMed] [Google Scholar]
- 23.Peinado H, Olmeda D, Cano A. Snail, zeb and bhlh factors in tumour progression: An alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 24.Karl T. The house mouse: Atlas of embryonic development. 1989. [Google Scholar]
- 25.Li F, Wang CH, Wang JG, Thai T, Boysen G, Xu L, Turner AL, Wolberg AS, Mackman N, Maeda N, Takahashi N. Elevated tissue factor expression contributes to exacerbated diabetic nephropathy in mice lacking enos fed a high fat diet. J Thromb Haemost. 2010;8:2122–2132. doi: 10.1111/j.1538-7836.2010.03976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 27.Wedgwood S, Black SM. Endothelin-1 decreases endothelial nos expression and activity through eta receptor-mediated generation of hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol. 2005;288:L480–487. doi: 10.1152/ajplung.00283.2004. [DOI] [PubMed] [Google Scholar]
- 28.Danielyan L, Gembizki O, Proksch B, Weinmann M, Morgalla M, Wiesinger H, Buniatian GH, Gleiter CH. The blockade of endothelin a receptor protects astrocytes against hypoxic injury: Common effects of bq-123 anderythropoietin on the rejuvenation of the astrocyte population. Eur J Cell Biol. 2005;84:567–579. doi: 10.1016/j.ejcb.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 29.Spinella F, Caprara V, Garrafa E, Di Castro V, Rosano L, Natali PG, Bagnato A. Endothelin axis induces metalloproteinase activation and invasiveness in human lymphatic endothelial cells. Can J Physiol Pharmacol. 2010;88:782–787. doi: 10.1139/Y10-050. [DOI] [PubMed] [Google Scholar]
- 30.Wolf D, Tseng N, Seedorf G, Roe G, Abman SH, Gien J. Endothelin-1 decreases endothelial ppargamma signaling and impairs angiogenesis after chronic intrauterine pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2014;306:L361–371. doi: 10.1152/ajplung.00277.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woods AK, Hoffmann DS, Weydert CJ, Butler SD, Zhou Y, Sharma RV, Davisson RL. Adenoviral delivery of vegf121 early in pregnancy prevents spontaneous development of preeclampsia in bph/5 mice. Hypertension. 2011;57:94–102. doi: 10.1161/HYPERTENSIONAHA.110.160242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer KR, Tong S, Kaitu’u-Lino TJ. Placental-specific sflt-1: Role in pre-eclamptic pathophysiology and its translational possibilities for clinical prediction and diagnosis. Mol Hum Reprod. 2017;23:69–78. doi: 10.1093/molehr/gaw077. [DOI] [PubMed] [Google Scholar]
- 34.Kurihara Y, Kurihara H, Suzuki H, Kodama T, Maemura K, Nagai R, Oda H, Kuwaki T, Cao WH, Kamada N, et al. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368:703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- 35.Speed JS, Pollock DM. New clues towards solving the mystery of endothelin and blood pressure regulation. Hypertension. 2015;66:275–277. doi: 10.1161/HYPERTENSIONAHA.115.05277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rautureau Y, Coelho SC, Fraulob-Aquino JC, Huo KG, Rehman A, Offermanns S, Paradis P, Schiffrin EL. Inducible human endothelin-1 overexpression in endothelium raises blood pressure via endothelin type a receptors. Hypertension. 2015;66:347–355. doi: 10.1161/HYPERTENSIONAHA.115.05168. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad S, Hewett PW, Wang P, Al-Ani B, Cudmore M, Fujisawa T, Haigh JJ, le Noble F, Wang L, Mukhopadhyay D, Ahmed A. Direct evidence for endothelial vascular endothelial growth factor receptor-1 function in nitric oxide-mediated angiogenesis. Circ Res. 2006;99:715–722. doi: 10.1161/01.RES.0000243989.46006.b9. [DOI] [PubMed] [Google Scholar]
- 38.Ajne G, Wolff K, Fyhrquist F, Carlstrom K, Hemsen-Mortberg A, Nisell H. Endothelin converting enzyme (ece) activity in normal pregnancy and preeclampsia. Hypertens Pregnancy. 2003;22:215–224. doi: 10.1081/PRG-120024025. [DOI] [PubMed] [Google Scholar]
- 39.Dupuis J, Schwab AJ, Simard A, Cernacek P, Stewart DJ, Goresky CA. Kinetics of endothelin-1 binding in the dog liver microcirculation in vivo. Am J Physiol. 1999;277:G905–914. doi: 10.1152/ajpgi.1999.277.4.G905. [DOI] [PubMed] [Google Scholar]
- 40.Fukuroda T, Fujikawa T, Ozaki S, Ishikawa K, Yano M, Nishikibe M. Clearance of circulating endothelin-1 by etb receptors in rats. Biochem Biophys Res Commun. 1994;199:1461–1465. doi: 10.1006/bbrc.1994.1395. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence MG, Altenburg MK, Sanford R, Willett JD, Bleasdale B, Ballou B, Wilder J, Li F, Miner JH, Berg UB, Smithies O. Permeation of macromolecules into the renal glomerular basement membrane and capture by the tubules. Proc Natl Acad Sci U S A. 2017;114:2958–2963. doi: 10.1073/pnas.1616457114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amraoui F, Spijkers L, Hassani Lahsinoui H, Vogt L, van der Post J, Peters S, Afink G, Ris-Stalpers C, van den Born BJ. Sflt-1 elevates blood pressure by augmenting endothelin-1-mediated vasoconstriction in mice. PLoS One. 2014;9:e91897. doi: 10.1371/journal.pone.0091897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaitu’u-Lino TJ, Tong S, Beard S, Hastie R, Tuohey L, Brownfoot F, Onda K, Hannan NJ. Characterization of protocols for primary trophoblast purification, optimized for functional investigation of sflt-1 and soluble endoglin. Pregnancy Hypertens. 2014;4:287–295. doi: 10.1016/j.preghy.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Chakraborty C, Barbin YP, Chakrabarti S, Chidiac P, Dixon SJ, Lala PK. Endothelin-1 promotes migration and induces elevation of [ca2+]i and phosphorylation of map kinase of a human extravillous trophoblast cell line. Mol Cell Endocrinol. 2003;201:63–73. doi: 10.1016/s0303-7207(02)00431-8. [DOI] [PubMed] [Google Scholar]
- 45.Liu S, Li Q, Na Q, Liu C. Endothelin-1 stimulates human trophoblast cell migration through cdc42 activation. Placenta. 2012;33:712–716. doi: 10.1016/j.placenta.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Fisher SJ. The placental problem: Linking abnormal cytotrophoblast differentiation to the maternal symptoms of preeclampsia. Reprod Biol Endocrinol. 2004;2:53. doi: 10.1186/1477-7827-2-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winterhager E, Gellhaus A, Blois SM, Hill LA, Barr KJ, Kidder GM. Decidual angiogenesis and placental orientation are altered in mice heterozygous for a dominant loss-of-function gja1 (connexin43) mutation. Biol Reprod. 2013;89:111. doi: 10.1095/biolreprod.113.111690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.