Abstract
Aim
While the course of psychopathology has been explored from an index mental health diagnosis onwards, there are few detailed, prospective studies of the occurrence of clinical psychopathology in youth with familial risk for severe mental illnesses such as psychosis. We sought to describe the appearance of Axis I psychopathology in a unique sample of adolescents with a family history of schizophrenia (FHR).
Methods
162 first- and second-degree relatives (mean age 15.7 ± 3.6; range 8–25) of probands with schizophrenia or schizoaffective disorder were assessed at baseline and annual intervals for up to three years, focusing on DSM-IV-TR Axis I psychopathology.
Results
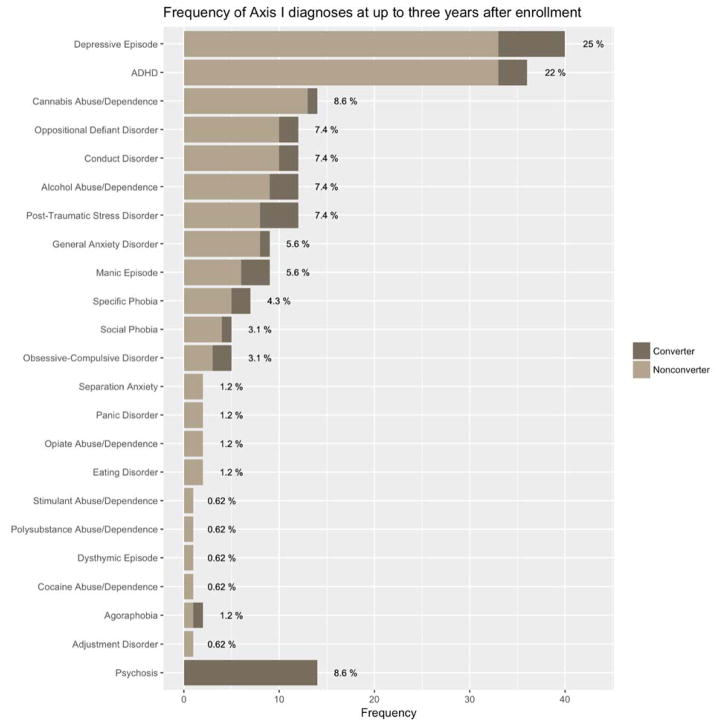
14 individuals (8.6%) developed a psychotic disorder. 105 subjects (65%) met criteria for an Axis I disorder over the course of the study, the most common of which was a depressive episode (40 subjects; 25%). Of the 148 individuals who did not develop psychosis, 91 (61%) had a comorbid Axis I disorder compared with 10/14 converters (71%). Ordered by increasing age of onset, diagnoses included cognitive and externalizing disorders, anxiety disorders, affective disorders, substance use disorders and psychotic disorders.
Conclusions
In addition to an elevated risk of psychosis, young FHR relatives manifest a broad range of non-psychotic Axis I psychopathology in childhood and adolescence. The appearance of this breadth of diagnoses has implications for the structure and function of clinical services for young people.
Keywords: psychosis, familial high-risk, psychopathology, emergence, diagnosis, characteristics
INTRODUCTION
The appearance of clinical psychopathology is a key variable of interest in investigations of the risk, onset and course of mental illness in young people. Such studies can ultimately inform risk estimates, research approaches, and even service provision through prevention and other forms of early intervention (EI). In psychosis, longitudinal follow-up of diagnoses over time has primarily been carried out on those with an established first episode of psychosis (FEP) (Bromet et al., 2011; Haahr et al., 2008; Heslin et al., 2015; Pope, Joober, & Malla, 2013). However, recent examinations of clinical psychopathology leading up to a FEP, mostly in subjects at ultra or clinical high-risk (CHR) for psychosis, have shed important light on trajectories of illness in youth (Addington et al., 2011; Cannon et al., 2008; DeVylder et al., 2014; Fusar-Poli et al., 2012; 2013a; Nelson et al., 2013; Ruhrmann et al., 2010).
In contrast to the relatively heterogeneous and help-seeking FEP and CHR populations, there has been comparatively little work on the appearance and characteristics of psychopathology in young people identified due to their family history of psychosis (familial high-risk, FHR) (Shah, Tandon, & Keshavan, 2013). The FHR group, which is more etiologically homogenous, includes first- and second-degree relatives of individuals with a schizophrenia-spectrum disorder, who have an 8–12% and 3–4% lifetime risk of developing psychosis respectively (Sullivan, 2005). Recent longitudinal FHR studies have demonstrated a broad spectrum of baseline psychopathology in this population (Johnstone, Russell, Harrison, & Lawrie, 2003; Keshavan, Diwadkar, Montrose, Stanley, & Pettegrew, 2004). While not exhaustive, there is also accumulating evidence of impairments in cognition, including verbal learning and memory, verbal fluency, executive functioning, and social cognition (Bhojraj et al., 2009; Bhojraj et al., 2010; Eack et al., 2010; Johnstone et al., 2005; Keshavan et al., 2010); alterations in brain structures such as gray matter, auditory association areas, language areas, superior temporal gyrus, prefrontal cortex, and hippocampus, among others (Bhojraj et al., 2009; Bhojraj et al., 2011a; Bhojraj et al., 2011b; Job et al., 2006; Rajarethinam et al., 2004; Thermenos et al., 2013); and metabolism in the thalamus, caudate and anterior cingulate (N. Tandon et al., 2013). Finally, despite an assumed ‘genetic’ vulnerability, a variety of socioenvironmental factors have been shown to be relevant for prediction of psychosis in this population (Gilbert et al., 2003; Padmanabhan et al., 2017; Shah et al., 2013).
Individuals at FHR are difficult to recruit and retain in longitudinal observational or intervention studies; psychopathology in young FHR subjects therefore remains poorly understood. However, such populations are of additional importance as they are not already seeking clinical care, have few of the confounds associated with medication exposure (particularly for antipsychotic medication) or illness duration, and present an opportunity to examine genotypic and endophenotypic risk factors before psychosis-like symptoms emerge. A recent meta-analysis concluded that children of parents with severe mental illness (SMI) are at substantial risk for developing a number of psychiatric disorders, not just the illness experienced by the parent (Rasic, Hajek, Alda, & Uher, 2014). However, few studies have comprehensively followed youth at FHR for psychosis at regular intervals to determine Axis I psychopathology during a critical window (Birchwood, Todd, & Jackson, 1998).
We sought to investigate this issue with the aim of understanding which diagnoses develop in this population, at what age and frequency, and whether these characteristics differ between those who later do versus do not develop psychosis.
METHODS
Sample
A sample of 162 relatives (119 offspring, 20 siblings, and 23 second-degree relatives) of individuals with schizophrenia were identified and evaluated using the Structured Clinical Interview for DSM-IV (SCID) (First, Spitzer, Gibbon, & Williams, 2002) supplemented by the Schedule for Affective Disorders and Schizophrenia-Child Version (K-SADS) (Ambrosini, Metz, Prabucki, & Lee, 1989). At baseline, participants were an average age of 15.7 ± 3.6 years, and consisted of 80 males and 82 females (79 Caucasian, 80 African-American, 3 Asian).
Inclusion criteria for the study were an age range of 8–25, and having either a first- and/or second-degree relative affected with schizophrenia or schizoaffective disorder. Exclusion criteria included existence of mental retardation, lifetime evidence of any previous psychotic disorder (not including a clinical high-risk stage), prior exposure to antipsychotic medications, significant medical or neurological conditions, or recent substance abuse/dependence. Evidence of a previous psychotic disorder at baseline was an exclusion criterion to entry into the study, in order to identify individuals who were still at risk and had not yet developed a psychotic illness. Individuals who subsequently developed a full-threshold psychotic disorder were referred to as “converters”.
Procedure
Study participants were recruited through advertisements or referral by clinicians in Pittsburgh, PA. After a SCID assessment confirmed diagnosis of schizophrenia or schizoaffective disorder in the relative, written informed consent regarding FHR subjects was acquired from subjects; for participants aged 18 or less, consent was provided by parents or guardians, with participants providing assent. A baseline consensus diagnosis utilized the SCID/K-SADS combined with detailed review of available health records and interviews with key informants (parents, guardians) to identify current diagnoses, lifetime diagnoses prior to enrollment in the study, and ruled out presence of a previous psychotic disorder. In subjects over the age of 15, the SCID was supplemented by the Developmental Disorders modules of the K-SADS. IQ was determined using the Revised Wechsler Adult Intelligence Scale (Wechsler, 1981) and socioeconomic status was determined using the Hollingshead index of social position (Hollingshead, 1965). This research was approved by the University of Pittsburgh Medical Center’s Institutional Review Board.
All participants were followed up at approximately annual intervals for up to three years. At follow-up visits, SCID/K-SADS interviews were conducted by trained clinicians (DM or DMM) with the FHR subjects; retention was approximately 80% at each follow-up time-point. Age of onset for previous (since the last visit) or new diagnoses was determined using historical data acquired at baseline, subsequent evaluations, chart reviews, and collateral information from patients, families and guardians where available. Where possible, structured diagnostic interviews, interval histories and relevant medical records were obtained by the same trained clinicians who carried out the baseline assessments. All available and relevant information was used in regular consensus diagnostic meetings, which were chaired by a senior clinician (MSK or DMM).
Data analysis
Sociodemographic variables for the overall sample, converters and nonconverters, and completers (those obtained assessments at the year 3 timepoint) and noncompleters (those lost to follow-up before year 3) were analyzed with appropriate chi-squared and t-tests to determine sample characteristics. Occurrence of psychopathology (DSM-IV-TR diagnoses) over time was compiled. The overall psychopathologic outcomes between converters and nonconverters were compared using the Fisher’s exact test.
RESULTS
Sample Characteristics
This FHR population has been described in detail elsewhere (Keshavan et al., 2004; 2008). Here, baseline characteristicswere compiled for the 162 subjects described here (Table 1). There were no differences in baseline sociodemographics between the 14 subjects who did and the 148 who did not develop a psychotic illness over the course of the study. Those who completed the year 3 assessment of the study had significantly higher baseline IQ (t-statistic 2.07, p < 0.05) than noncompleters (Table 1).
Table 1.
Sociodemographic characteristics of the FHR population
| Total FHR (baseline) | Converters | Nonconverters | p-value | test-stat | Year 3 completers | Year 3 noncompleters | p-value | test-stat | |
|---|---|---|---|---|---|---|---|---|---|
| n | 162 | 14 | 148 | NA | NA | 70 | 92 | NA | NA |
| Age (yrs) | 15.7 (3.6) | 17.0 (3.1) | 15.6 (3.6) | 0.16 | 1.41 | 15.6 (3.5) | 15.7 (3.7) | 0.87 | −0.16 |
| Yrs ed | 9.1 (3.3) | 10.3 (2.8) | 9 (3.4) | 0.18 | 1.35 | 9.1 (3.3) | 9.1 (3.4) | 0.92 | 0.10 |
| IQ | 102.2 (15.7) | 100.8 (15.3) | 102.4 (15.8) | 0.72 | −0.37 | 105.1 (15.4) | 99.9 (15.7) | 0.04 | 2.07 |
| Sex | 82F/162 | 8F/14 | 74F/148 | 0.82 | 0.05 | 37F/70 | 45F/92 | 0.62 | .25 |
| SES | 33.8 (15.7) | 33.0 (15.3) | 33.9 (15.8) | 0.84 | −0.21 | 35.6 (15.4) | 32.4 (15.7) | 0.19 | 1.71 |
| Race | 79CA/162 | 5CA/14 | 74CA/148 | 0.31 | 1.04 | 36CA/70 | 43CA/92 | 0.55 | 0.35 |
Axis I Diagnoses
105 subjects (65%) met criteria for an Axis I psychiatric disorder at some point over the course of the study. There was no significant difference in the frequency of developing new Axis I diagnoses between offspring versus non-offspring (siblings/second-degree relatives), or between females versus males. Despite year 3 completers having higher baseline IQ than noncompleters, there were no significant differences in development of Axis I diagnoses between these two subgroups.
The frequencies at which specific diagnoses occurred are documented in Figure 1. Depressive episodes were the most common diagnosis, followed by attention deficit/hyperactivity disorder and marijuana abuse. 71% of those who converted to psychosis (10/14) had comorbid Axis I disorders, compared with 61% (91/148) of nonconverters.
Figure 1.
Frequency of Axis I diagnoses at up to three years after intake.
Converters vs Nonconverters
There was an overall but non-significant difference in the distribution of diagnoses: converters developed Axis I psychopathology at higher rates than did nonconverters (p = 0.0667, Fisher’s exact test). Due to the limited number of converters, it was not possible to compare outcomes for specific disorder categories.
Age of Onset (Axis I Diagnoses)
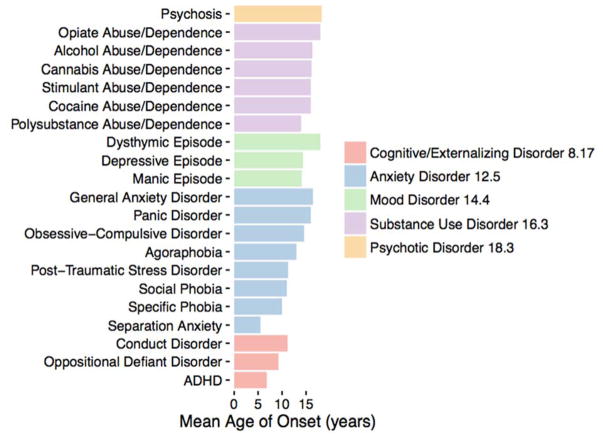
Ages of onset for specific disorders were grouped into five categories. In order of increasing mean age of onset, these groups were: cognitive/externalizing disorders (attention-deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, intermittent explosive disorder), anxiety disorders (separation anxiety, specific phobia, social phobia, PTSD, agoraphobia, OCD, panic disorder and generalized anxiety disorder), mood disorders (manic episode, depressive episode, dysthymic disorder), substance use disorders (including use of polysubstances, cocaine, stimulants, cannabis, alcohol, and opiates), and psychotic disorders (Figure 2). Psychosis-spectrum diagnoses that emerged were schizophrenia, schizoaffective disorder, schizophreniform disorder, and psychosis NOS. The onset of psychosis in this group occurred at a mean age of 18.3 (SD 3.2).
Figure 2.
Mean age of onset of Axis I diagnoses in FHR subjects for disorders developed by three or more subjects.
DISCUSSION
In this descriptive report, we provide the first detailed picture of the appearance and evolution of clinical psychopathology in a sample of youth at familial high-risk for psychosis over a three year period. Most prominently, the FHR group as a whole developed a range of Axis I psychopathology that itself changed and accumulated over a three year period – including cognitive/externalizing disorders, anxiety disorders, mood disorders, substance use disorders, and affective and nonaffective psychosis. 14 subjects (8.6%) transitioned to psychosis, consistent with the known conversion rate for first- and second-degree relatives of index cases with psychosis. Although not statistically significant, converters developed non-psychotic Axis I psychopathology more frequently than did nonconverters.
Our observations build on knowledge regarding populations at variable risk for psychosis and are therefore of relevance to both researchers and clinicians. In comparison to general populations, the ages of onset in this FHR cohort are similar to those from the WHO World Mental Health (WMH) Surveys for anxiety and impulse control disorders, but fall below the WMH range for mood disorders, substance use and psychotic disorders (Kessler et al., 2005; Kessler et al., 2007). Similarities and differences also emerge when comparing this FHR population to CHR populations: although a pluripotentiality of outcomes in CHR might be expected due to the heterogenous nature of this state (with its various subgroups), a recent meta-analysis of FHR studies also reveals substantial variability in long-term clinical outcomes in offspring of parents with SMI (Rasic et al., 2014). CHR researchers have reported that only a minority of subjects later transition to a psychotic illness (Fusar-Poli et al., 2013a) and that many non-psychotic Axis I conditions (especially depression, anxiety and substance use disorders) develop at a significantly higher prevalence than psychosis in that group (Addington et al., 2011; Simon et al., 2011). Our detailed study supports these findings in an FHR population of non-psychotic offspring of parents with schizophrenia: depression, anxiety and substance use are more common diagnostic endpoints than psychosis itself. While externalizing disorders such as ADHD, oppositional defiant disorder, and conduct disorder were also relatively common in our younger FHR population, there is limited knowledge available regarding their prevalence in CHR (Mazzoni et al., 2009).
Clinicians encountering young people with a family history of psychosis should therefore bear in mind that FHR status is a significant risk factor for any mental disorder and not just for psychosis itself. This is relevant for two reasons. First, clinical instruments for the psychosis CHR state (N. Tandon, Shah, Keshavan, & Tandon, 2012a) include first-degree relatives with sub-threshold symptoms and/or functional impairments as criteria. Second, even without explicit help-seeking, youth with a family history of severe mental illness are at risk for a range of disorders over time. Together, this raises questions regarding whether clinical infrastructures for at-risk states should be built solely for specific, diagnostically-bound ‘exit disorders’ or whether more general, transdiagnostic clinical settings for these vulnerable youth would better address both their specific developmental needs and the emerging evidence regarding long-term outcomes (Cross et al., 2014; Fusar-Poli, Yung, McGorry, & van Os, 2013b; McGorry & Nelson, 2016).
Because FHR individuals are not necessarily help-seeking, they are challenging to identify and then follow over time. A unique strength of these observations, then, is that they were conducted in a non-clinical sample and then repeated roughly annually for up to three years in a detailed and systematic fashion, in the midst of a critical neurodevelopmental period. Limitations include the fact that consensus was achieved (using subject self-report, collateral information from a relative or chart review) regarding diagnoses that emerged prior to enrollment in the study; later consensus diagnostic meetings were not blind to subject, and may have become unintentionally biased towards maintaining pre-existing diagnoses. Despite the relatively high retention rate, attrition may have been non-random: subjects who could not be reached at one or more follow-up points may be disproportionately likely to have experienced a significant change in their psychopathology. Finally, since there is no control group against which the FHR cohort’s emergence of clinical psychopathology can be compared, the descriptive nature of the analysis makes it difficult to draw conclusions about trajectories: depending on their age of enrollment in the study, individuals were more or less likely to develop specific kinds of disorders, particularly for age-dependent diagnoses such as ADHD or conduct disorder. Additional psychopathology may have developed before or after the three-year follow-up period and was therefore unable to be captured in the current analysis.
The study also began before the widespread use of instruments to distinguish between supra-threshold psychotic disorders and their CHR states. Thus, even though the researchers were aware of the existence of baseline sub-threshold symptoms as opposed to a threshold psychosis (Shah et al., 2012; Tandon et al., 2012b), consensus diagnostic meetings may have identified a severe CHR state as a threshold psychotic disorder. Detailed comparison between converters and nonconverters is limited by the fact that the FHR population included those who were observed before (not during) the period of highest risk, and since older adolescents or young adults who developed psychosis were excluded at baseline and not eligible as a control group.
Nonetheless, these initial results regarding the emergence and evolution of Axis I psychopathology in a population of adolescents at FHR for psychosis have significant implications for prevention and early intervention strategies, in psychosis specifically and youth mental health more generally. Given the broad range of psychopathology (in conjunction with the higher rates of incidence) seen in young people at various forms of risk for psychosis, they call attention to the need for additional support and closer monitoring for FHR youth who are seeking help. They also imply that clinical infrastructures for those at risk (both CHR and FHR) should nonetheless be flexible and adept in providing easily accessible, youth-friendly care for any mental illness that develops in young people. This represents a crucial challenge to be tackled by next-generation early intervention platforms for youth mental health.
Acknowledgments
National Institutes of Health (NIH) MH01180, MH64023, MH78113 to MSK; National Alliance for Research on Schizophrenia and Depression (Independent Investigator Award to MSK); FRSQ Clinician-Scientist Award (JLS). We thank Reviewer 1, EIP for their valuable comments regarding the manuscript.
References
- Addington J, Cornblatt BA, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, et al. At clinical high risk for psychosis: outcome for nonconverters. The American Journal of Psychiatry. 2011;168(8):800–805. doi: 10.1176/appi.ajp.2011.10081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini PJ, Metz C, Prabucki K, Lee JC. Videotape reliability of the third revised edition of the K-SADS. J Am Acad Child Adolesc Psychiatry. 1989;28(5):723–728. doi: 10.1097/00004583-198909000-00013. [DOI] [PubMed] [Google Scholar]
- Bhojraj TS, Francis AN, Rajarethinam R, Eack S, Kulkarni S, Prasad KM, Montrose DM, Dworakowski D, Diwadkar V, Keshavan MS. Verbal fluency deficits and altered lateralization of language brain areas in individuals genetically predisposed to schizophrenia. Schizophrenia Research. 2009;115(2–3):202–208. doi: 10.1016/j.schres.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojraj TS, Diwadkar VA, Sweeney JA, Prasad KM, Eack SM, Montrose DM, Keshavan MS. Longitudinal alterations of executive function in non-psychotic adolescents at familial high risk for schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(3):469–74. doi: 10.1016/j.pnpbp.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojraj TS, Sweeney JA, Prasad KM, Eack S, Rajarethinam R, Francis AN, et al. Progressive alterations of the auditory association areas in young non-psychotic offspring of schizophrenia patients. Journal of Psychiatric Research. 2011a;45(2):205–212. doi: 10.1016/j.jpsychires.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojraj TS, Sweeney JA, Prasad KM, Eack SM, Francis AN, Miewald JM, Montrose DM, Keshavan MS. Gray matter loss in young relatives at risk for schizophrenia: relation with prodromal psychopathology. Neuroimage. 2011b;54(s1):S272–9. doi: 10.1016/j.neuroimage.2010.04.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchwood M, Todd P, Jackson C. Early intervention in psychosis: The critical period hypothesis. Br J Psychiatry Suppl. 1998;172(33):53–59. [PubMed] [Google Scholar]
- Bromet EJ, Kotov R, Fochtmann LJ, Carlson GA, Tanenberg-Karant M, Ruggero C, Chang S. Diagnostic shifts during the decade following first admission for psychosis. American Journal of Psychiatry. 2011;168(11):1186–1194. doi: 10.1176/appi.ajp.2011.11010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross SPM, Hermens DF, Scott EM, Ottavio A, McGorry PD, Hickie IB. A clinical staging model for early intervention youth mental health services. Psychiatric Services (Washington, DC) 2014;65(7):939–943. doi: 10.1176/appi.ps.201300221. [DOI] [PubMed] [Google Scholar]
- DeVylder JE, Muchomba FM, Gill KE, Ben-David S, Walder DJ, Malaspina D, Corcoran CM. Symptom trajectories and psychosis onset in a clinical high-risk cohort: the relevance of subthreshold thought disorder. Schizophrenia Research. 2014;159(2–3):278–83. doi: 10.1016/j.schres.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Mermon DE, Montrose DM, Miewald J, Gur RE, Gur RC, Sweeney JA, Keshavan MS. Social cognition deficits among individuals at familial high risk for schizophrenia. Schizophrenia Bulletin. 2010;36(6):1081–8. doi: 10.1093/schbul/sbp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, patient edition. New York: Biometric Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rössler A, Schultze-Lutter F, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry (Chicago, Ill) 2013a;70(1):107–120. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Cappucciati M, Rutigliano G, Heslin M, Stahl D, Brittenden Z, et al. Diagnostic Stability of ICD/DSM First Episode Psychosis Diagnoses: Meta-analysis. Schizophrenia Bulletin. 2016 doi: 10.1093/schbul/sbw020. [DOI] [PMC free article] [PubMed]
- Fusar-Poli P, Yung AR, McGorry P, van Os J. Lessons learned from the psychosis high-risk state: towards a general staging model of prodromal intervention. Psychological Medicine. 2013b:1–8. doi: 10.1017/S0033291713000184. [DOI] [PubMed]
- Gilbert AR, Montrose DM, Sahni SD, Diwadkar VA, Keshavan MS. Obstetric complications correlate with neurobehavioral and brain structural alterations in young relatives at risk for schizophrenia. Annals of the New York Academy of Sciences. 2003;1008:269–275. doi: 10.1196/annals.1301.030. [DOI] [PubMed] [Google Scholar]
- Haahr U, Friis S, Larsen TK, Melle I, Johannessen JO, Opjordsmoen S, et al. First-episode psychosis: diagnostic stability over one and two years. Psychopathology. 2008;41(5):322–329. doi: 10.1159/000146070. [DOI] [PubMed] [Google Scholar]
- Heslin M, Lomas B, Lappin JM, Donoghue K, Reininghaus U, Onyejiaka A, et al. Diagnostic change 10 years after a first episode of psychosis. Psychological Medicine. 2015;45(13):2757–2769. doi: 10.1017/S0033291715000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A. Two-Factor Index of Social Position. New Haven: Yale University Press; 1965. [Google Scholar]
- Job DE, Whalley WC, McIntosh AM, Owens DGC, Johnstone EC, Lawrie SM. Grey matter changes can improve the prediction of schizophrenia in subjects at high risk. BMC Med. 2006;4:29. doi: 10.1186/1741-7015-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone EC, Russell KD, Harrison LK, Lawrie SM. The Edinburgh High Risk Study: current status and future prospects. World Psychiatry : Official Journal of the World Psychiatric Association (WPA) 2003;2(1):45–49. [PMC free article] [PubMed] [Google Scholar]
- Johnstone EC, Ebmeier KP, Miller P, Owens DGC, Lawrie SM. Predicting schizophrenia: findings from the Edinburgh High-Risk Study. Br J Psychiatry. 2005;186:18–25. doi: 10.1192/bjp.186.1.18. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Montrose DM, Stanley JA, Pettegrew JW. Premorbid characterization in schizophrenia: the Pittsburgh High Risk Study. World Psychiatry : Official Journal of the World Psychiatric Association (WPA) 2004;3(3):163–168. [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Kulkarni S, Bhojraj T, Francis A, Diwadkar V, Montrose DM, et al. Premorbid cognitive deficits in young relatives of schizophrenia patients. Front Hum Neurosci. 2010;3:62. doi: 10.3389/neuro.09.062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan M, Montrose DM, Rajarethinam R, Diwadkar V, Prasad K, Sweeney JA. Psychopathology among offspring of parents with schizophrenia: relationship to premorbid impairments. Schizophrenia Research. 2008;103(1–3):114–120. doi: 10.1016/j.schres.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorders: A review of recent literature. Curr Opin Psychiatry. 2007;20(4):359–364. doi: 10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni P, Kimhy D, Khan S, Posner K, Maayan L, Eilenberg M, Messinger J, Kestenbaum C, Corcoran C. Childhood onset diagnoses in a case series of teens at clinical high risk for psychosis. Journal of Child and Adolescent Psychopharmacology. 2009;19(6):771–6. doi: 10.1089/cap.2008.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGorry P, Nelson B. Why We Need a Transdiagnostic Staging Approach to Emerging Psychopathology, Early Diagnosis, and Treatment. JAMA Psychiatry (Chicago, Ill) 2016:1–2. doi: 10.1001/jamapsychiatry.2015.2868. [DOI] [PubMed]
- Nelson B, Yuen HP, Wood SJ, Lin A, Spilotacopoulos D, Bruxner A, et al. Long-term follow-up of a group at ultra high-risk (“prodromal”) for psychosis: the PACE 400 study. JAMA Psychiatry. 2013;70(8):793–802. doi: 10.1001/jamapsychiatry.2013.1270. [DOI] [PubMed] [Google Scholar]
- Padmanabhan JL, Shah JL, Tandon N, Keshavan MS. The “polyenvironmental risk score”: Aggregating environmental risk factors predicts conversion to psychosis in familial high-risk subjects. Schizophrenia Research. 2017;181:17–22. doi: 10.1016/j.schres.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope MA, Joober R, Malla AK. Diagnostic stability of first-episode psychotic disorders and persistence of comorbid psychiatric disorders over 1 year. Can J Psychiatry. 2013;58(10):588–594. doi: 10.1177/070674371305801008. [DOI] [PubMed] [Google Scholar]
- Rajarethinam R, Sahni S, Rosenberg DR, Keshavan MS. Reduced superior temporal gyrus volume in young offspring of patients with schizophrenia. Am J Psychiatry. 2004;161(6):1121–4. doi: 10.1176/appi.ajp.161.6.1121. [DOI] [PubMed] [Google Scholar]
- Rasic D, Hajek T, Alda M, Uher R. Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta-analysis of family high-risk studies. Schizophrenia Bulletin. 2014;40(1):28–38. doi: 10.1093/schbul/sbt114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrmann S, Schultze-Lutter F, Salokangas RK, Heinimaa M, Linszen D, Dingemans P, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the prospective European prediction of psychosis study. Arch Gen Psychiatry. 2010;67(3):241–251. doi: 10.1001/archgenpsychiatry.2009.206. [DOI] [PubMed] [Google Scholar]
- Shah J, Eack SM, Montrose DM, Tandon N, Miewald JM, Prasad KM, et al. Multivariate prediction of emerging psychosis in adolescents at high risk for schizophrenia. Schizophrenia Research. 2012;141:189–196. doi: 10.1016/j.schres.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah JL, Tandon N, Keshavan MS. Psychosis prediction and clinical utility in familial high-risk studies: selective review, synthesis, and implications for early detection and intervention. Early Intervention in Psychiatry. 2013;7(4):345–360. doi: 10.1111/eip.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AE, Velthorst E, Nieman DH, Linszen D, Umbricht D, de Haan L. Ultra high-risk state for psychosis and non-transition: a systematic review. Schizophrenia Research. 2011;132(1):8–17. doi: 10.1016/j.schres.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Sullivan PF. The genetics of schizophrenia. PLoS Medicine. 2005;2(7):e212. doi: 10.1371/journal.pmed.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon N, Bolo NR, Sanghavi K, Mathew IT, Francis AN, Stanley JA, Keshavan MS. Brain metabolite alterations in young adults at familial high risk for schizophrenia using proton magnetic resonance spectroscopy. Schizophrenia Research. 2013;148(1–3):59–66. doi: 10.1016/j.schres.2013.05.024. [DOI] [PubMed] [Google Scholar]
- Tandon N, Shah J, Keshavan MS, Tandon R. Attenuated psychosis and the schizophrenia prodrome: current status of risk identification and psychosis prevention. Neuropsychiatry. 2012a;2(4):345–353. doi: 10.2217/npy.12.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon N, Montrose D, Shah J, Rajarethinam RP, Diwadkar VA, Keshavan MS. Early prodromal symptoms can predict future psychosis in familial high-risk youth. Journal of Psychiatric Research. 2012b;46:105–110. doi: 10.1016/j.jpsychires.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thermenos HW, Keshavan MS, Juelich RJ, Molokotos E, Whitfield-Gabrieli S, Brent BK, Makris N, Seidman LJ. A review of neuroimaging studies of young relatives of individuals with schizophrenia: a developmental perspective from schizotaxia to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2013;162B(7):604–35. doi: 10.1002/ajmg.b.32170. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale -- revised. New York: Psychological Corp; 1981. [Google Scholar]