Abstract
Transfer of antibiotic resistance genes from probiotic bacteria to pathogens poses a safety concern. Orally administered probiotics are exposed to stressful conditions during gastrointestinal transit. In this study, filter mating experiments were performed to investigate the potential role of exposure of Bifidobacterium isolates to acid and bile stress on the transfer of a tetracycline resistance gene, tet(W), to Enterococcus faecalis ATCC 51299. No E. faecalis transconjugants were obtained after mating with either stressed or unstressed Bifidobacterium, thereby suggesting that tet(W) could not be transferred as a result of exposure to gastrointestinal stresses.
Keywords: Bifidobacterium, gastrointestinal stress, probiotics, tet(W), antimicrobial resistance
Bifidobacteria are commonly incorporated into foods as probiotics because of their associated health benefits [1]. Intrinsic resistance to antibiotics is desirable in probiotics when they are co-administered with antibiotics for the prevention of antibiotic-associated diarrhea. However, it becomes a safety issue if there is a risk of horizontal transfer of resistance genes to pathogens [2]. The addition of probiotic bacteria to various products makes them a potential source for the spread of antibiotic resistance genes [3].
Tetracycline resistance is common in bifidobacteria due to tet genes, especially tet(W), located on the bacterial chromosome [4]. Although integrated in the chromosome, the tet(W) gene of Bifidobacterium may be surrounded by genes coding for transposases that catalyze gene movement. This may suggest that tet(W) can be transferred to other bacteria under certain conditions. However, there is currently no evidence to demonstrate this [5].
Orally administered probiotic bifidobacteria encounter stressful conditions in the gastrointestinal tract, such as acid in the stomach and bile in the small intestine [6]. Such conditions may modify their physiological properties, leading to various consequences, which may include antibiotic resistance gene transfer [7]. Therefore, the aim of this study was to investigate the effect of exposure to acid and bile stresses on the transmissibility of tet(W) from Bifidobacterium isolates to Enterococcus faecalis ATCC 51299.
Products containing Bifidobacterium were purchased from stores in Coventry, UK (Table 1). In addition, a freeze-dried commercial culture, Nu-Trish Bifidobacterium BB-12, was kindly provided by Chr. Hansen (Berkshire, UK). Bifidobacteria were isolated on Reinforced Clostridial Agar (RCA) (CM0151, Oxoid, Hampshire, UK) containing 50 mg/l of lithium mupirocin (69732, Sigma-Aldrich, Switzerland) (RCA-MUP). Cultures of Escherichia coli K12 and E. faecalis ATCC 51299 were provided by Coventry University. Prior to experiments, cultures of Bifidobacterium were prepared on RCA incubated under anaerobic conditions (10% CO2, 10% H2, 80% N2) in an anaerobic workstation (Don Whitley Scientific, Shipley, UK) for 48 hr at 37°C. Escherichia coli K12 and E. faecalis ATCC 51299 were prepared on nutrient agar (CM0309, Oxoid) incubated under aerobic conditions for 24 hr at 37°C.
Table 1. Sources of bifidobacteria used in this study.
| Isolate code | Product type | Bifidobacterium description on product |
|---|---|---|
| 1 | Yogurt | B. longum |
| 2 | Yogurt | B. lactis |
| 3 | Yogurt | Bifidobacterium |
| 4 | Yogurt | Bifidobacterium |
| 5 | Capsule | B. animalis ssp. lactis |
| 6 | Freeze-dried BB-12 | B. animalis ssp. lactis |
The minimum inhibitory concentration (MIC) of tetracycline for each culture was determined by broth microdilution. Inocula were prepared by suspending colonies obtained from each solid medium in normal saline (8.5 g/l NaCl). A stock solution of tetracycline (tetracycline hydrochloride, T-3383, Sigma) at a concentration of 1,280 µg/ml was prepared in sterile distilled water. From this stock, a twofold dilution series (128–0.25 µg/ml) of tetracycline in 150 µl of Reinforced Clostridial Medium (RCM, CM0149, Oxoid) or Iso-Sensitest Broth (ISB, CM0473, Oxoid) was prepared in round-bottomed 96-well microtiter plates for Bifidobacterium and E. faecalis 51299/E. coli K12, respectively. Wells were inoculated with 10 µl of bacterial suspension. Negative controls were 150 µl of uninoculated RCM or ISB, and positive controls were inoculated broths without tetracycline. Plates were incubated with lids under appropriate conditions for each culture. The MIC was identified as the lowest antibiotic concentration at which no growth (turbidity) was observed. The bacteria were classified as resistant or susceptible to tetracycline based on defined break points [8].
Bifidobacterium isolates were phenotypically characterized by Gram staining and catalase reaction. All cultures were genotypically characterized by a polymerase chain reaction based on 16S-23S rDNA internal transcribed spacer region (ITS-PCR), according to the method described by Xu et al. [9], with modifications. Extraction of genomic DNA was carried out using InstaGene Matrix (7326030, Bio-Rad, Hertfordshire, UK) according to the manufacturer’s instructions.
The 25 µl reaction mixture contained 2.5 µl of 10x PCR buffer including 20 mM MgCl2 (B9004S, New England Biolabs, Hertfordshire, UK), 4 µl of 1.25 mM dNTP mix (N0447, New England Biolabs), 0.1 µl of 5U Taq DNA polymerase (M0267X, New England Biolabs), 1 µl each of 10 pmol/µl forward and reverse primer (Table 2), 15.4 µl of sterile Milli-Q water, and 1 µl of DNA template. PCR amplification was carried out in an Eppendorf Master Cycler (22331, Eppendorf, Germany) using the following temperature program: initial denaturation (1 min, 94°C) followed by 35 cycles of denaturation (1 min, 94°C), annealing (1 min, 55°C), and elongation (1 min, 72°C) and then final extension (7 min, 72°C).
Table 2. Primer sequences used in this study.
| Gene | Forward primer | Reverse primer | Annealing temperature (°C) |
|---|---|---|---|
| 16S-23S rDNA ITS | GTCGTAACAAGGTAGCCGTA | CAAGGCATCCACCGT | 55 |
| tet(W) | TGGAATTCTTGCCCATGTAGACG | GAACATATGGCGCACCTTGTCC | 64 |
| trp-tet(W)* | ATTCAGCGACGAACTGGCACAG | CGCTTGAATGGTAATCCCACG | 63 |
*Primers amplify trp gene with 5′ end of tet(W).
Cultures were then screened for the presence of tet(W) and trp coding for the tetracycline resistance gene and the flanking transposase gene, respectively, by a PCR using primers in Table 2 [4], according to the method described by Masco et al. [10], with modifications. The 50 µl PCR assay mix contained 32.8 µl of sterile Milli-Q water, 1 µl of 1.25 mM dNTP mix (N0447, New England Biolabs), 5 µL of 10x PCR buffer including 20 mM MgCl2 (B9004S, New England Biolabs), 3 µl each of 10 pmol/µl forward and reverse primer (Table 2), 0.2 µl of 5U Taq DNA polymerase (M0267X, New England Biolabs), and 5 µl of DNA template. PCR amplification was carried out using the following temperature program: initial denaturation (5 min, 95°C) followed by 25 cycles of denaturation (45 sec, 94°C), annealing (1 min, at primer-specific temperature [Table 2]), and elongation (1 min, 72°C) and then final extension (10 min, 72°C).
All PCR amplicons were visualized by electrophoresis on 1% (1.2% for ITS-PCR) agarose gels containing 2 µl GelRed stain (41003, Biotium, Fremont, CA, USA); wells were loaded with 10 µl of PCR product + 2 µl of loading dye (R0611, Thermo Fisher Scientific, Loughborough, UK). The gels were run in 1x Tris-Borate-EDTA (TBE) buffer for 1 hr at 120 V. The sizes of the PCR products were estimated using DNA molecular size markers (SM1113, Thermo Fisher Scientific; N3234, New England Biolabs). Gel images were taken under UV light in a UV transilluminator (Gel Doc EZ Imager, Bio-Rad).
Stress conditions for three Bifidobacterium isolates (isolates 1, 2, and 6) were determined according to the method described by Amund et al. [7], with modifications. RCM was acidified with 1 M hydrochloric acid (HCl) to pH values of 3 and 4, supplemented with bovine bile (B3883, Sigma-Aldrich) to 0.5% and 1% (w/v), or unadjusted as the control. Suspensions of 48-hr cultures in normal saline (approximately 108 cfu/ml) were prepared, from which 1 ml volumes were inoculated into 9 ml each of RCM and incubated anaerobically at 37°C. Enumeration on RCA was carried out immediately after inoculation (Time 0) and at predetermined intervals (1, 2, 3, and 24 hr). Stressful conditions were determined as those where there was no growth after 24 hr of incubation. Whereas unadjusted controls showed growth after 24 hr, adjusted RCM (acid, bile) resulted in a decline or no growth after 24 hr. Furthermore, after 1 hr of incubation, there was no significant change in numbers observed in any of the stress treatments (results not shown). Therefore, the conditions chosen to induce stress in the bifidobacteria for the conjugation experiments were pH 3 and 1% (w/v) bovine bile for 1 hr.
Fresh colonies of donor bacteria (Bifidobacterium) were suspended in 5 ml of RCM that had been acidified to pH 3 with 1 M HCl, supplemented with 1% (w/v) bovine bile, or unadjusted, until a turbid suspension was reached. Suspensions were incubated anaerobically at 37°C for 1 hr in order to achieve acid-stressed, bile-stressed, and unstressed (control) treatments, respectively [7]. After incubation, cells were harvested by centrifugation at 4,400 rpm for 5 min. The supernatants were discarded, and the remaining pellets were resuspended in normal saline to achieve a turbidity equivalent to a 2 McFarland standard (approximately 6 × 108 cfu/ml).
Conjugation experiments were carried out between the bifidobacteria and E. faecalis ATCC 51299 by the filter mating method described by Ouoba et al. [11], with modifications. Fresh colonies of recipient bacteria (E. faecalis ATCC 51299) were suspended in normal saline until a similar turbidity to the donor suspensions was reached (2 McFarland standard). Subsequently, a 1:10 dilution of the recipient suspension was made (approximately 107 cfu/ml). One milliliter of each donor suspension was mixed with 1 ml of diluted recipient suspension (thereby achieving an approximate donor-recipient ratio of 10:1). The mixtures were each filtered through a membrane filter (pore size 0.45 µm, diameter 47 mm) (63069, GN-6 Metricel Membrane, Pall Life Sciences, Portsmouth, UK), using a filter holder and vacuum pump. To trap the bacteria in the membrane, the filters were washed with 2 ml of normal saline. Finally, the filters with donor and recipient bacteria were placed on Brain Heart Infusion (BHI) agar (CM1135, Oxoid) and incubated overnight under aerobic conditions at 37°C. Afterwards, each filter was transferred into a 15 ml tube containing 5 ml of normal saline and vortexed to resuspend bacteria. To detect potential E. faecalis transconjugants, 100 µl of suspension was spread on MacConkey agar (CM0007, Oxoid) supplemented with 10 µg/ml of tetracycline and incubated under aerobic conditions. Suspensions were also plated on RCA-MUP and MacConkey agar to confirm viability of donors and recipients (results not shown).
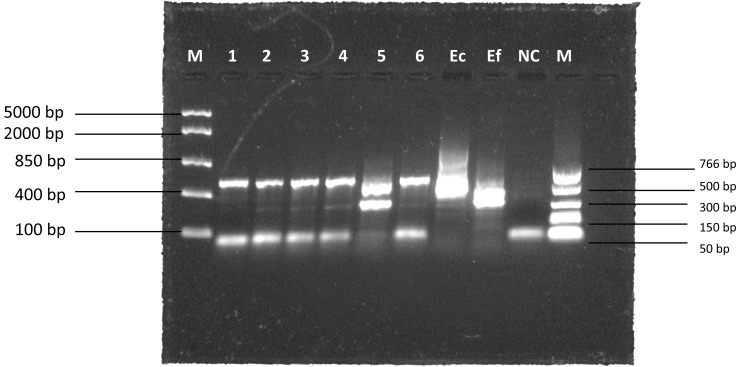
All Bifidobacterium isolates were phenotypically characterized as Gram-positive, catalase-negative rods. Genotypic characterization by ITS-PCR showed all isolates except one exhibited similar banding patterns, with a primary band around 500 bp in size (Fig. 1).
Fig. 1.
ITS-PCR banding patterns. M: marker; Ec: E. coli K12; Ef: E. faecalis ATCC 51299; NC: negative control.
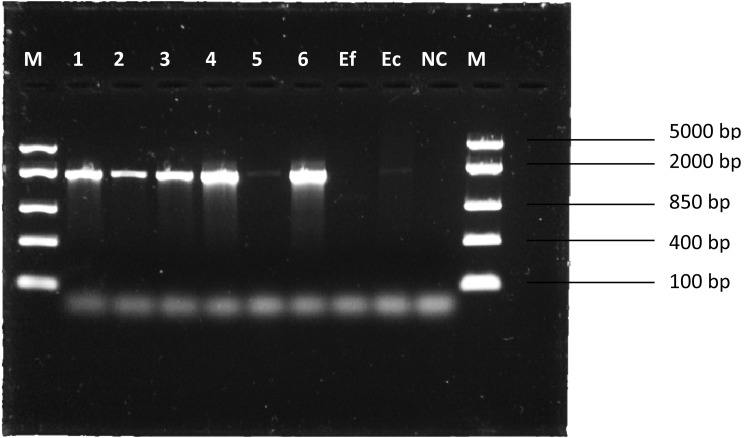
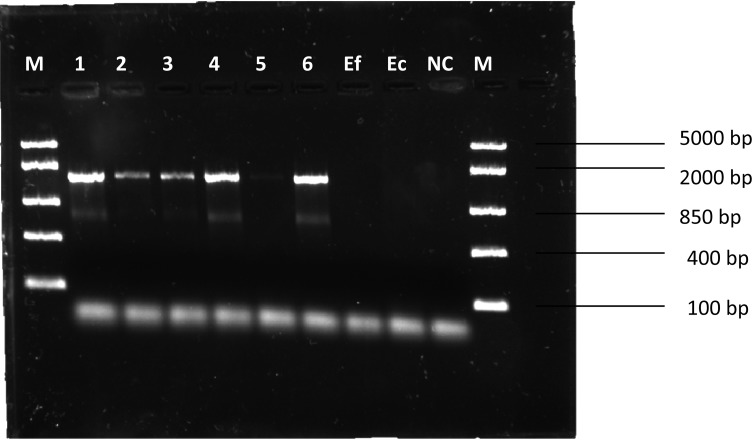
Bifidobacterium isolates were confirmed to be resistant to tetracycline (Table 3), and tet(W) and trp were detected (Fig. 2 and Fig. 3). PCR amplicons had sizes of approximately 1,888 bp and 1,474 bp, respectively, which is consistent with Gueimonde et al. [4]. E. coli K12 was found to be sensitive to tetracycline (Table 3) but positive for tet(W) (Fig. 2). E. faecalis ATCC 51299 was found to be sensitive to tetracycline (Table 3) and lacked tet(W) (Fig. 2). E. coli K12 was therefore excluded from subsequent conjugation experiments due to the detection of tet(W).
Table 3. Tetracycline MICs and detection of tet(W) and trp in Bifidobacterium isolates and recipient bacteria.
| Bacteria | Break point (µg/ml) [8] | MIC (µg/ml) | R or S* | tet(W) | trp |
|---|---|---|---|---|---|
| 1 | 8 | 32 | R | + | + |
| 2 | 8 | 16 | R | + | + |
| 3 | 8 | 16 | R | + | + |
| 4 | 8 | 16 | R | + | + |
| 5 | 8 | >128 | R | + | + |
| 6 | 8 | 32 | R | + | + |
| E. coli K12 | 8 | 4 | S | + | – |
| E. faecalis ATCC 51299 | 4 | 0.5 | S | – | – |
*R: resistant; S: susceptible.
Fig. 2.
PCR amplicons of tet(W). M: marker; Ef: E. faecalis ATCC 51299; Ec: E. coli K12; NC: negative control.
Fig. 3.
PCR amplicons of trp-tet(W). M: marker; Ef: E. faecalis ATCC 51299; Ec: E. coli K12; NC: negative control.
Filter mating of E. faecalis ATCC 51299 with stressed or unstressed Bifidobacterium isolates resulted in no tetracycline resistant transconjugants, thereby suggesting that exposure to gastrointestinal stress conditions such as acid and bile may not lead to mobilization and subsequent transfer of the tet(W) gene to other enteric bacteria.
Sublethal food preservation stress conditions (pH, osmotic, high/low temperature) have been demonstrated to significantly increase plasmid transmission rates between plasmid-bearing E. coli donor cultures and recipient E. coli and Salmonella Typhimurium strains in vitro [12]. Transposon movement and transposition in E. coli has also been shown to be influenced by nutritional stress [13]. The aforementioned may justify the need to evaluate candidate probiotic bacteria for not just the presence and transferability of antibiotic resistance genes but also the presence and transferability of antibiotic resistance genes with the added consideration of environmental stress, including gastrointestinal stress.
Based on the ITS-PCR banding patterns and the Bifidobacterium BB-12 commercial culture as the reference strain, the Bifidobacterium isolates (donors) in the experiments could be considered to be B. animalis ssp. lactis. While low frequency transfer of tet(W) between Bifidobacterium strains has been demonstrated in vitro [14], to our knowledge, no studies have been able to demonstrate transfer of tet(W) from B. animalis ssp. lactis to other bacteria or other bifidobacteria. Strains of B. animalis ssp. lactis are the most commonly used bifidobacteria commercially [15] and are considered to be safe [16].
It should be noted that there appeared to be some instances of mismatches between the ITS-PCR banding pattern and the Bifidobacterium description on the product label (i.e., isolates 1 and 5) (Fig. 1). Such findings have been reported in other studies on probiotic products [1, 15, 17] and have been suggested to be due to misidentification or undeclared bacterial cultures. Correct identification and labelling are crucial in ensuring safety and functionality [15].
It has been highlighted in previous research that many in vitro experimental conditions do not fully represent the complex conditions of the gut, and the impact of these conditions on the transfer of antibiotic resistance is still unclear [4]. Furthermore, standardized conjugation protocols for in vitro experiments with bifidobacteria have yet to be developed [18]. To our knowledge, this report represents the first attempt at examining the relationship between gastrointestinal conditions and antibiotic resistance gene transfer by bifidobacteria. Further investigations should examine a wider range of Bifidobacterium strains and recipient bacteria and include variations in incubation conditions (aerobic, anaerobic) and donor-recipient ratios, among others.
References
- 1.Jayamanne VS, Adams MR. 2006. Determination of survival, identity and stress resistance of probiotic bifidobacteria in bio-yoghurts. Lett Appl Microbiol 42: 189–194. [DOI] [PubMed] [Google Scholar]
- 2.Amund OD. 2016. Exploring the relationship between exposure to technological and gastrointestinal stress and probiotic functional properties of lactobacilli and bifidobacteria. Can J Microbiol 62: 715–725. [DOI] [PubMed] [Google Scholar]
- 3.D’Aimmo MR, Modesto M, Biavati B. 2007. Antibiotic resistance of lactic acid bacteria and Bifidobacterium spp. isolated from dairy and pharmaceutical products. Int J Food Microbiol 115: 35–42. [DOI] [PubMed] [Google Scholar]
- 4.Gueimonde M, Flórez AB, van Hoek AHAM, Stuer-Lauridsen B, Strøman P, de los Reyes-Gavilán CG, Margolles A. 2010. Genetic basis of tetracycline resistance in Bifidobacterium animalis subsp. lactis. Appl Environ Microbiol 76: 3364–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gueimonde M, Sánchez B, G de los Reyes-Gavilán CG, Margolles A. 2013. Antibiotic resistance in probiotic bacteria. Front Microbiol 4: 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Dea Lindner J, Canchaya C, Zhang Z, Neviani E, Fitzgerald GF, van Sinderen D, Ventura M. 2007. Exploiting Bifidobacterium genomes: the molecular basis of stress response. Int J Food Microbiol 120: 13–24. [DOI] [PubMed] [Google Scholar]
- 7.Amund OD, Ouoba LII, Sutherland JP, Ghoddusi HB. 2014. Assessing the effects of exposure to environmental stress on some functional properties of Bifidobacterium animalis ssp. lactis. Benef Microbes 5: 461–469. [DOI] [PubMed] [Google Scholar]
- 8.European Food Safety Authority (EFSA).2012. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J 10: 2740. [Google Scholar]
- 9.Xu YZ, Anyogu A, Ouoba LII, Sutherland JP. 2010. Genotypic characterization of Brochothrix spp. isolated from meat, poultry and fish. Lett Appl Microbiol 51: 245–251. [DOI] [PubMed] [Google Scholar]
- 10.Masco L, Van Hoorde K, De Brandt E, Swings J, Huys G. 2006. Antimicrobial susceptibility of Bifidobacterium strains from humans, animals and probiotic products. J Antimicrob Chemother 58: 85–94. [DOI] [PubMed] [Google Scholar]
- 11.Ouoba LII, Lei V, Jensen LB. 2008. Resistance of potential probiotic lactic acid bacteria and bifidobacteria of African and European origin to antimicrobials: determination and transferability of the resistance genes to other bacteria. Int J Food Microbiol 121: 217–224. [DOI] [PubMed] [Google Scholar]
- 12.Mc Mahon MAS, Blair IS, Moore JE, Mc Dowell DA. 2007. The rate of horizontal transmission of antibiotic resistance plasmids is increased in food preservation-stressed bacteria. J Appl Microbiol 103: 1883–1888. [DOI] [PubMed] [Google Scholar]
- 13.Twiss E, Coros AM, Tavakoli NP, Derbyshire KM. 2005. Transposition is modulated by a diverse set of host factors in Escherichia coli and is stimulated by nutritional stress. Mol Microbiol 57: 1593–1607. [DOI] [PubMed] [Google Scholar]
- 14.Kazimierczak KA, Flint HJ, Scott KP. 2006. Comparative analysis of sequences flanking tet(W) resistance genes in multiple species of gut bacteria. Antimicrob Agents Chemother 50: 2632–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raeisi SN, Ouoba LII, Farahmand N, Sutherland J, Ghoddusi HB. 2013. Variation, viability and validity of bifidobacteria in fermented milk products. Food Control 34: 691–697. [Google Scholar]
- 16.European Food Safety Authority (EFSA).2011. Scientific opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed (2011 update). EFSA J 9: 2497. [Google Scholar]
- 17.Gueimonde M, Delgado S, Mayo B, Ruas-Madiedo P, Margolles A, de los Reyes-Gavilan CG. 2004. Viability and diversity of probiotic Lactobacillus and Bifidobacterium populations included in commercial fermented milks. Food Res Int 37: 839–850. [Google Scholar]
- 18.Huys G, Botteldoorn N, Delvigne F, De Vuyst L, Heyndrickx M, Pot B, Dubois JJ, Daube G. 2013. Microbial characterization of probiotics--advisory report of the Working Group “8651 Probiotics” of the Belgian Superior Health Council (SHC). Mol Nutr Food Res 57: 1479–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]