Abstract
Japanese inpatients with schizophrenia have a higher mortality risk due to underweight compared with the general population. The aim of this study was to investigate the effect of 4G-β-D-galactosylsucrose on body weight in underweight schizophrenia inpatients. The study population consisted of 5 male and 11 female subjects aged 63.0 ± 10.9 years. The subjects had ingested 3.0 g/day 4G-β-D-galactosylsucrose for 6 months. BMI increased significantly, from 20.9 ± 3.7 kg/m2 to 22.3 ± 4.3 kg/m2, and this was accompanied by a significant increase in Bifidobacterium in the fecal microbiota, which increased from 16.1 ± 12.6% to 21.5 ± 13.9%. Although 4G-β-D-galactosylsucrose appears to have no significant effects on nutritional indicators such as serum albumin, it may alleviate underweight in inpatients with schizophrenia. Body weight may be related to fecal microbiota composition.
Keywords: underweight, schizophrenia, 4G-β-D-galactosylsucrose, Bifidobacterium
Many studies have reported a high prevalence of obesity in patients with schizophrenia compared with the general population [1]. However, underweight and obesity are characteristics of Japanese schizophrenia inpatients [2]. In schizophrenia patients, underweight is accompanied by physical complications such as pneumonia and is associated with long-term hospitalization [3]. Japanese inpatients with schizophrenia have a higher mortality risk due to underweight compared with the general population [4]. The aim of this study was to investigate the efficacy and tolerability of 4G-β-D-galactosylsucrose and its effect on body weight in underweight Japanese schizophrenia inpatients. The ethics committee of Sunlight Brain Research Center and Osaka Institute of Psychiatry Shin-Abuyama Hospital approved this study, and written informed consent was obtained from all subjects.
The study population consisted of 5 male and 11 female subjects with the following characteristics: age, 63.0 ± 10.9 years; body mass index (BMI), 20.9 ± 3.7 kg/m2; length of hospital stay, 3,053 ± 2,805 days; and antipsychotic chlorpromazine equivalent dose, 729.7 ± 510.2 mg. The subjects ingested 3.0 g/day 4G-β-D-galactosylsucrose as a Food for Specified Health Use (FOSHU) beverage manufactured by H+B Life Science with their medication and dietary composition unchanged. The study period was 6 months. We examined BMI, fasting blood glucose levels, serum triglyceride levels, total cholesterol levels, serum albumin levels, psychotic symptoms, adverse reactions, and fecal microbiota composition before and 6 months after the initiation of 4G-β-D-galactosylsucrose. Psychotic symptoms were assessed using the Brief Psychiatric Rating Scale (BPRS), which assesses the levels of 18 symptom constructs, such as hostility, suspiciousness, hallucination, and grandiosity [5]. Adverse reactions such as fever, abdominal pain, constipation, and diarrhea were carefully observed during the study period. The fecal microbiota was analyzed by using terminal restriction fragment length polymorphism (T-RFLP) at Techno Suruga Lab. The 16S rRNA gene was amplified from fecal bacterial DNA of the patients by using the fluorescently labeled 516f (5′-(6-FAM)-TGCCAGCAGCCGCGGTA-3′), and 1492r (5′-GGTTACCTTGTTACGACTT-3′) primers. The amplified DNA was purified and verified by electrophoresis. The restriction enzymes were selected according to Nagashima et al. [6]. The resultant DNA fragments, namely, fluorescent-labeled terminal restriction fragments (T-RFs), were analyzed by next-generation sequencing genetic analyzer, and their lengths and peaks area were determined by using the GeneMapper genotyping software. The T-RFs were divided into 29 operational taxonomic units (OTUs). The bacteria were predicted for each classification unit, and the corresponding OTU was identified according to reference human fecal microbiota profiling. The statistical analysis was performed using the Wilcoxon signed-rank test, and differences were considered statistically significant at p<0.05.
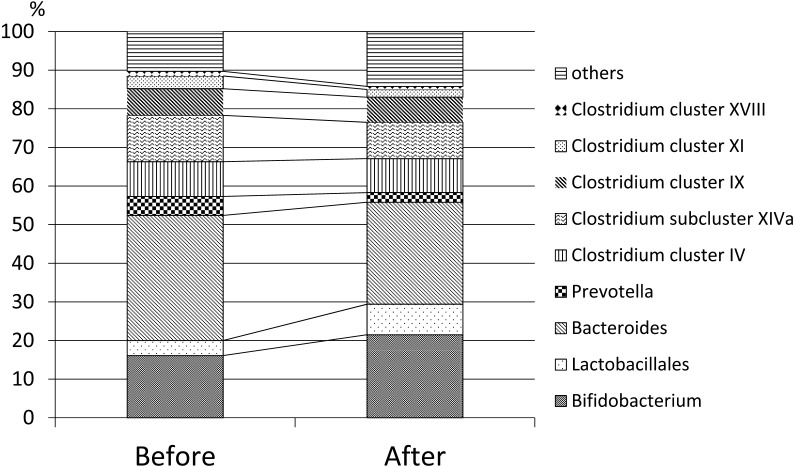
The obtained results were as follows: (1) Weight and BMI increased significantly from 51.5 ± 9.1 kg to 55.2 ± 4.3 kg and from 20.9 ± 3.7 kg/m2 to 22.3 ± 4.3 kg/m2 (p=0.01), respectively, whereas there was no significant change in blood glucose and triglyceride levels (Table 1). (2) The fecal microbiota composition was significantly changed after 6 months of treatment with 4G-β-D-galactosylsucrose. From the compositional standpoint, Bifidobacterium increased significantly (from 16.1 ± 12.6% to 21.5 ± 13.9%; p=0.007), and Clostridium subcluster XIVa decreased significantly (from 12.0 ± 4.1% to 9.4 ± 3.9%; p=0.01). Bacteroides decreased from 32.4 ± 13.6% to 26.4 ± 11.4%, but it did not reach the level of statistical significance (p=0.06). Figure 1 shows the changes in fecal microbiota composition between before and 6 months after 4G-β-D-galactosylsucrose ingestion. (3) 4G-β-D-galactosylsucrose did not result in any observed time-sequential changes in BPRS scores statistically (Table 1), although one patient showed a marked improvement of psychotic symptoms such as hallucination. (4) The most common adverse events of 4G-β-D-galactosylsucrose included mild abdominal discomfort in 4 of the 16 patients, and soft stool in 3 of the 16 patients, however, most of the adverse events were mild in degree and did not differ significantly from those observed in previous clinical studies [7].
Table 1. Body weights, blood examinations, and psychotic symptom scores before and after 6 months of 4G-β-D-galactosylsucrose ingestion.
| Before | After | p-value | |
|---|---|---|---|
| Body weight (kg) | 51.5 ± 9.1 | 55.2 ± 4.3 | 0.01 |
| BMI (kg/m2) | 20.9 ± 3.7 | 22.3 ± 4.3 | 0.01 |
| Blood glucose (mg/dl) | 76.3 ± 8.0 | 72.1 ± 19.0 | 0.43 |
| Triglyceride (mg/dl) | 92.1 ± 42.6 | 82.5 ± 37.4 | 0.54 |
| Total cholesterol (mg/dl) | 160.7 ± 70.3 | 183.9 ± 40.8 | 0.35 |
| Serum albumin (g/dl) | 3.8 ± 0.2 | 3.8 ± 0.3 | 0.83 |
| BPRS | 42.1 ± 8.6 | 38.8 ± 7.9 | 0.41 |
Fig. 1.
Changes in fecal microbiota composition before and after 6 months of 4G-β-D-galactosylsucrose ingestion.
The important finding of our study is that 4G-β-D-galactosylsucrose was shown to have a weight gain effect in underweight schizophrenia inpatients that was accompanied by a bifidobacteria-enhancing effect. It is reported that 4G-β-D-galactosylsucrose has a weight-reducing effect, not a weight-gain effect. In an oral triolein tolerance test on rats, 4G-β-D-galactosylsucrose reduces adipose tissue accumulation by inhibiting intestinal lipid absorption, resulting in significantly decreased weight [8]. This mechanism may work via a direct interaction of 4G-β-D-galactosylsucrose with triglyceride, because nuclear magnetic resonance spectroscopy (NMR spectroscopy) showed that 4G-β-D-galactosylsucrose formed an intermolecular complex with triolein [8]. However, triglyceride and total cholesterol levels in our study did not change, and body weight increased significantly. Thus, the effect of 4G-β-D-galactosylsucrose on body weight in underweight schizophrenia inpatients may not be associated with lipid metabolism. In chronic schizophrenia patients, dysbiosis of the gut microbiota, injury of the intestinal mucosal barrier, and bacterial translocation occurs as underweight progresses, which might enhance inflammation and aggravate underweight, resulting in worsening of psychotic symptoms [9]. 4G-β-D-galactosylsucrose passes undigested into the large intestine and stimulates bacterial fermentation in the colon. Therefore, it is able to modify the gut microbiota composition in terms of colonic levels, such as by increasing Bifidobacterium, which might alleviate gut permeability and improve immune function [10].
Although this study has limitations such as a small sample size and no control group, it indicates that the change in microbiota composition might have influenced body weight in underweight schizophrenia inpatients. Body weight may be related to fecal microbiota composition. The human gastrointestinal tract is colonized by a total of 1014 bacterial cells of 400 to 1,000 species to form a gut microbiota that has an important influence on the nutritional and health status of the host [11]. The diverse microbial communities that colonize the colon are thought to be intimately related to aspects of physiology and the pathology of human health [12]. To our knowledge, this is the first report evaluating fecal microbiota composition by using T-RFLP in underweight schizophrenia inpatients. Further studies are needed to elucidate the details of how 4G-β-D-galactosylsucrose increases body weight in underweight schizophrenia inpatients, especially the relationship between microbiota composition and gut permeability.
Disclosure Statement
Drs Nagamine, Ido, Nakamura, and Okamura contributed to the concept/design. Drs Nagamine and Ido contributed to the data acquisition and the analysis/data interpretation. Drs Nagamine, Ido, and Nakamura drafted this article. No funding was received for the preparation of this article. The authors have no conflicts of interest relevant to the content of the article. This content has not been published or submitted for publication elsewhere.
References
- 1.Manu P, Dima L, Shulman M, Vancampfort D, De Hert M, Correll CU. 2015. Weight gain and obesity in schizophrenia: epidemiology, pathobiology, and management. Acta Psychiatr Scand 132: 97–108. [DOI] [PubMed] [Google Scholar]
- 2.Inamura Y, Sagae T, Nakamachi K, Murayama N. 2012. Body mass index of inpatients with schizophrenia in Japan. Int J Psychiatry Med 44: 171–181. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki Y, Sugai T, Fukui N, Watanabe J, Ono S, Tsuneyama N, Saito M, Someya T. 2014. High prevalence of underweight and undernutrition in Japanese inpatients with schizophrenia. Psychiatry Clin Neurosci 68: 78–82. [DOI] [PubMed] [Google Scholar]
- 4.Sugai T, Suzuki Y, Yamazaki M, Shimoda K, Mori T, Ozeki Y, Matsuda H, Sugawara N, Yasui-Furukori N, Minami Y, Okamoto K, Sagae T, Someya T. 2015. High prevalence of underweight and undernutrition in Japanese inpatients with schizophrenia: a nationwide survey. BMJ Open 5: e008720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overall JE, Gorham DR. 1962. The brief psychiatric rating scale. Psychol Rep 10: 799–812. [Google Scholar]
- 6.Nagashima K, Hisada T, Sato M, Mochizuki J. 2003. Application of new primer-enzyme combinations to terminal restriction fragment length polymorphism profiling of bacterial populations in human feces. Appl Environ Microbiol 69: 1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goetze O, Fruehauf H, Pohl D, Giarrè M, Rochat F, Ornstein K, Menne D, Fried M, Thumshirn M. 2008. Effect of a prebiotic mixture on intestinal comfort and general wellbeing in health. Br J Nutr 100: 1077–1085. [DOI] [PubMed] [Google Scholar]
- 8.Mizote A, Taniguchi Y, Takei Y, Koya-Miyata S, Kohno K, Iwaki K, Kurose M, Oku K, Chaen H, Fukuda S. 2009. Lactosucrose inhibits body fat accumulation in rats by decreasing intestinal lipid absorption. Biosci Biotechnol Biochem 73: 582–587. [DOI] [PubMed] [Google Scholar]
- 9.Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. 2016. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry 21: 738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. 2009. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58: 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. 2005. Diversity of the human intestinal microbial flora. Science 308: 1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau JT, Whelan FJ, Herath I, Lee CH, Collins SM, Bercik P, Surette MG. 2016. Capturing the diversity of the human gut microbiota through culture-enriched molecular profiling. Genome Med 8: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]