Abstract
Purpose
The National Cancer Institute is the only referral centre in Malaysia that provides 68Ga-DOTA-peptide imaging. The purpose of this study is to determine the impact of 68Ga-DOTA-peptide PET/CT on the management of gastrointestinal neuroendocrine tumours (GI-NET).
Materials and Methods
A cross-sectional study was performed to review the impact of 68Ga-DOTA-peptide (68Ga-DOTATATE or 68Ga-DOTATOC) PET/CT on patients with biopsy-proven GI-NET between January 2011 and December 2015. Suspected NET was excluded. Demographic data, tumoral characteristics, change of disease stage, pre-PET intended management and post-PET management were evaluated.
Results
Over a 5-year period, 82 studies of 68Ga-DOTA-peptide PET/CT were performed on 44 GI-NET patients. The most common primary site was the rectum (50.0%) followed by the small bowel, stomach and colon. Using WHO 2010 grading, 40.9% of patients had low-grade (G1) tumour, 22.7% intermediate (G2) and 4.5% high (G3). Of ten patients scheduled for pre-operative staging, 68Ga-DOTA-peptide PET/CT only led to therapeutic change in three patients. Furthermore, false-negative results of 68Ga-DOTA-peptide PET/CT were reported in one patient after surgical confirmation. However, therapeutic changes were seen in 20/36 patients (55.6%) scheduled for post-surgical restaging or assessment of somatostatin analogue (SSA) eligibility. When 68Ga-DOTA-peptide PET/CT was used for monitoring disease progress during systemic treatment (sandostatin, chemotherapy, everolimus and PRRT) in metastatic disease, impact on management modification was seen in 19/36 patients (52.8%), of which 84.2% had inter-modality change (switch to everolimus, chemotherapy or PRRT) and 15.8% had intra-modality change (increased SSA dosage).
Conclusions
68Ga-DOTA-peptide PET/CT has a significant impact on management decisions in GI-NET patients as it can provide additional information on occult metastasis/equivocal lesions and supply the clinician an opportunity to select patients for targeted therapy.
Keywords: 68Ga-DOTA-peptide, 68Ga-DOTA-TATE, PET/CT, Neuroendocrine tumour
Introduction
Neuroendocrine tumours (NETs) are a heterogenous group of neoplasms with characteristic features of somatostatin receptor (SSTR) over-expression [1]. As targeted somatostatin receptor-based therapy is recognised as an important component in the treatment for NETs, the ability to demonstrate and predict the response to these therapies is extremely useful [2, 3]. Hence, functional imaging, which specifically targets these receptors, plays a pivotal role in the detection, mapping, monitoring as well as treatment planning of these diseases.
68Ga-DOTA peptides are a group of PET tracers that specifically bind to SSTR [4]. Three main peptides are available: TOC, NOC and TATE [4]. The main difference among these peptides is the variable affinity to SSTR subtypes. All peptides show good affinity to SSTR 2 and 5, whereas only -NOC shows additional good binding to SSTR 3 [5]. However, there is currently no evidence of a clinical impact of these differences on receptor binding affinity, and therefore there is no indication suggesting the preferential use of one compound over the others [6].
Recently, somatostatin receptor scintigraphy (SRS), which was previously regarded as the gold standard somatostatin receptor imaging in gastro-entero-pancreatic neuroendocrine tumours (GEP-NET), has been largely replaced by 68Ga-DOTA-peptide PET/CT. This substitution is expected as 68Ga-DOTA-peptide PET/CT is found to provide simpler patient preparation and a lower radiation dose to the patient, and, most importantly, it provides superior image quality, which results in higher sensitivity in detecting GEP-NET as compared to SRS [7, 8].
As high detection rates do not necessarily translate into a change in the therapeutic approach, few studies have addressed the impact of 68Ga-DOTA-peptide PET/CT on the management of neuroendocrine tumours [9, 10]. This study aims to establish the local data on the role of 68Ga-DOTA-peptide PET/CT on the management of gastrointestinal neuroendocrine tumours (GI-NET) in the Malaysian population.
Materials and Methods
Patients
Patients with histologically proven GI-NET who underwent 68Ga-DOTA-peptide PET/CT at the National Cancer Institute between January 2011 and December 2015 were included in this study. Suspected NET based on symptoms alone without histological confirmation, cases of NET with unknown primary and pancreatic NETs were excluded from this study. Reasons for excluding pancreatic NETs were due to their distinct presentation, symptom and treatment characteristics.
The histopathological report, other investigations/imaging (i.e. ultrasound, CT, endoscopy), indication for 68Ga-DOTA-peptide PET/CT and pre-PET management strategies were examined.
Based on their indication for 68GaDOTA-peptide PET/CT, patients were categorised into three groups: pre-operative staging, post-operative restaging and monitoring of systemic treatment response. In the post-operative restaging group, patients would undergo surgical resection based on conventional imaging modalities. 68GaDOTA-peptide PET/CT was performed after the surgery for further re-staging. The reasons for such practice are the long waiting time for 68GaDOTA-peptide PET/CT appointments and that surgical resection of the primary tumour remains the mainstay of treatment even in the presence of metastatic disease.
In the first and second groups, the pre-PET disease stage, post-PET disease stage and treatment modification post-PET were analysed. The stage of the patient was determined using the American Joint Committee on Cancer (AJCC) staging system.
In the third group, PET/CT was performed to assess treatment response in patients receiving some form of systemic therapy such as cold octreotide therapy, everolimus, chemotherapy or peptide receptor radionuclide therapy. Apart from using the Response Evaluation Criteria in Solid Tumours(RECIST) 1.1, new non-physiological uptake detected on 68Ga-DOTA-peptide PET/CT was considered as disease progression in this study. Raised chromogranin A levels or worsening of symptoms were not considered to indicate disease progression in this study.
The study was approved by the local ethics committee and adhered to Good Clinical Practice guidelines.
Radiopharmaceutical Preparation
Throughout the study period, either DOTA-TOC or DOTA-TATE peptide was used. 68Ga-DOTA-peptide PET/CT service was started in June 2010 using 68Ga-DOTA-TATE as radiotracer. In October 2013, 68Ga-DOTA-TOC replaced 68Ga-DOTA-TATE. In November 2015, we switched back to 68Ga-DOTA-TATE.
Both 68Ga-DOTA-TATE and 68Ga-DOTA-TOC were prepared using similar methods. A 68Ge/68Ga generator was eluted using 0.05 M HCl. 68Ga was then mixed with a reactive vial containing 25 ug of DOTA-TATE or DOTA-TOC. Synthesis was carried out at approximately 90 °C for 10 min. Then, the reactive vial was allowed to cool to approximately 50 °C; 0.3 ml of 1 M HEPES buffer was then mixed into the reactive vial. Activity of the final product was measured and time recorded.
68Ga-DOTA-peptide PET/CT Imaging Protocol
Cold octreotide therapy was not withheld and no specific patient preparations were required prior to imaging. Activities administered were between 148 to 185 MBq (4–5 mCi). Imaging was performed 45 to 60 min post-injection of the 68Ga-DOTA-peptide. PET/CT imaging was acquired on a GE Discovery ST scanner with a PET unit and eight-slice CT unit from vertex to mid-thigh. The CT component was carried out without contrast with exposure factors of 120 kVp and 80 mA in 0.8 s. Emission PET images were acquired in 3D mode with 4 min per bed position and three-slice overlap between consecutive bed positions. The PET data were reconstructed using iterative reconstruction and the CT images were reconstructed to axial slices of 3.3 mm thickness.
An experienced nuclear medicine physician examined PET/CT images. Positive uptake was based on visual assessment of areas that showed non-physiological increased tracer uptake. The maximum standardised uptake value (SUVmax) corrected for body weight was also documented.
Results
A total of 44 patients with histologically proven GI-NET underwent 68Ga-DOTA-peptide PET/CT during the study period, comprising 16 females and 28 males with a median age of 55 years (range 19–73). Of the tumours, 27.3% arose from the foregut, 15.9% from the midgut and 26.8% from the hindgut. Based on the World Health Organisation (WHO) histopathology grading, 40.9% of patients had grade 1 NET, 22.7% had grade 2 NET, 4.5% had grade 3 NET and in 31.8% of patients the grading was unknown. The chromogranin level (cutoff taken at 94 ng/ml) was elevated on presentation in 20.5% of patients, increasing in trend in 45.5% and never raised in 13.6%; the levels were unknown in 61.4% of patients (Table 1).
Table 1.
Patient chracteristics
| Characteristics | n = 44 | |
|---|---|---|
| Gender, n (%) | Female Male |
16 (36.4%) 28 (63.6%) |
| Age, median years (range) | 55 (19–73) | |
| Primary site, n (%) | Foregut-stomach 7, doudenum 5 Midgut-small bowel (4), appendix (3) Hindgut-rectal 22, sigmoid 2, colon 1 |
12 (27.3%) 7 (15.9%) 25 (56.8%) |
| WHO grading, n (%) | G1 G2 G3 Unknown |
18 (40.9%) 10 (22.7%) 2 (4.5%) 14 (31.8%) |
| Raised CgA (ng/ml), n (%) Cutoff value: <94 |
High on presentation From normal to high Never raised Unknown |
9 (20.5%) 2 (45.5%) 6 (13.6%) 27 (61.4%) |
| Number of times scanning performed per patient | 1 2 3 4 5 6 |
24 10 5 3 1 1 |
From the 44 patients, 82 studies of 68Ga-DOTA-peptide PET/CT were performed. Frequency of scans performed per patient is shown in Table 1. Of 82 68Ga-DOTA-peptide PET/CTs performed, 36 examinations were performed using 68Ga-DOTA-TATE, and the remaining 46 were performed using 68Ga-DOTA-TOC.
A wide range of SUVmax was demonstrated in tumoral lesions with the SUVmax of tumoral lesions in the soft tissue range (9.6 to 76.8), node (3.3 to 64.7), liver (9.2 to 66.6), adrenal gland (9.7 to 23.1), bone (2.8 to 61.1) and extra-orbital muscle (8.5 to 19.7). Interestingly, no lung metastasis was detected.
In the first group of patients, the staging of five (50%) of the ten patients who had 68Ga-DOTA-peptide PET/CT for pre-operative staging were changed following PET, whereby one patient was upstaged and the remaining four were downstaged. In the downstaged group, three patients (30%) who were initially diagnosed with metastatic disease had a change in decision for primary tumour resection only. The remaining one downstaged patient was shown to have a false-negative PET/CT on subsequent EUS study. This patient had D1 tumour, which measured 1.5 cm in size and invaded the submucosa. Subsequent biopsy results showed a WHO grade 1 neuroendocrine tumour. In per patient analysis, the sensitivity and specificity were 87.5% and 100% respectively, whereas in per lesion analysis, the sensitivity and specificity were 94.1% and 90.0% respectively.
In the second group of patients, a total of 19 (52.8%) of 36 patients who had 68Ga-DOTA-peptide PET/CT for post-operative restaging had their staging changed. Ten patients were upstaged and nine were downstaged. Twenty patients (55.6%) had their therapy changed and 11 patients (30.6%) had no change in therapy. The remaining five patients (13.9%) were lost to follow-up (Table 2). This included commencement of octreotide therapy, repeat surgery, palliative radiotherapy and adoption of a watch-and-wait strategy.
Table 2.
Impact on post-operative 68Ga-DOTA-peptide PET/CT on staging and management (n = 36)
| Impact on stage | 19 (52.8%) |
|---|---|
| Upstaging Downstaging |
10 9 (including 2 false negatives) |
| Impact on therapy | 20 (55.6%) |
| Omit further Tx Start cold octreotide Re-surgery Palliative RT |
6 11 2 1 |
| No impact on therapy | 11 (30.6%) |
| Unknown impact because lost to follow-up | 5 (13.9%) |
In the third group of patients, a total of 36 patients had 68Ga-DOTA-peptide PET/CT for monitoring of treatment response. Seventeen patients (47.2%) showed disease progression, whereas 19 patients (52.8%) showed stable disease. A total of 19 patients (52.8%) had a significant change in their therapy (Table 3). This was either commencement/change in dosage of octreotide therapy, starting chemotherapy/everolimus or peptide receptor radionuclide therapy.
Table 3.
Impact of 68Ga-DOTA-peptide PET/CT on the surveillance and monitoring response post-chemo/sandostatin/everolimus/PRRT (n = 36)
| PET/CT showed progression | 17 (47.2%) |
| PET/CT showed non-progression | 19 (52.8%) |
| Impact on therapy | 19 (52.8%) |
| Start octreotide Increase octreotide dosage Start chemotherapy Start everolimus Start PRRT |
2 3 4 3 7 |
| No impact on therapy | 16 (44.4%) |
| Continue sandostatin dosage Continue everolimus Continue PRRT |
11 1 4 |
| Unknown impact because lost to follow-up | 1 (2.8%) |
It is worth noting that the therapeutic alteration decision was not based on imaging alone. As NET is considered a slow-growing tumour, some clinicians adopt a conservative approach, whereas some opt for therapeutic change even in stable metastatic disease.
Discussion
To date, five studies have demonstrated a high impact of 68Ga-DOTA-peptide PET/CT in the management of NET, ranging from 19.0% to 60.0% (Table 4) [9–13]. Our findings were consistent with these results, where an impact on management was seen in 30% in the pre-treatment group and approximately 50% in the post-treatment group (including both the post-operative restaging group and post-systemic treatment group).
Table 4.
Studies on impact of 68Ga-DOTA-peptide PET/CT in the management of NET
In patients who had 68Ga-DOTA-peptide PET/CT for pre-operative assessment, there was a substantial change in their disease staging post-PET. As an adjunct to conventional imaging (multiphase CT/ MRI/ EUS), 68Ga-DOTA-peptide PET/CT offers further delineation on the N and M staging and characterises equivocal lesions detected on CT or MRI. However, the impact of 68Ga-DOTA-peptide on the treatment approach was less striking. This is partly due to the fact that the change of disease stage may not necessarily be followed by a change of the treatment strategy especially in patients with known disseminated disease. Furthermore, 68Ga-DOTA-peptide PET/CT could be falsely negative in small lesions. Hence, conventional imaging for initial staging still remains mandatory as it provides precise anatomical information and guides biopsy.
The impact of 68Ga-DOTA-peptide PET/CT on both stage and management change was particularly evident when it was performed for post-operative restaging purposes. This was partly due to more accurate patient re-stratification and subsequently led to additional or no treatment. It was also interesting to note that 25% of patients had their disease down-staged, which subsequently led to omission from further treatment in 16% of patients. This indicates that 68Ga-DOTA-peptide PET/CT has an important role in excluding disease, which prevents unnecessary treatment and saves costs. However, we should keep in mind the possibility of false-negative findings, which were also noted in this study. In this study, two patients demonstrated false-negative results on 68Ga-DOTA-peptide PET/CT as one patient had small lesions and another patient harboured a WHO grade 3 de-differentiated tumour. Due to potential false-negative findings on 68Ga-DOTA-peptide PET/CT, which may result in a negative impact, further analysis of the patient outcomes is needed in future studies.
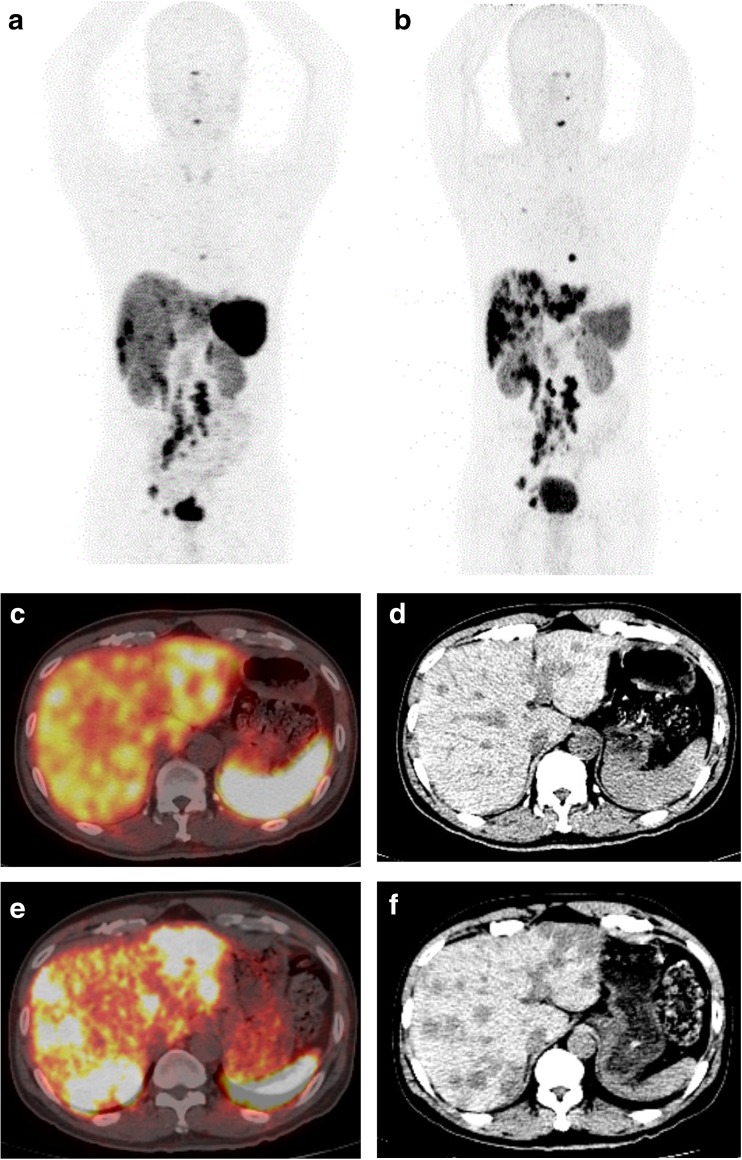
By identifying tumours with somatostatin receptor expression, 68Ga-DOTA-peptide PET/CT is particularly useful in identifying patients who will likely benefit from cold octreotide or peptide receptor radionuclide therapy [3, 14]. However, somatostatin receptor expression in NET is heterogenous and dynamic, especially the evolution of tumoral characteristic from somatostatin receptor expression to glucose hypermetabolism over time [15, 16]. Therefore, dual tracer PET/CT using both 68Ga-DOTA-peptide and 18F–FDG has been suggested to allow real-time comprehensive assessment of overall tumoral characteristics and stage, particularly high grade 2 and grade 3 tumours (Fig. 1).
Fig. 1.
A 51-year-old male with rectal NET who underwent low anterior resection. Post-surgery Ga-DOTATATE PET/CT showed extensive somatostatin receptor-avid metastases in the liver, abdominal and pelvic lymph nodes, and bones (A, C and D). He was started on subcutaneous long-acting octreotide. Six months later, his Ga-DOTATATE PET/CT showed progression of disease particularly in the liver (B, E and F). He was subsequently referred for peptide receptor chemoradionuclide therapy (PRCRT)
Regarding the surveillance and monitoring of the treatment response group, 68Ga-DOTA-peptide PET/CT influenced patient’s stage and management in half of the studied population. Although the use of standardised uptake values (SUV) for treatment monitoring is uncertain and requires further studies [14, 17], the high sensitivity of 68Ga-DOTA-peptide PET/CT has placed it in an important position in identifying occult new lesions during follow-up, which might not be demonstrated by CT. However, current standard response criteria have proven suboptimal for the assessment of the antiproliferative effect of many targeted agents, particularly in the context of slow-growing tumours such as well-differentiated NETs [18]. Again, tumoral heterogeneity may hinder the use of single agent 68Ga-DOTA-peptide PET/CT during follow-up. We suggest performing a dual tracer approach at the initial staging, followed by a single tracer for future monitoring if no glucose hypermetabolism lesion is demonstrated on 18F–FDG PET/CT. However, more studies are needed to establish the appropriate use of the dual tracer approach with consideration of initial histopathological grading and subsequent tumour progression.
As this is a cross-sectional cohort study, the main drawbacks would include unavailable or missing data due to loss to follow-up. The evolving classification, diagnosis and treatment algorithm of NETs also contribute to the difficulties faced in this study. For example, the use of the Ki67 index for tumour grading was only used routinely in recent years. In addition to this, many centres adopt diverse diagnostic and treatment strategies because of limitations in specialties, resources and experience.
Conclusion
In conclusion, 68Ga-DOTA-peptide PET/CT has a significant impact on management decisions in GI-NET patients as it can provide additional information on occult metastasis/equivocal lesions and give the clinician an opportunity to select patients for targeted therapy.
Acknowledgments
We are grateful to Dr. Asmayani Khalib and all our staff for their assistance in this study. We also thank the Director General of the Ministry of Health of Malaysia for permission to publish this paper.
Compliance with Ethical Standards
Conflicts of Interest
Teik Hin Tan, Ching Yee Boey and Boon Nang Lee declare that they have no conflicts of interest.
Ethics Statement
Informed consent was obtained from all patients for being included in the study. This study is approved by the local ethics committee.
Ethics Approval
All procedures performed in studies were in accordance with the ethical standards of the national Medical Research and Ethics Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
This retrospective study was approved by the national ethics committee, and the requirement to obtain informed consent was waived.
References
- 1.Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumors: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30:781–793. doi: 10.1007/s00259-003-1184-3. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni HR, Baum RP. Theranostics with Ga-68 somatostatin receptor PET/CT: monitoring response to peptide receptor radionuclide therapy. PET Clin. 2014;9:91–97. doi: 10.1016/j.cpet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni HR, Baum RP. Patient selection for personalized peptide receptor radionuclide therapy using Ga-68 somatostatin receptor PET/CT. PET Clin. 2014;9:83–90. doi: 10.1016/j.cpet.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Deppen SA, Liu E, Blume JD, et al. Safety and efficacy of 68Ga DOTATATE PET/CT for diagnosis, staging, and treatment management of neuroendocrine tumors. J Nucl Med. 2016;57:708–714. doi: 10.2967/jnumed.115.163865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC, Maecke H. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging. 2007;34:982–993. doi: 10.1007/s00259-006-0317-x. [DOI] [PubMed] [Google Scholar]
- 6.Ambrosini V, et al. 68Ga-labelled peptides for diagnosis of gastroenteropancreatic NET. Eur J Nucl Med Mol Imaging. 2012;39:S52–S60. doi: 10.1007/s00259-011-1989-4. [DOI] [PubMed] [Google Scholar]
- 7.Buchmann I, Henze M, Engelbrecht S, Eisenhut M, Runz A, Schäfer M, Schilling T, Haufe S, Herrmann T, Haberkorn U. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2007;34:1617–1626. doi: 10.1007/s00259-007-0450-1. [DOI] [PubMed] [Google Scholar]
- 8.Krausz Y, Freedman N, Rubinstein R, Lavie E, Orevi M, Tshori S, Salmon A, Glaser B, Chisin R, Mishani E, Gross JD. 68Ga-DOTA-NOC PET/CT imaging of neuroendocrine tumors: comparison with 111In-DTPA-octreotide (OctreoScan®) Mol Imaging Biol. 2011;13:583–593. doi: 10.1007/s11307-010-0374-1. [DOI] [PubMed] [Google Scholar]
- 9.Naswa N, Sharma P, Kumar A, Nazar AH, Kumar R, Chumber S, Bal C. Gallium-68-DOTA-NOC PET/CT of patients with gastroenteropancreatic neuroendocrine tumors: a prospective single-center study. AJR Am J Roentgenol. 2011;197:1221–1228. doi: 10.2214/AJR.11.7298. [DOI] [PubMed] [Google Scholar]
- 10.Hofman MS, Kong G, Neels OC, Eu P, Hong E, Hicks RJ. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol. 2012;56:40–47. doi: 10.1111/j.1754-9485.2011.02327.x. [DOI] [PubMed] [Google Scholar]
- 11.Ambrosini V, Campana D, Bodei L, et al. 68Ga-DOTANOC PET/CT clinical impact in patients with neuroendocrine tumors. J Nucl Med. 2010;51:669–673. doi: 10.2967/jnumed.109.071712. [DOI] [PubMed] [Google Scholar]
- 12.Herrmann K, Czernin J, Wolin EM, et al. Impact of 68Ga-DOTATATE PET/CT on the management of neuroendocrine tumors: the referring physician's perspective. J Nucl Med. 2015;56:70–75. doi: 10.2967/jnumed.114.148247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skoura E, Michopoulou S, Mohmaduvesh M, et al. The impact of 68Ga-DOTATATE PET/CT imaging on management of patients with neuroendocrine tumors: experience from a National Referral Center in the United Kingdom. J Nucl Med. 2016;57:34–40. doi: 10.2967/jnumed.115.166017. [DOI] [PubMed] [Google Scholar]
- 14.Kratochwil C, Stefanova M, Mavriopoulou E, et al. SUV of [68Ga]DOTATOC-PET/CT predicts response probability of PRRT in neuroendocrine tumors. Mol Imaging Biol. 2015;17:313–318. doi: 10.1007/s11307-014-0795-3. [DOI] [PubMed] [Google Scholar]
- 15.Kayani I, Bomanji JB, Groves A, Conway G, Gacinovic S, Win T, et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1,Tyr3-octreotate) and 18F-FDG. Cancer. 2008;112(11):2447–2455. doi: 10.1002/cncr.23469. [DOI] [PubMed] [Google Scholar]
- 16.Oh S, Prasad V, Lee DS, Baum RP. Effect of peptide receptor radionuclide therapy on Somatostatin receptor status and glucose metabolism in neuroendocrine tumors: Intraindividual comparison of Ga-68 DOTANOC PET/CT and F-18 FDG PET/CT. Int J Mol Imaging. 2011;2011 [DOI] [PMC free article] [PubMed]
- 17.Gabriel M, Oberaurl A, Dobrozemskyl G, et al. 68Ga-DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J Nucl Med. 2009;50:1427–1434. doi: 10.2967/jnumed.108.053421. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Carbonero R, et al. Imaging approaches to assess the therapeutic response of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): current perspectives and future trends of an exciting field in development. Cancer Metastasis Rev. 2015;34:823–842. doi: 10.1007/s10555-015-9598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]