Abstract
Purpose
We aimed to evaluate the prognostic values of radiography, F-18 FDG PET, and I-131 whole body scans in patients with lung-only metastasis from differentiated thyroid carcinoma (DTC).
Methods
Between 1998 and 2013, we included 31 patients (F: 26, M: 5) with lung-only metastasis from DTC who had been treated with I-131 and underwent PET. Lung metastasis was categorized according to the size (macronodular ≥1.0 cm vs. micronodular <1.0 cm), FDG avidity (avid vs. non-avid), and I-131 avidity (avid vs. non-avid). Progression-free survival (PFS) was evaluated for each patient.
Results
Among 31 patients, seven (23%) had macronodular lung metastasis, 26 (84%) had FDG avid lung metastasis, and 16 (52%) had I-131 avid lung metastasis. During the median follow-up period of 9.4 y, median PFS was 6.1 y. Based on Kaplan-Meier analysis, macronodular lung metastasis (p = 0.017) and I-131 non-avid lung metastasis (p = 0.059) were significantly associated with worse outcomes, but FDG avid lung metastasis was not (p = 0.135). Patients with FDG non-avid lung metastasis did not experience disease progression during follow-up, while 11 of 26 patients (42%) experienced disease progression. Based on univariate analysis, the hazard ratio for a poor prognosis was 3.78 (p = 0.029) for macronodular lung metastasis and 3.29 (p = 0.079) for I-131 non-avid lung metastasis.
Conclusions
Macronodular and I-131 non-avid lung metastasis were associated with a poor prognosis in lung-only metastasis from DTC. Although FDG avid lung metastasis may be associated with a poor prognosis, a larger-scale study is needed.
Keywords: Differentiated thyroid carcinoma, Lung metastasis, F-18 FDG avidity, I-131 avidity, Micronodular, Macronodular
Introduction
Papillary and follicular carcinomas of the thyroid gland, often referred together as differentiated thyroid carcinoma (DTC), are one of the most curable cancers and are associated with a favorable prognosis. However, 4–23% of patients with DTC experience distant metastases and lung and bone are common sites of distant metastasis [1–3]. It has been reported that lung plus bone metastasis from DTC is associated with a poorer prognosis than lung-only metastasis from DTC [2, 4]. Although high active I-131 treatment is the main therapeutic modality for metastasis with DTC patients, two-thirds of such patients eventually become refractory to I-131 treatment [4]. Among these, those who did not show iodine uptake in the whole body scan after I-131 treatment had a poor prognosis, and their 10-year survival rate was only around 10% [2, 4].
Alternative therapies have been tried in cases of I-131 refractory DTC. For example, retinoic acid for redifferentiation and sorafenib for targeted therapy are being investigated. To determine the use of these alternative therapies instead of I-131 treatment, prognostic factors for patients with lung-only metastasis need to be identified. Several previous studies have shown that prognostic factors for the survival of distant metastasis from DTC include the size of the metastatic lesions [5, 6] and I-131 avidity [6–8]. F-18 FDG-PET and F-18 FDG-PET/CT have been widely used for staging, response assessment, and monitoring for many types of cancers since the 1990s. Thyroid cancer with high F-18 FDG uptake is known to be less I-131 avid and therefore relatively refractory to I-131 treatment [9]. However, Pitoia et al. reported that the lesion size, F-18 FDG avidity, and I-131 avidity was associated with prognosis in a small number of patients (n = 24) with lung-only metastasis from DTC, but overall survival was evaluated within a relatively short follow-up period (5 years or more) [10]. Otherwise, there has been no report on the long-term prognostic values of these imaging features in lung-only metastasis from DTC.
The aim of this study was to investigate the prognostic values of imaging parameters representing lesion size, I-131 avidity and F-18 FDG avidity of metastatic lung nodules in lung-only metastasis from DTC.
Materials and Methods
Patients
We retrospectively reviewed our institutional data of 9235 patients with thyroid carcinoma (International Classification of Diseases code C73) between 1998 and 2013. The inclusion criteria are as follows: 1) patients who were treated with total thyroidectomy following high dose I-131 (≥ 100 mCi), 2) a lung metastasis was diagnosed before or after I-131 treatment, 3) chest X-ray and chest CT (all patients), F-18 FDG-PET (PET) (n = 10), or F-18 FDG-PET/CT (PET/CT) (n = 21) were performed before I-131 treatment, and 4) follow-up for 2 y or more in patients who did not experience disease progression. Therefore, 47 patients were selected. Among them, the following 16 patients were excluded. 1) patients with metastasis to other than the regional lymph nodes or lung, 2) patients with other coexisting primary malignancy, and 3) patients with active inflammatory lung disease (which can cause false-positive I-131 uptake) at the time of I-131 treatment. Finally, a total of 31 patients were analyzed in the present study. Our study protocol was approved by our Institutional Review Board (IRB No.: K-1511-002-003).
Diagnosis of Lung Metastasis
The diagnosis of lung metastasis was based on one or more following imaging or histopathological findings: 1) chest X-ray or chest CT, 2) PET or PET/CT, 3) I-131 lung uptake in diagnostic or therapeutic I-131 whole-body scans (WBSs), or 4) pathological confirmation of lung metastasis (n = 2). Lung metastasis was diagnosed initially in 13 patients and during follow-up in the remaining 18 patients.
Image Analysis
Based on the chest CT, we divided 31 patients into macronodular lung metastasis (largest nodule ≥1.0 cm in diameter) and micronodular lung metastasis (largest nodule <1.0 cm in diameter) groups. The F-18 FDG avidity for metastatic lung nodules was assessed by using PET or PET/CT. Any lung nodule showing F-18 FDG uptake higher than adjacent normal lung parenchyma was considered to be F-18 FDG-avid lung metastasis. F-18 FDG avid lung metastases were further divided into two groups: 1) increased F-18 FDG uptake on all discernable lung nodules on chest CT (FDG all-avid lung metastasis) and 2) increased F-18 FDG uptake in parts of discernable lung nodules on chest CT (FDG partial-avid lung metastasis). As for WBS, lung uptake was divided into three groups: 1) no abnormal uptake (I-131 non-avid), 2) diffuse increased uptake, and 3) focal increased uptake [5]. PET was performed using an ECAT EXACT HR+ PET scanner (CTI/Siemens, Knoxville, TN, USA), and PET/CT was performed using a Discovery PET/CT scanner (Discovery LS; GE Medical Systems, Milwaukee, WI, USA) or a Biographs 6 PET/CT scanner (Biograph6; SiemensMedical Solutions, Malvern, PA, USA). PET, PET/CT, and WBS were visually analyzed by two experienced nuclear medicine physicians in consensus. Quantitative analysis was not performed for PET, PET/CT, or WBS.
Treatment and Follow-Up Protocol
All patients were treated with a high fixed dose of I-131 (> 180 mCi). I-131 treatment was repeated until I-131 lung uptake disappeared after an interval of 12 months. Before I-131 treatment, thyroxin was withdrawn for 5–6 weeks to stimulate thyroid stimulating hormone. After 2 days of I-131 treatment, WBS was performed. Patients were followed up every 3 to 6 months and additional imaging studies (chest X-ray, chest CT, PET or PET/CT, and diagnostic WBS) were performed every 6 to 12 months [11].
Definition of Progression-Free Survival
During follow up, progression of disease (PD) was defined as 1) an increase of lung nodule size ≥5 mm based on chest CT or PET/CT or 2) newly diagnosed lung metastasis on imaging modalities or histopathological finding. We defined progression-free survival (PFS) as the time from the diagnosis of lung metastasis until the identification of PD [11].
Statistical Analysis
Categorical variables are shown as numbers and percentages, and continuous variables are shown as means with standard deviations or median values with a range. Student’s t-test was used to compare continuous variables and a chi-square or Fisher’s exact test was used to estimate the differences between groups. The Kaplan–Meier method was performed to generate survival curves, and log-rank tests were used to evaluate differences in survival curves between groups. The Cox proportional hazard model was conducted to assess the association of related prognostic factors with progression-free survival (PFS). The hazard ratio (HR), 95% confidence interval (CI), and p-value were reported. All reported p-values are two-sided, and p-values <0.1 were defined as significant because only a small number of patients were included in the present study [12]. All statistical analyses were performed using MedCalc version 17.8.1 (MedCalc Software, Belgium).
Results
Patient Characteristics
The clinicopathological characteristics, image findings and follow-up results of all patients included in this study are detailed in Table 1 and the characteristics according to the progression of disease are summarized in Table 2. Of the 31 patients, 11 (35%) had disease progression during follow-up. The mean age at diagnosis of lung metastasis was 52.7 years (range, 17–74 years). Thirty patients were diagnosed with papillary thyroid carcinoma of a primary lesion, and only one patient was diagnosed with follicular thyroid carcinoma. Lung metastasis was confirmed in two patients by a histopathological method and in 29 patients by an imaging method. Out of 31 patients, seven (23%) had macronodular lung metastasis, and 26 of 31 patients (84%) had F-18 FDG avid lung metastasis. Subgroup analysis was performed on 26 patients. Among them, 11 patients (42%) showed I-131 avid lung metastasis and 15 patients (58%) showed I-131 non-avid lung metastasis. In addition, 13 patients (50%) had FDG all-avid lung metastasis and another 13 patients (50%) had FDG partial-avid lung metastasis. Out of 31 patients, 16 (52%) had I-131 avid lung metastasis. Of these 16 patients, 11 patients (69%) had FDG avid lung metastasis and five patients (31%) had FDG non-avid lung metastasis. In addition, eight patients had diffusely increased I-131 lung uptake and eight patients had focally increased I-131 lung uptake, respectively.
Table 1.
Characteristics of all patients investigated in this study
| Patient number | Gender | Age (years) | Pathology | Diagnosis time of lung metastasis | Initial size | FDG avidity | I-131 avidity | Follow-up result | PFS time (years) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | female | 48 | papillary | late | micronodular | FDG avid | I-131 avid | non-PD | 8 |
| 2 | female | 60 | papillary | late | micronodular | FDG avid | I-131 non-avid | non-PD | 14 |
| 3 | female | 57 | papillary | late | micronodular | FDG avid | I-131 avid | PD | 1 |
| 4 | female | 50 | papillary | late | macronodular | FDG avid | I-131 non-avid | PD | 4 |
| 5 | female | 44 | papillary | late | micronodular | FDG avid | I-131 non-avid | non-PD | 13 |
| 6 | female | 59 | papillary | late | micronodular | FDG non-avid | I-131 avid | non-PD | 9 |
| 7 | female | 67 | papillary | late | micronodular | FDG avid | I-131 non-avid | non-PD | 5 |
| 8 | female | 61 | papillary | late | micronodular | FDG avid | I-131 non-avid | non-PD | 13 |
| 9 | female | 74 | papillary | initial | micronodular | FDG avid | I-131 non-avid | PD | 5 |
| 10 | female | 41 | papillary | initial | micronodular | FDG avid | I-131 avid | non-PD | 14 |
| 11 | female | 66 | papillary | late | micronodular | FDG avid | I-131 non-avid | non-PD | 9 |
| 12 | female | 17 | papillary | initial | micronodular | FDG avid | I-131 avid | non-PD | 11 |
| 13 | female | 40 | papillary | initial | micronodular | FDG avid | I-131 avid | non-PD | 10 |
| 14 | female | 24 | papillary | initial | micronodular | FDG non-avid | I-131 avid | non-PD | 3 |
| 15 | female | 67 | papillary | initial | macronodular | FDG avid | I-131 avid | non-PD | 7 |
| 16 | male | 66 | papillary | initial | micronodular | FDG avid | I-131 non-avid | PD | 3 |
| 17 | female | 49 | papillary | initial | micronodular | FDG non-avid | I-131 avid | non-PD | 4 |
| 18 | female | 67 | papillary | initial | micronodular | FDG avid | I-131 avid | non-PD | 5 |
| 19 | male | 53 | papillary | late | micronodular | FDG avid | I-131 avid | non-PD | 4 |
| 20 | female | 57 | papillary | late | micronodular | FDG avid | I-131 non-avid | non-PD | 11 |
| 21 | female | 43 | papillary | initial | micronodular | FDG non-avid | I-131 avid | non-PD | 6 |
| 22 | female | 65 | papillary | late | macronodular | FDG avid | I-131 non-avid | PD | 3 |
| 23 | female | 50 | papillary | late | micronodular | FDG avid | I-131 non-avid | PD | 2 |
| 24 | male | 47 | papillary | initial | micronodular | FDG non-avid | I-131 avid | non-PD | 10 |
| 25 | male | 49 | papillary | late | macronodular | FDG avid | I-131 non-avid | PD | 1 |
| 26 | female | 68 | papillary | late | macronodular | FDG avid | I-131 non-avid | PD | 2 |
| 27 | female | 55 | papillary | late | micronodular | FDG avid | I-131 non-avid | PD | 6 |
| 28 | female | 39 | follicular | initial | macronodular | FDG avid | I-131 avid | PD | 8 |
| 29 | female | 42 | papillary | late | micronodular | FDG avid | I-131 avid | PD | 5 |
| 30 | male | 61 | papillary | initial | macronodular | FDG avid | I-131 non-avid | non-PD | 9 |
| 31 | female | 47 | papillary | late | micronodular | FDG avid | I-131 avid | non-PD | 12 |
FDG Fluorodeoxyglucose, PD Progression of disease, PFS Progression-free survival
Table 2.
Clinicopathological and image characteristics according to the progression of disease
| Total (%) | non-PD | PD | p | |
|---|---|---|---|---|
| Number | 31 | 20 (65) | 11 (35) | |
| Age (years) | 52.7 ± 13.0 | 50.9 ± 13.8 | 55.9 ± 11.2 | 0.313 |
| Male (%) | 5 (16) | 3 (15) | 2 (18) | 1.000 |
| Size of primary thyroid tumor (cm) | 2.8 ± 2.3 | 2.1 ± 1.6 | 5.5 ± 2.6 | 0.016 |
| Lung metastasis at diagnosis (%) | 13 (42) | 10 (50) | 3 (27) | 0.275 |
| Macronodular lung metastasis (%) | 7 (23) | 2 (10) | 5 (45) | 0.067 |
| FDG avid lung metastasis (%) | 26 (84) | 15 (75) | 11 (100) | 0.133 |
| I-131 avid/I-131 non-avid | 11 (42)/15(58) | 8 (53)/7 (47) | 3 (27)/8 (73) | 0.246 |
| FDG all-avid/partial-avid | 13 (50)/13 (50) | 8 (53)/7 (47) | 5 (45)/6 (55) | 1.000 |
| I-131 avid lung metastasis (%) | 16 (52) | 13 (65) | 3 (27) | 0.066 |
| FDG avid/FDG non-avid | 11 (69)/5 (31) | 8 (62)/5 (38) | 3 (100)/0(0) | 0.509 |
| Number of I-131 treatment (IQR) | 4 (3; 5) | 3 (3; 4) | 4 (4; 7.5) | 0.027 |
| Cumulative activity of I-131 treatment (mCi, IQR) | 740 (576; 917) | 600 (560; 788) | 800 (736; 1782) | 0.016 |
PD Progression of disease, FDG Fluorodeoxyglucose, IQR Inter-quartile range
The size of the primary thyroid tumors was significantly greater in the PD group (5.5 ± 2.6 cm) than in the non-PD group (2.1 ± 1.6 cm, p = 0.016). The PD group (4 times, IQR: 4–7.5 times) received significantly more I-131 treatment than the non-PD group (3 times, IQR: 3–4 times, p = 0.027). The cumulative I-131 treatment dose was higher in the PD group than in the non-PD group (800 mCi vs. 600 mCi, p = 0.016). The PD group had significantly more macronodular lung metastasis (45% vs. 10%, p = 0.067) and less I-131 avid lung metastasis (27% vs. 65%, p = 0.066) than the non-PD group. There were no significant differences in age, sex, the proportion of lung metastasis at diagnosis, or F-18 FDG avid lung metastasis between the two groups.
Progression-Free Survival of Lung Metastasis
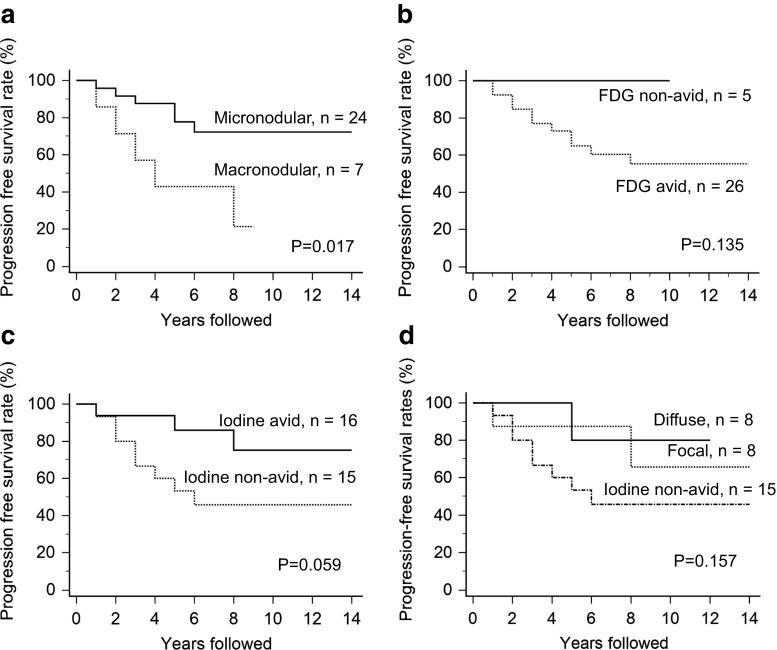
The median follow-up period of the patients was 9.4 years (range, 2.3–15.9 years). Median PFS time was 6.1 years (range, 0.9–14.3 years) and the 5-year and 10-year PFS rates were 69.9% and 61.1%, respectively. Patients with macronodular lung metastasis (n = 7) showed significantly worse outcomes than patients with micronodular lung metastasis (n = 24) (Fig. 1a; p = 0.017, by a log-rank test). The 5-year and 10-year PFS rates were 43% and 21%, respectively, for macronodular lung metastasis and 78% and 72%, respectively, for micronodular lung metastasis.
Fig. 1.
Progression-free survival for the patients with differentiated thyroid cancer with lung metastasis by a lung nodule size on radiological examination, b FDG avid, c I-131 avid, and d I-131 uptake pattern for lung metastasis
Patients with F-18 FDG non-avid lung metastasis (n = 5) had no progression of disease during the follow-up period. On the contrary, 11 of 26 patients with F-18 FDG avid lung metastasis (n = 26) experienced progression of disease (Fig. 1b; p = 0.135, by a log-rank test). The 5-year and 10-year PFS rates were both 100% for FDG non-avid lung metastasis and 65% and 55%, respectively, for FDG avid lung metastasis. In addition, we further divided 31 patients into four groups according to the FDG avidity and size of lung metastasis: 1) FDG avid macronodular lung metastasis (n = 7), 2) FDG avid micronodular lung metastasis (n = 19), 3) FDG non-avid macronodular lung metastasis (n = 0), and 4) FDG non-avid micronodular lung metastasis (n = 5). The 5-year/10-year PFS rates were 43%/21%, 73%/66%, and 100%/100% for each group 1, 2, and 4, respectively.
Patients with I-131 non-avid lung metastasis (n = 15) had significantly worse outcomes than patients with I-131 avid lung metastasis (n = 16) (Fig. 1c; p = 0.059, by a log-rank test). The 5-year and 10-year PFS rates were 53% and 46%, respectively, for I-131 non-avid lung metastasis and 86% and 75%, respectively, for I-131 avid lung metastasis. When the I-131 uptake pattern for lung metastasis was further subdivided into focal (n = 8), diffuse (n = 8), and non-avid patterns (n = 15), patients with diffuse or focal uptake patterns showed similar outcomes (Fig. 1d; p = 0.157, by a log-rank test).
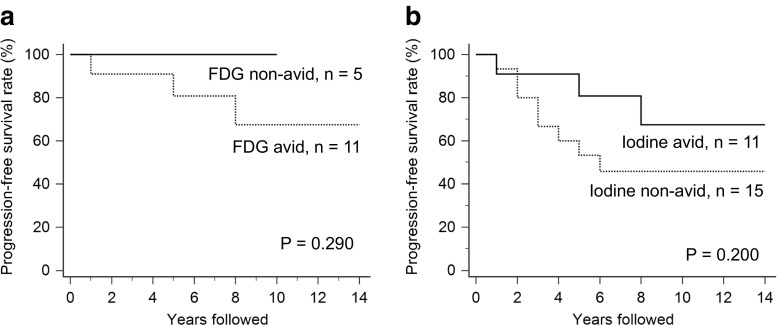
Patients with I-131 avid lung metastases were further analyzed for survival by F-18 FDG avid and F-18 FDG non-avid status. The 5-year and 10-year PFS rates were both 100% for the F-18 FDG non-avid group and 81% and 67%, respectively, for F-18 FDG avid group (Fig. 2a, p = 0.290, by a log-rank test). Likewise, patients with F-18 FDG avid lung metastases were again subjected to survival analysis according to I-131 avid and I-131 non-avid status. The 5-year and 10-year PFS rates were 53% and 46%, respectively, for I-131 non-avid group and 81% and 67%, respectively, for I-131 avid group (Fig. 2b, p = 0.200, by a log-rank test). Representative images are presented in Figs. 3 and 4.
Fig. 2.
a Progression-free survival according to F-18 FDG avidity in the I-131 avid group and b progression-free survival according to I-131 avidity in the F-18 FDG avid group
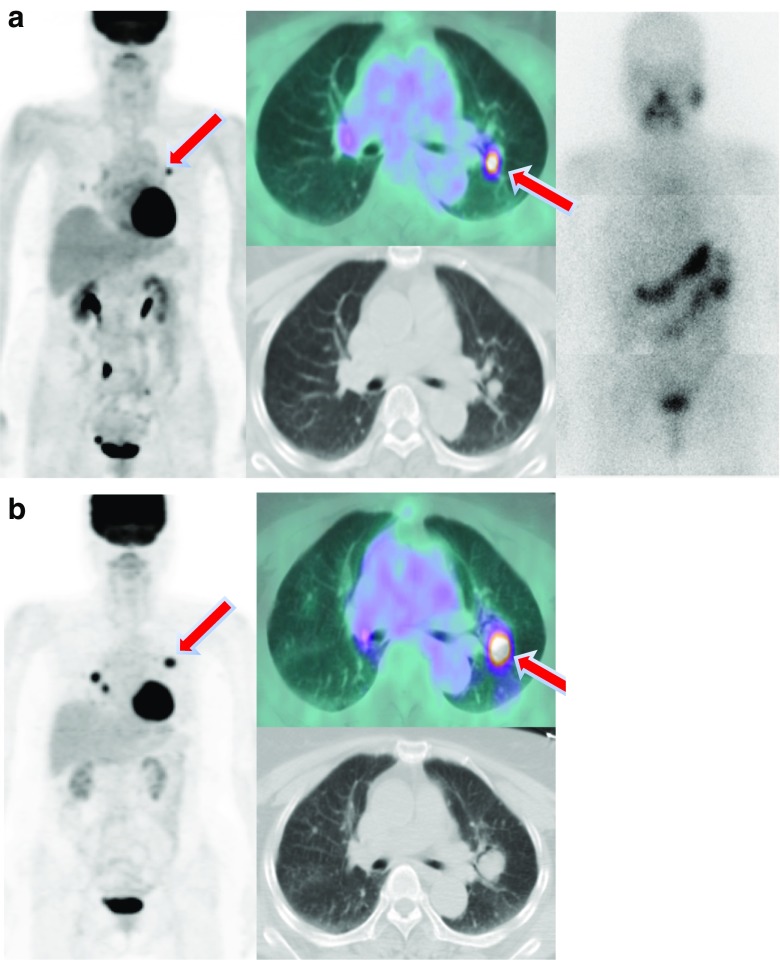
Fig. 3.
Whole body images after I-131 treatment for lung metastasis and a series of changes in FDG-PET/CT. a Multiple nodules were detected on FDG-PET/CT in a 47-year-old male patient. FDG uptake was not observed in these lesions, and histological examination revealed metastatic papillary carcinoma. Diffuse lung uptake was observed in the whole body scan. b The patient underwent five treatment cycles of I-131 within 7 years (cumulative dose: 940 mCi). The patient has been followed for 10 years, and the number of metastatic lung nodules has decreased in FDG-PET/CT
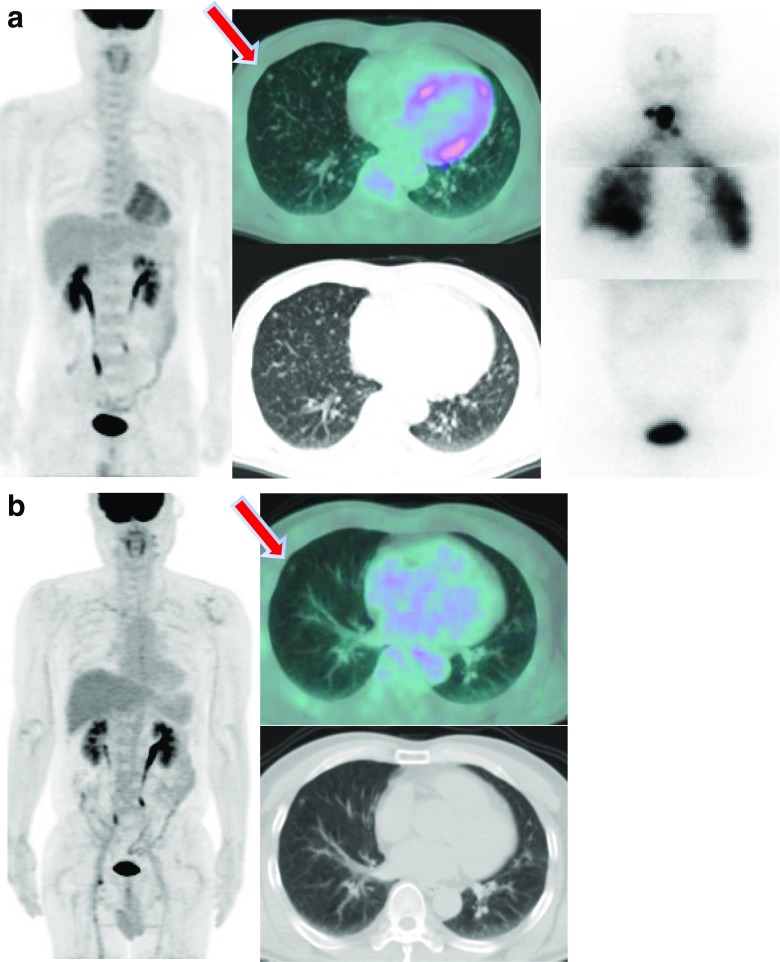
Fig. 4.
Whole body images after I-131 treatment for lung metastasis and a series of changes in FDG-PET/CT. a A micronodule was detected on FDG-PET/CT in a 47-year-old male patient. FDG uptake was not observed in this lesion. Histological examination revealed metastatic papillary carcinoma. The patient underwent I-131 treatment (200 mCi). Diffuse lung uptake was observed in the whole body scan. b The patient has been followed for 10 years, and the lesion of the lung has maintained without significant change in FDG-PET/CT
Risk Factors for Prognosis of Lung Metastasis
Based on the univariate analysis for progression-free survival, macronodular lung metastasis was significantly associated with a poor prognosis (HR 3.78 [CI 1.15–12.45.]. p = 0.029). I-131 non-avid lung metastasis was also associated with a poor prognosis (HR 3.29 [CI 0.87–12.41], p = 0.079). Other variables such as age, male sex, size of the primary thyroid tumor, late lung metastasis, and F-18 FDG avid lung metastasis were not associated with progression-free survival (Table 3).
Table 3.
Clinicopathological factors and image findings associated with progression-free survival in univariate analysis
| Variables | HR (95% CI) | p |
|---|---|---|
| Age (≥ 45 years) | 1.96 (0.42, 9.09) | 0.391 |
| Male | 1.44 (0.31, 6.71) | 0.641 |
| Size of primary thyroid tumor | 6.56E + 05 (5.85E-224, 7.35E + 234) | 0.960 |
| Late lung metastasis | 2.23 (0.59, 8.42) | 0.236 |
| Macronodular lung metastasis | 3.78 (1.15,12.45) | 0.029 |
| FDG avid lung metastasis | 3.34E + 05 (7.20E-198, 15.75E + 207) | 0.957 |
| I-131 non-avid lung metastasis | 3.29 (0.87, 12.41) | 0.079 |
HR Hazard ratio, CI Confidence interval, FDG Fluorodeoxyglucose
Discussion
We found that patients with lung-only metastasis from DTC had poor outcomes when they had macronodular, F-18 FDG avid, and I-131 non-avid lung metastasis. As far as we know, this is the largest study on the prognostic values of imaging parameters including radiography, PET or PET/CT, and WBS in DTC with lung-only metastasis.
In the present study, larger lesion size and I-131 non-avid lung metastasis were associated with patient outcomes, which is consistent with previous studies [5, 6, 11]. Pacini et al. reported that patients with I-131 avid lung and bone metastasis that were not detected by radiography had better outcomes than those with I-131 non-avid or radiography-detected lung and bone metastasis [13]. The expression of the sodium-iodide symporter (NIS) has been reported to be crucial for I-131 to concentrate in thyroid cancer cells. The dedifferentiation of DTC induces the down regulation of NIS genes, which results in I-131 non-avidity and a decreased therapeutic response [14]. In addition, the relatively short range of the β-particle of I-131 in tissue (0.08–2.3 mm) may be associated with a decreased therapeutic response in macronodular lung metastasis [15]. Recently, Kim et al. has shown that the size of the metastatic lesion is the most important prognostic factor in patients with lung only metastasis from DTC [11]. However, they did not include F-18 FDG-PET in their study. In the present study, we included F-18 FDG avidity of lung metastasis as well as lesion size or I-131 avidity as a prognostic factor. Because we could not perform multivariate analysis, further studies with more patients are needed to evaluate the predictive values of the combinations of these imaging parameters.
In this study, F-18 FDG non-avid lung metastasis was not statistically significantly associated with patient outcomes (p = 0.135). Nonetheless, patients with F-18 FDG non-avid lung metastasis did not experience PD while more than 40% of patients with F-18 FDG avid lesions experienced PD during the follow-up period. It has been reported that higher F-18 FDG uptake by a tumor is associated with poor prognosis in many types of cancers as well as DTC [16–18]. Kim et al. reported that GLUT-1 gene expression was higher in less-differentiated papillary thyroid cancer compared to well-differentiated papillary thyroid cancer [19]. Dedifferentiated thyroid cancer cells are known to be less I-131 avid and therefore refractory to I-131 treatment. As mentioned above, dedifferentiated thyroid cancer cells show high F-18 FDG uptake and low iodine uptake, which is known as the “flip-flop phenomenon” [15]. In addition, patients with FDG non-avid micronodular metastasis (n = 5) had a tendency to show a better prognosis than those with FDG avid micronodular lung metastasis (n = 19) in the current study (the 5- year and 10-year PFS rates of 100% and 100% vs. 73% and 66%, respectively). These findings indicate that the FDG avidity of lung metastasis may be useful for the prediction of prognosis in micronodular pulmonary metastasis; however, further study with a larger number of patients is needed to confirm these findings.
We included patients with lung-only metastasis from DTC because it had been reported that patients with lung plus other distant metastasis showed poor outcomes and required a different therapeutic strategy compared to those with lung-only metastasis [20]. Casara et al. reported that the 5-year survival rate of patients with lung-only metastasis and lung plus bone metastasis from DTC was 69% and 36%, respectively [2]. According to the American Thyroid Association guideline, I-131 avid lung metastasis should be treated with I-131 as long as the tumor cells concentrate I-131, because high rates of complete remission are reported in these patients. On the contrary, I-131 avid bone metastases are not usually curative with I-131, and therefore, surgery or external radiotherapy should be considered [20].
Alternative therapeutic approaches are necessary for I-131 treatment-refractory lung metastasis from DTC because of I-131 induced lung and bone marrow toxicity. Clinical trials for I-131 refractory DTC have been performed by using redifferentiation therapy or targeted therapy. Oh et al. reported that about 21% of patients with I-131 refractory papillary thyroid cancer showed a response to combined therapy with I-131 and retinoic acid to induce the redifferentiation of thyroid cancer cells [21]. Recently, redifferentiation of advanced thyroid cancer with selumetinib, a mitogen-activated protein kinase inhibitor, resulted in clinically meaningful increases in I-131 uptake [22]. It also has been reported that sorafenib, an oral tyrosine kinase inhibitor, improved survival in patients with progressive I-131 refractory DTC [23]. To decide on treatment methods for lung-only metastasis from DTC, we need to be able to predict the response to I-131 therapy. If a poor response to I-131 is expected, alternative treatment (redifferentiation therapy or targeted therapy) is more suitable.
Based on our results, the prognosis of patients with lung-only metastasis from DTC might be predicted by using PET/CT and WBS. These imaging parameters have the advantage of providing a non-invasive assessment for biological status of lung metastases (e.g., size, metabolic status, and I-131 avidity). In addition, multiple metastatic nodules that may have different biological characteristics can be accurately evaluated by using these imaging modalities.
The present study has several limitations. First, only a small number of patients were enrolled. Therefore, multivariate analysis for prognostic factors could not be performed. Further multicenter studies are required. Second, the follow-up period was relatively short, and thus overall survival analysis was not performed. Third, we did not perform a quantitative analysis. Wang et al. reported that metabolic tumor volume measured by F-18 FDG PET was significantly correlated with survival in thyroid cancer [24]. In the present study, however, an older dedicated PET scanner (HR+) that is not currently in use in our institution was used instead of a PET/CT scanner (Discovery or Biograph6) in ten of 31 patients. Hence, it was not possible to calibrate data between the machines for quantification. Fourth, we did not use biochemical parameters such as Tg in survival analysis. At the time of lung metastasis, Tg and Tg antibody values were measured immediately before RAI treatment, but there were five patients without Tg or Tg antibody test results and another four patients with positive Tg antibody test results. Thus, Tg analysis was not performed in this study due to the lack of data from these nine patients. Fifth, we did not perform a lesion-based analysis because the endpoint of this study was to evaluate the prognostic values of image findings.
Conclusion
Among patients with lung-only metastasis from DTC, those with macronodular and I-131 non-avid lung metastasis showed a poor prognosis. These imaging parameters might be helpful for treatment planning for the patients with lung-only metastasis from DTC. Although FDG avid lung metastasis may be associated with a poor prognosis, a larger-scale study is needed to validate the findings.
Acknowledgements
This study was supported by a grant from the Korea Institute of Radiological and Medical Sciences (KIRAMS), funded by the Ministry of Science, ICT and Future Planning, Republic of Korea (711045543/50462-2017) and by the Establishment and Operation of Research Infrastructure for Radioisotope Application (50461-2017), funded by the Ministry of Science, ICT and Future Planning, Republic of Korea.
Compliance with Ethical Standards
Conflict of Interest
Joon Ho Choi, Byung Hyun Byun, Ilhan Lim, Hansol Moon, Jihyun Park, Kyoung Jin Chang, Byung Il Kim, Chang Woon Choi, and Sang Moo Lim have no conflicts of interest to declare.
Ethics Statement
This study was approved by the Institutional Review Board at the Korea Cancer Center Hospital (IRB No. K-1511-002-003). The manuscript contains a statement that the study was approved by an institutional review board or equivalent and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects in the study gave written informed consent or the institutional review board waived the need to obtain informed consent.
References
- 1.Lang BH, Wong KP, Cheung CY, Wan KY, Lo CY. Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol. 2013;20:1329–1335. doi: 10.1245/s10434-012-2711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casara D, Rubello D, Saladini G, Masarotto G, Favero A, Girelli ME, et al. Different features of pulmonary metastases in differentiated thyroid cancer: natural history and multivariate statistical analysis of prognostic variables. J Nucl Med. 1993;34:1626–1631. [PubMed] [Google Scholar]
- 3.Chopra S, Garg A, Ballal S, Bal CS. Lung metastases from differentiated thyroid carcinoma: prognostic factors related to remission and disease-free survival. Clin Endocrinol. 2015;82:445–452. doi: 10.1111/cen.12558. [DOI] [PubMed] [Google Scholar]
- 4.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 5.Cho SW, Choi HS, Yeom GJ, Lim JA, Moon JH, Park DJ, et al. Long-term prognosis of differentiated thyroid cancer with lung metastasis in Korea and its prognostic factors. Thyroid. 2014;24:277–286. doi: 10.1089/thy.2012.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song HJ, Qiu ZL, Shen CT, Wei WJ, Luo QY. Pulmonary metastases in differentiated thyroid cancer: efficacy of radioiodine therapy and prognostic factors. Eur J Endocrinol. 2015;173:399–408. doi: 10.1530/EJE-15-0296. [DOI] [PubMed] [Google Scholar]
- 7.Casara D, Rubello D, Saladini G, Gallo V, Masarotto G, Busnardo B. Distant metastases in differentiated thyroid cancer: long-term results of radioiodine treatment and statistical analysis of prognostic factors in 214 patients. Tumori. 1991;77:432–436. doi: 10.1177/030089169107700512. [DOI] [PubMed] [Google Scholar]
- 8.Sampson E, Brierley JD, Le LW, Rotstein L, Tsang RW. Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer. 2007;110:1451–1456. doi: 10.1002/cncr.22956. [DOI] [PubMed] [Google Scholar]
- 9.Hong CM, Ahn BC, Jeong SY, Lee SW, Lee J. Distant metastatic lesions in patients with differentiated thyroid carcinoma. Clinical implications of radioiodine and FDG uptake. Nuklearmedizin. 2013;52:121–129. doi: 10.3413/Nukmed-0541-12-11. [DOI] [PubMed] [Google Scholar]
- 10.Pitoia F, Bueno F, Cross G. Long-term survival and low effective cumulative radioiodine doses to achieve remission in patients with 131Iodine-avid lung metastasis from differentiated thyroid cancer. Clin Nucl Med. 2014;39:784–790. doi: 10.1097/RLU.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 11.Kim M, Kim WG, Park S, Kwon H, Jeon MJ, Lee JJ, et al. Initial size of metastatic lesions is best prognostic factor in patients with metastatic differentiated thyroid carcinoma confined to the lung. Thyroid. 2017;27:49–58. doi: 10.1089/thy.2016.0347. [DOI] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pacini F, Cetani F, Miccoli P, Mancusi F, Ceccarelli C, Lippi F, et al. Outcome of 309 patients with metastatic differentiated thyroid carcinoma treated with radioiodine. World J Surg. 1994;18:600–604. doi: 10.1007/BF00353775. [DOI] [PubMed] [Google Scholar]
- 14.Filetti S, Bidart JM, Arturi F, Caillou B, Russo D, Schlumberger M. Sodium/iodide symporter: a key transport system in thyroid cancer cell metabolism. Eur J Endocrinol. 1999;141:443–457. doi: 10.1530/eje.0.1410443. [DOI] [PubMed] [Google Scholar]
- 15.Kim DH, Jung JH, Son SH, Kim CY, Hong CM, Jeong SY, et al. Difference of clinical and radiological characteristics according to radioiodine avidity in pulmonary metastases of differentiated thyroid cancer. Nucl Med Mol Imaging. 2014;48:55–62. doi: 10.1007/s13139-013-0239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robbins RJ, Wan Q, Grewal RK, Reibke R, Gonen M, Strauss HW, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006;91:498–505. doi: 10.1210/jc.2005-1534. [DOI] [PubMed] [Google Scholar]
- 17.Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European lung cancer working party for the IASLC lung cancer staging project. J Thorac Oncol. 2008;3:6–12. doi: 10.1097/JTO.0b013e31815e6d6b. [DOI] [PubMed] [Google Scholar]
- 18.Wu Z, Zhao J, Gao P, Song Y, Sun J, Chen X, et al. Prognostic value of pretreatment standardized uptake value of F-18-fluorodeoxyglucose PET in patients with gastric cancer: a meta-analysis. BMC Cancer. 2017;17:275. doi: 10.1186/s12885-017-3271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, Chung JK, Min HS, Kang JH, Park DJ, Jeong JM, et al. Expression patterns of glucose transporter-1 gene and thyroid specific genes in human papillary thyroid carcinoma. Nucl Med Mol Imaging. 2014;48:91–97. doi: 10.1007/s13139-013-0249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh SW, Moon SH, Park DJ, Cho BY, Jung KC, Lee DS, et al. Combined therapy with 131I and retinoic acid in Korean patients with radioiodine-refractory papillary thyroid cancer. Eur J Nucl Med Mol Imaging. 2011;38:1798–1805. doi: 10.1007/s00259-011-1849-2. [DOI] [PubMed] [Google Scholar]
- 22.Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–632. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–328. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Larson SM, Fazzari M, Tickoo SK, Kolbert K, Sgouros G, et al. Prognostic value of [18F]fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancer. J Clin Endocrinol Metab. 2000;85:1107–1113. doi: 10.1210/jcem.85.3.6458. [DOI] [PubMed] [Google Scholar]