Abstract
Basella is an important green leafy vegetable species of Chenopodiaceae family and is known for its medicinal properties. Hydroxy-benzoic acids, hydroxy-cinnamic acids and flavones groups were identified and characterized from the aqueous stem extracts of B. alba and B. rubra species. Higher values of phenolics as well as antioxidant activity were noted from B. alba species extracts. The evaluation of the cytoxicity of these extracts on A431 (epidermoid carcinoma), Hep G2 (hepatocellular carcinoma) and MG 63 (osteosarcoma) cells indicated anti-proliferative activity against all the cell lines. B. alba extract showed higher anti-proliferative activity (37.95–84.86%). Chick embryo chorioallantoic membrane (CAM) assay revealed inhibition of neo-vessels formation. Significant suppression was found with extracts of B. alba at 7 mg/ml compared to that of B. rubra. This is the first study to report the anti-angiogenic activity of Basella species. These studies indicate that Basella sps can be used as a source of natural antioxidants and can be of high significance in pharmaceutical and nutraceutical industries.
Keywords: Basella, Phenolic compounds, Antioxidants, HPLC–ESI–MS, Anti-angiogenesis, Cytotoxicity
Introduction
The mechanism of correlating improved health to consumption of vegetables has become evident in the past decade due to the significant amount of research carried out in the fruits and vegetable species (Gupta and Prakash 2009). Green leafy vegetables (GLVs) are rich sources of many nutrients and antioxidant compounds and thus constitute a major category of vegetable groups which are designated as ‘nature’s anti-aging wonders’ (Gupta and Prakash 2009). The potential health benefits of GLVs are due to the presence of dietary fibers vitamins, minerals, essential amino acids and phytochemicals, such as polyphenols, phenolic acids, flavonoids, sterols, carotenoids and tannins responsible for their high antioxidant nature (Karam et al. 2016). Phenolics and flavonoids constitute a large group of structurally and functionally diverse compounds. They are known to be triggered in abiotic and biotic stress response in plants (Padmavati et al. 1998; Padmavati and Reddy 1999). They occur naturally as whole food and play crucial role in any well-balanced diet with high nutritional value (Kumar 2017). GLVs are widely consumed as a part of regular diet in Asia.
Basella is an important leafy vegetable species and recognized as a medicinal plant. Basella alba and Basella rubra have been used in traditional medicine system in India for several years and widely consumed leafy vegetable in India (Kumar et al. 2015a, b, c). B. alba leaves are used in Ayurveda system of medicine as soothing agents, laxative, demulcent, astringent and the cooked roots are used in the treatment of diarrhoea (Kumar et al. 2013). The tender stem of both the species contains gelatinous or mucilaginous substances (Kumar et al. 2016). In-vitro assays, preclinical and clinical studies have shown that Basella exhibits anti-bacterial, anti-hyperglycemic, anti-inflammatory, cytotoxic and anti-proliferative activity (Priya et al. 2015; Kumar et al. 2015a, b, c; Kilari et al. 2016). These pharmacological properties of Basella species may be ascribed to several phenolic substances. Despite such potent bioactivities, the phytochemicals in these plant species remain to be comprehensively characterized, and their biological properties are to be explored to the full extent. Only limited studies are available with reference to Basella sps. To this end, characterization of the phenolic constituents present in Basella and activity testing was done. The cytotoxic and anti-angiogenic activity of the stem extracts was tested. The findings of this study are an important contribution to the evaluation of Basella species for their potential use mainly in pharmaceutical and food industries.
Materials and methods
Chemicals and reagents
Querectin, Kaempferol, Myricetin, Luteolin, Naringenin, trans-Cinnamic acid, p- Coumaric acid, Caffeic acid, Ferulic acid Gallic acid, Protocatechuic acid and Vanillic acid standards were purchased from (Sigma Chemical Co, St Louis, MO, USA). Tri-carboxylic acetic acid (TCA), Folin–Ciocalteau (FC) reagent, Aluminium chloride, Potassium ferricyanide was obtained from (Merck Specialties Private Limited, Dr. Annie Besant Road, Worli, Mumbai, India). DPPH was obtained from (Sigma Chemical Co, St Louis, MO, USA). DMEM medium for cell culture assays were purchased from (Gibco Invitrogen, USA). Cell lines were procured from National Centre for Cell Science (NCCS) Pune, Maharashtra, India. Remaining solvents and chemicals were of analytical grade and obtained from (Sisco Research Laboratories Pvt Ltd, Navketan Ind., M.C.Rd, Andheri (E), Mumbai, India).
Plant material
Seeds of B. alba and Basella rubra were obtained from (National Seeds Corporation, Government of India, Kolkata, West Bengal). Seeds (50 grams) of both of the plants were washed and soaked overnight in water at Room temperature (RT). Next day, the soaked seeds were sown in germination boxes (15 cm × 12 cm) filled with loamy soil, and sprinkled with water. The germination boxes were kept at RT, and the growth was observed. Seedlings (approx. 3 cm in length) on the 6th day were transplanted to field for cultivation. Seedlings at two leaf stage were exposed to sunlight for 3–4 h on the first day of transplantation. They were sowed 5 cm apart from each other in a row. Plant maturity is achieved (40–50 days) post germination. The plants were grown during the months of February–May. (http://iitkgp.vlab.co.in/?sub=79&brch=262&sim=1299&cnt=4)
Extraction
Adult stems of B alba and B. rubra were collected, washed, air dried in sunlight. Fresh weight of the stem was calculated (gram/dry weight). Stems were cut into small pieces and immediately crushed under liquid nitrogen in a mortar and pestle. The powder was stored in a plastic container and kept at 4 °C. The powdered form of stem (200 g) was weighed and dissolved in water and aqueous methanol. The extraction was carried out at room temperature (37 °C) for 24 h in a shaker. Further all the extracts were filtered through 4 layers of cheese cloth. The filtered extracts were further treated with chloroform, n-hexane, to remove lipids and insoluble particles. The obtained filtrate was evaporated to dryness using a lyophilizer. Further, the extracts were kept in liquid form with respective solvents and were stored at 4 °C until use. The yield was calculated for both the water and aqueous methanol extracts. The yield of the water extract was found to be slightly higher than methanol extracts. Further the qualitative analysis revealed the same number of compounds in the plant extracts of both the solvents.
Analysis of the phenolic content and flavonoid content in Basella sps extracts
The phenolic content of the plant samples was determined using the Folin–Ciocalteu (FC) assay with some modifications (Siddhuraju and Becker 2003). Briefly, aliquots (100 µl) of the crude extracts of different concentrations (10, 20, 30, 40 and 50 µg/ml) were prepared. Then the samples were adjusted to a final volume of 1 ml with water and then mixed with 0.5 ml of freshly prepared FC reagent diluted with water (1:1, v/v). The mixtures were vortexed and allowed to react for 5 min. Then 2.5 ml of 20% sodium carbonate in water (w/v), mixed and kept for incubation. After 40 min of incubation in the dark, the absorbance was measured at 725 nm. Water was used as a blank and the results were expressed as mg of Gallic acid equivalents (GAE) per gram of dry weight of the extract.
Flavonoids content was determined for the plant samples with slight modifications (Zhishen et al. 1999). 0.5 ml of each plant extract was taken and 0.5 ml of water, and 0.15 ml of 5% NaNO2 was added and vortexed. After 5 min, 0.15 ml of 10% AlCl3 was added to the solution and vortexed. At 6 min, 1 ml of 1 M NaOH was added to the mixture, and finally, the total volume of the solution was made to 3 ml with water. Absorbance of the samples was read at 510 nm and the results were expressed as mg of Quercetin per gram of the dry weight.
Antioxidant assays
The radical scavenging activity of the plant extracts against the DPPH radical was performed in methanol with slight modifications (Brand-Williams et al. 1995). For the assay in methanol, an aliquot (1 ml) of the plant extract at different concentrations (50, 100, 200, 300 and 400 µg/ml) were prepared and added to 1 ml of DPPH in methanol (0.1 mM) and kept in the dark conditions for 30 min. The absorbance was measured at 517 nm. Ascorbic acid was used as the standard. The percentage inhibition was calculated using the formula:
The reducing power of the samples was analyzed by the reduction of Fe3+ to Fe2+ for the ferricyanide method with slight modifications (Oyaizu 1986). For the method, reaction mixtures (1.2 ml) containing various concentrations of 0.2 ml of ascorbic acid (10–100 µM) and 0.2 ml of plant extract (15–90 µg/ml) 1), 0.5 ml of 0.1 M phosphate buffer (pH 6.6) and 0.5 ml of (1% w/v) potassium ferricyanide were incubated for 20 min at 50 °C in a water bath. After incubation, the resulting reaction mixture was cooled and mixed with 0.5 ml of (10% w/v) TCA and 0.2 ml of (0.1% w/v) FeCl3 for 10 min. The absorbance of the final solution was measured at 700 nm.
LC–MS
The HPLC system (CBM-20 A lite, Shimadzu, Japan) consisted of a binary pump (LC-20AD), autosampler (SIL-20A), oven (CTO-20A), fraction collector (FRC-10A) and UV–Vis detector (SPD-20A). Reversed phase HPLC separation was carried out using a C18 column (250 × 4.6 mm) at room temperature. The mobile phase components were 5% acetonitrile (A) and 95% acetonitrile (B), both containing 0.1% TFA. The flow rate was set at 1.0 ml/minute. The mobile phase gradient was set in the following order:—B conc 10% (0–3 min), 22% (3.01–6 min), 35% (6.01–9 min), 48% (9.01–13 min), 60% (13.01–17 min), 73% (17.01–21 min), 85% (21.01–24 min), 95% (24.01–27 min), 100% (27.01–30 min), and 10% (30.01–35 min). All the phenolic and flavonoid standards were weighed for 1 mg/ml and dissolved either in 100% or 80% methanol, 100% ethanol and 95% acetonitrile depending on their solubility. The injection volume of the standard compounds and plant sample was 15 μl. The detection wavelength for all the standards and plant extracts were set at 254, 280 and 330 nm.
Electrospray ionization Mass Spectrometry (ESI–MS) was performed using Waters 2695 separation module coupled with Quattromicro™ API mass spectrometer (Waters, Milford, MA, USA). Data acquisition and analysis were carried out in waters MASS LYNX 4.1 software (Waters). The mass spectrometer was run in positive and negative ionization mode to acquire spectra in the range of 50–1000 m/z with the following parameters: capillary voltage, 3.0 kV; cone voltage, 30 V; and extractor, 3 V. Source temperature was 130 °C, desolvation, and cone gas flow rates were 450 and 80 l h−1 respectively. The scan time was 0.5 s and inter scan delay time was set as 0.1 s.
Cell culture and MTT assay
Cytotoxicity of the plant extracts was determined against human cancer cells using epidermoid carcinoma (A431) liver hepatocellular carcinoma (HEPG2) and osteosarcoma (MG63). The cell lines were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% of fetal bovine serum (FBS) and 1% of the penicillin–streptomycin solution. In-vitro cytotoxicity of the plant extracts was determined by cell viability study with the MTT reduction assay. Briefly, 5 × 104 cells per well were seeded into 96 well microtiter plate. Plates were incubated for 24 h at 37 °C with 5% CO2 and 95% relative humidity. The present culture media was removed, and fresh media was added after 24 h of seeding. Plant extracts were added at specific concentrations (2–10 mg/ml) to culture media in triplicates and re-incubated under same conditions mentioned above. Then culture media was removed from the plates, and 100 µl of freshly prepared MTT solution (0.5 mg/ml in media) was added to each well and incubated for 2 h. After that, 100 µl of DMSO solution was added under dark conditions and kept for 15 min at the room temperature. The absorbance of the formazan product was recorded at 595 nm in a microplate reader (Perkin Elmer, Waltham, MS, USA). The percentage viability of the cell was calculated by using the formula:
In-vivo CAM assay
The in vivo CAM assay was performed for the plant samples with slight modifications (Brooks et al. 1999). Fertilized duck eggs (0 days old) were procured from Midnapore poultry farm, West Bengal, India. They were cleaned and surface sterilized using 70% alcohol and then kept in an incubator under a humidified condition at 37 °C. 9 days old eggs were candled at blunt end to identify the air sac and to assure the existence of embryonic blood vessels. Then a small window was carefully created on the broader side of the egg. B. alba and B. rubra stem extract sample diluted in PBS (50 μl containing 7000 μg/egg) was then applied on a sterilized filter paper discs and allowed to dry at room temperature. After complete drying, discs were placed onto chorioallantoic membrane (CAM) of the egg. Windows were sealed with a permeable clear tape and eggs were further incubated for another 72 h (12th day). After that, CAM was photographed using a digital camera (Nikon Coolpix).
Statistical analysis
All experiments were carried out in triplicates (n = 3) and expressed as a mean ± standard deviation. The data were correlated using Pearson correlation coefficient at p < 0.05. The IC50 values were calculated using linear regression analysis.
Results and discussion
B. alba stem extracts show greater extractible yield of phenolics and flavonoids
The water extract of B. alba stem showed highest extraction yield (24%) compared to B. rubra stem (21%). The total phenolic content of B. alba and B. rubra in the present study was found to be 153.84 ± 19.23 and 134.61 ± 11.77 respectively in terms of Gallic acid equivalents (GAE)/g of dry weight. Compared to the earlier reports of (Kumar et al. 2015a, b, c; Olajire and Azeez 2011) higher phenolic content and greater value per gram of the dry weight of the extract was observed in this study. The flavonoid content was evaluated in terms of Quercetin/g of dry weight for B. alba and B. rubra and the values was found to be 115.64 ± 9.86 and 74.82 ± 11.77. The results clearly indicate substantial amounts of phenolics and flavonoids accumulation in these plant extracts. The higher amount of phenolic and flavonoid content in B. alba may be useful in fortification for exploring beneficial health effects.
Hydroxycinnamic acids, hydroxybenzoic acids and diosmetin accumulate in Basella sps
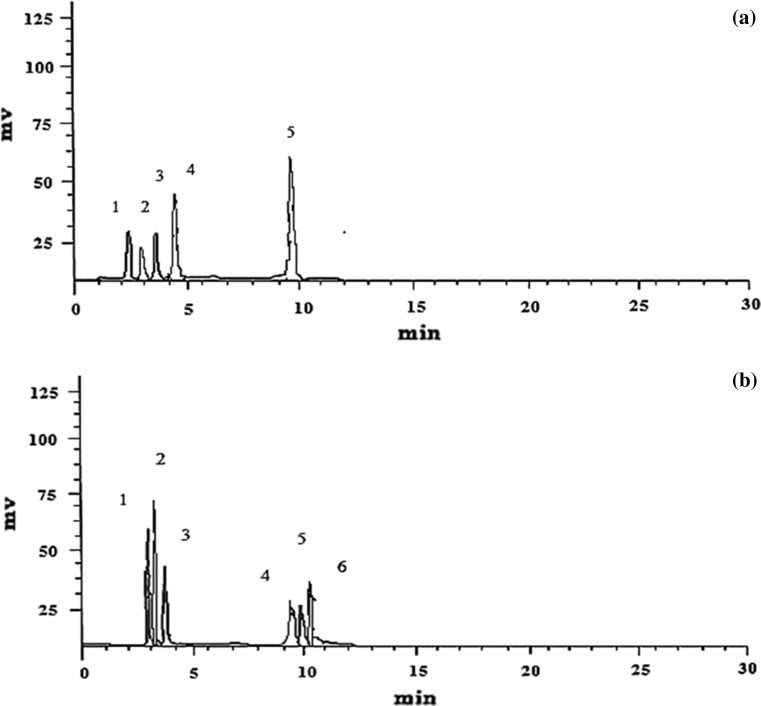
As shown in Fig. 1a and b, a total of five and six compound peaks have been detected in the stem extracts of both the plant species by HPLC. All the samples and standard compounds were injected and analyzed in triplicates to confirm the retention time of the isolated compound peaks in the HPLC chromatogram. The amount of individual separated phenolic compounds by HPLC is presented in Table 1. A higher concentration of p-Coumaric and caffeic acid than other phenolic compounds was observed in the aqueous extracts of Basella species.
Fig. 1.
HPLC separations of phenolic compounds from a B. alba stem (Peak 3—gallic acid; Peak 4—salicylic acid; Peak 5—ferulic acid) and b B. rubra stem (Peak 1—4-hydroxy benzaldehyde; Peak 2—caffeic acid; Peak 3—p-Coumaric acid; Peak 4—trans-cinnamic acid; Peak 5—diosmetin; Peak 6—galloyl shikimic acid)
Table 1.
Tentative identification and quantification of phenolic compounds in B. alba and B. rubra stem extracts by HPLC–ESI–MS method
| Tentative compound identity | Plant species | Molecular formula | Retention time (min) | [M+H]+ | [M–H]− | Total content (μg/g) |
|---|---|---|---|---|---|---|
| 4-hydroxy benzaldehyde | B. rubra | C7H6O2 | 2.51 | – | 121.1 | 9.35 ± 0.13 |
| Gallic acid | B. alba | C7H6O5 | 3.43 | – | 169.10 | 15.12 ± 0.07 |
| p-Coumaric acid | B. rubra | C9H803 | 3.85 | – | 163.0 | 59.65 ± 1.49 |
| Salicylic acid | B. alba | C7H6O3 | 4.36 | – | 137.02 | 16.16 ± 0.49 |
| Caffeic acid | B. rubra | C9H8O4 | 2.55 | – | 179.2 | 40.44 ± 1.04 |
| Ferulic acid | B. alba | C10H10O4 | 10.00 | 195.97 | – | 20.25 ± 0.40 |
| Trans-cinnamic acid | B. rubra | C9H8O2 | 9.25 | 149.3 | – | 15.08 ± 0.11 |
| Galloyl shikimic acid | B. rubra | – | 11.03 | – | 325.1 | 5.77 ± 0.34 |
| Diosmetin | B. rubra | C16H12O6 | 10.86 | 301.4 | – | 13.61 ± 0.18 |
LC–MS provides rapid identification of bioactive constituents in plant samples through efficient separation capabilities of HPLC and exact structural characterization by MS simultaneously (Kumar 2017). This method not only provides molecular mass of different constituents with different m/z values but can also differentiate the nature of those compounds by the position of functional groups present in them.
The evaluation of structural–functional relationships is essential to determine the biological effects exhibited by the bioactive compounds and to understand their mechanisms. These effects are generally due to the structural complexity (i.e. number and positioning of hydroxyl groups) of the phenolic compounds. Therefore, the potency of an individual bioactive compound can be altered by the modification of their functional groups for enhanced activity (Razzaghi-Asl et al. 2013). The present study reveals a range of phenolic acids in B. alba and B. rubra stem extracts identified for the first time by LC–MS. The simple phenolics of the phenylpropanoid pathway are important precursors for several complex structures that arise later in the branched pathways. These are also very reactive molecules. Their reactivity as individual as well as in the grouped state has been demonstrated in some systems. The structural change in compounds has been shown to increase or decrease the bioactivity of the initial phenolic acids (Piazzon et al. 2012). Hydroxy-cinnamic acids (HCAs) exhibits higher antioxidant activity than hydroxy-benzoic acids (HBAs) due to the presence of CH=CH–COOH group which ensures greater hydrogen donating ability and radical stabilizing ability (Balasundram et al. 2006).
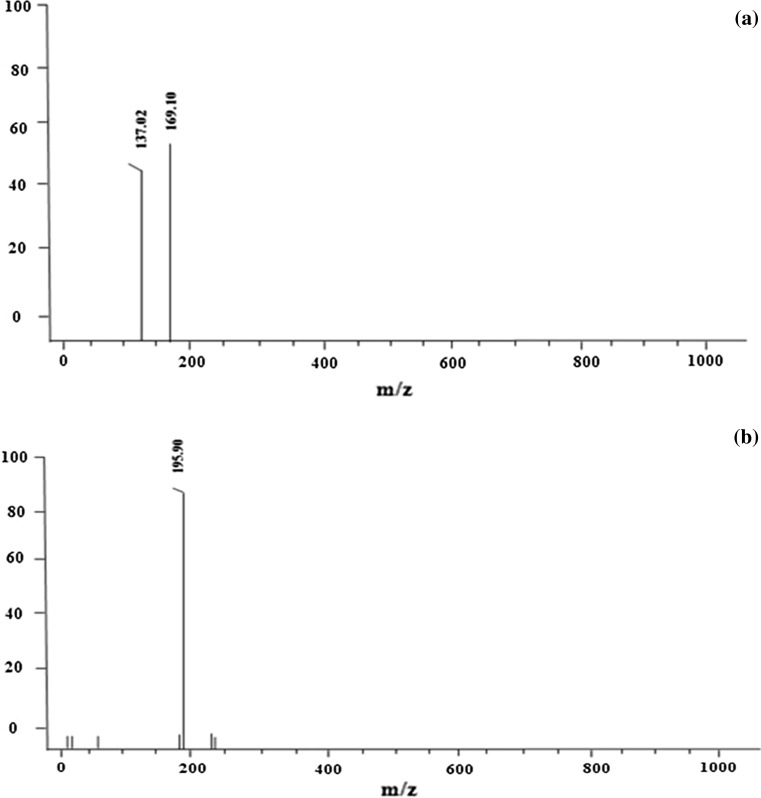
The analysis of phenolic acids is done in both ESI positive and negative ion modes. But based on the results of the present study, negative ionization was found to be more sensitive for the detection of these compounds. Three phenolic acids were tentatively identified and characterized for the first time in B. alba stem extract by LC–ESI–MS in both ESI (+ve) and ESI (−ve) mode are shown in Fig. 2a, b. ESI mode was preferred because it is favourable for the compounds eluting at polar phase. The identification was done based on the comparison of mass spectra of compounds characterized from plant samples with those of authentic standards. The identification of these led to their allocation in two structurally related groups, i.e., hydroxy- benzoic acids and hydroxy-cinnamic acids. Salicylic acid (Fig. 2a) was identified based on the characteristic product ion at m/z 137 ([salicylic acid−H]−). The same spectrum with m/z 169.10 was identified as gallic acid after comparing its retention time, UV spectra and mass spectra with those of standard compounds. Both these compounds were identified in negative ionization. Ferulic acid (Fig. 2b) with characteristic product ion was observed at m/z 195 [M+H]+. For all these identified compounds no fragment peaks were observed based on literature review. The phenolic acids tentatively identified by our LC–ESI–MS analysis are in agreement with reports from other plant species (Bertin et al. 2014; Ostrowski et al. 2014; Biswas and Roymon 2013) as there are no reports available on ESI–MS analysis of phenolic compounds from Basella.
Fig. 2.
Tentative identification of a salicylic acid ESI (−ve mode), b gallic acid ESI (−ve mode) and c ferulic acid ESI (+ve mode) in the stem extract of B. alba
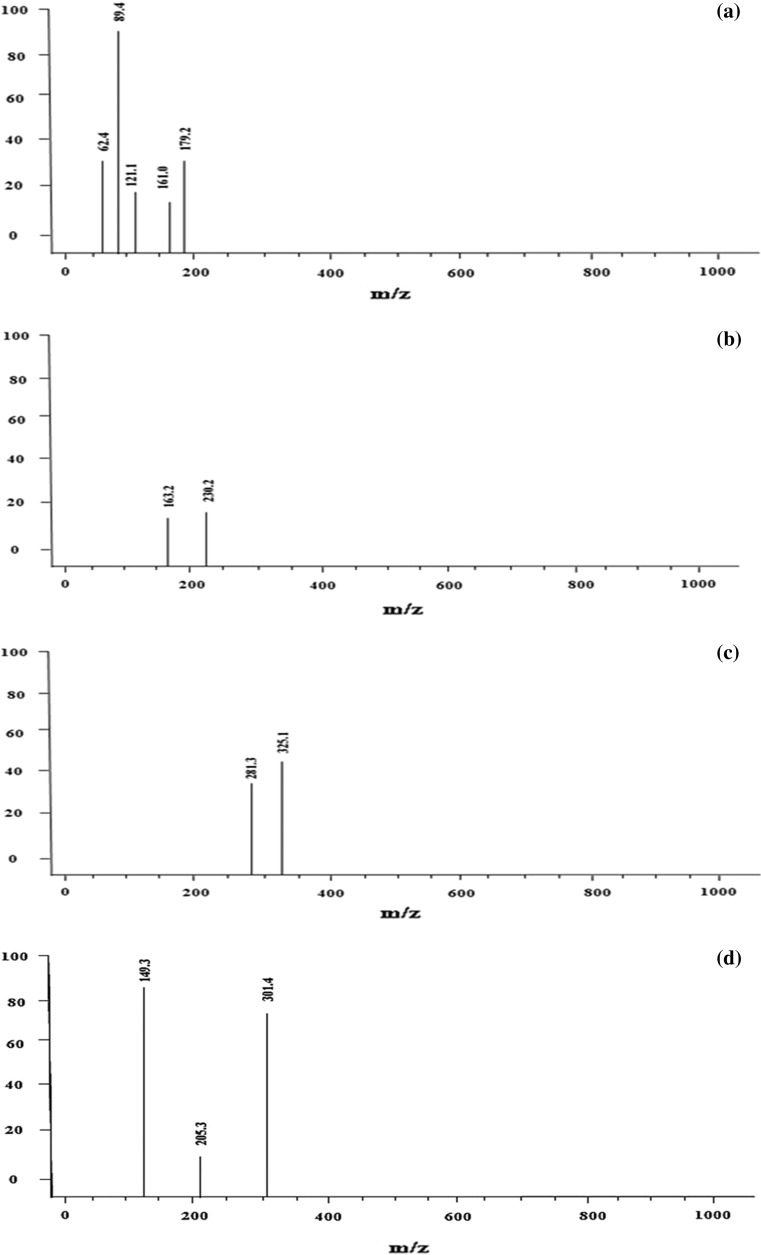
A total of five phenolic acids were tentatively identified in B. rubra stem extract represented in Fig. 3a–d m/z value of 179.12 (Fig. 3a) corresponds to Caffeic acid (Khanam et al. 2012). Further, the characteristic fragment ion of m/z 161.0 was observed due to the loss of water molecule [M–H–H2O]−. The same spectrum with compound peak at m/z 121.1 was identified as 4-hydroxy benzaldehyde which is a simple phenolic acid (Santi and Dipjyoti 2013). Hydroxy-benzaldehydes are regarded as an important group of natural products with good medicinal value and are of great industrial importance. They are known to exhibit anti-bacterial, anti-fungal, anti-tyrosinase, anti-acetylcholisterenase and anti-cancer properties from various medicinal plants (Anish and Adinpunya 2016). The characteristic fragment ion observed at m/z (89.4) and (62.4) corresponds to the loss of [M–H–CH3OH]− and [M–CO2+CH3]− respectively. Compound peak with parent ion [M–H]− at m/z 163.2 (Fig. 3b) was tentatively identified as the p-Coumaric acid in agreement with the reported literature (Stintzing et al. 2004). The parent ion [M–H]− at m/z 325.1 (Fig. 3c) corresponds to galloyl shikimic acid which formed fragment ion at m/z 281.3 indicates the neutral loss of a [M–H–CO2]−. The obtained m/z values are in agreement with the literature (Ersan et al. 2016). Figure 3d shows two compound peaks which was identified in ESI (+ve mode) corresponds to trans-cinnamic acid whose parent ion [M+H]+ was observed at m/z 149.3 (Slavin and Lloyd 2012) The only flavonoid to be tentatively identified from B. rubra stem was diosmetin, a O-methylated flavone whose m/z value was observed at 301.4 (Habib et al. 2017). This is the first study confirming the presence of methoxy flavones in Basella species. The identification of methylated flavones (Diosmetin) in this study is a novel finding.
Fig. 3.
Tentative identification of a 4-OH benzaldehyde; caffeic acid ESI (−ve mode), b p-Coumaric acid ESI (−ve mode), c galloyl shikimic acid ESI (−ve mode) and (d) trans-cinnamic; diosmetin ESI (+ve mode) in the stem extract of B. rubra
Both species of Basella possess good radical scavenging activity
The present study reports good radical scavenging activity in case of B. alba and B. rubra as compared to that reported by (Kumar et al. 2015a, b, c; Olajire and Azeez 2011). The percent inhibition of the extracts was calculated in the concentration range of (50–400 μg/ml). The results revealed a % inhibition value of 43.01 ± 5.47 and 34.48 ± 2.84 for B. alba and B. rubra stem extracts respectively. The higher content of phenolics and flavonoids in Basella stem extracts might be contributing to the free radical scavenging ability.
The reducing power of a biochemical compound is an important parameter to evaluate its antioxidant activity. The presence of antioxidants in the extract causes reduction of ferric form to its ferrous form which results in the change of the yellow colour of the reaction mixture to Prussian blue (Kumar et al. 2015a, b, c).
Basella alba and Basella rubra tested extracts showed higher reducing activity with increasing concentration. The reducing capacity of B. alba was found to be slightly higher than B. rubra. A single study on B. rubra fruits reported moderate reducing capacity of the water and methanol extracts at 25 mg/ml (Kumar et al. 2015a, b, c). Our plant extracts showed lesser absorbance values than the reference standard (Ascorbic acid). The results obtained from the antioxidant assays reveal that these plant extracts have the capability to act as good electron donors and can terminate the free radical chain reactions.
Anti-proliferative activity of Basella stem extracts
The extracts of B. alba and B. rubra against A431, Hep G2 and MG63 cell lines in a concentration-dependent manner (2–10 mg/ml). B. alba exhibited higher activity in these three cancer cell lines (IC50 = 3.66 ± 0.17 mg/ml for A431, 7.22 ± 0.13 mg/ml for HepG2, and 6.61 ± 0.01 mg/ml for MG63 cells) and revealed significantly higher growth inhibitory activity than the B. rubra (IC50 = 6.87 ± 0.15 mg/ml for A431, 7.67 ± 0.13 mg/ml for HepG2 and 7.31 ± 0.023 mg/ml for MG63). B. alba extract inhibited cell proliferation of epidermoid carcinoma (A431) cells from 37.95% at 2 mg/ml to 84.86% at 10 mg/ml, displaying a concentration-dependent tendency. The cytotoxic activity of both of the plant species was found almost similar for HepG2 cells. B. alba showed slightly higher growth inhibitory activity against MG63 cells.
For the first time, potential growth inhibitory activity of this species has been shown against epidermoid carcinoma, osteosarcoma, and liver hepatocellular carcinoma cell lines. The present study demonstrates more potent cytotoxic activity of Basella species at lesser concentrations. Some studies report the potential anti-cancer properties of ferulic acid, gallic acid, salicylic acid, caffeic acid and cinnamic acid has been found as potent anticancer agents (Anatharaju et al. 2016). It is possible that the phenolics present in Basella sps extracts may be contributing to the anti-proliferative activity.
Basella sps stem extracts show anti-angiogenic activity
Angiogenesis is the formation of new blood vessels from existing vasculature (Tai-Ping et al. 2006). Angiogenesis is a critical step in tumor growth, invasion, and metastasis and its inhibition is a promising strategy for cancer treatment (Shenbhagaraman et al. 2012).
The experiment was carried out to determine the effects of Basella extracts on the vascular density index of the 12 day eggs. The CAM model has certain advantages over other methods and is useful to observe changes in the ongoing process and provides easy manipulation of dose levels of the samples used (Chun-Hua et al. 2016). The CAM results indicated that the stem extracts of B. alba and B. rubra has suppressed angiogenesis on CAM (Fig. 4d). The results reveal that the plant extracts at dose level of 7 mg/ml has significantly reduced the number of vessels development compared with the control (PBS-treated group). Hence, we may conclude that aqueous stem extract of B. alba and B. rubra can be used as easily accessible sources of natural antioxidants for potential preventative therapies against cancer and angiogenesis-related diseases.
Fig. 4.
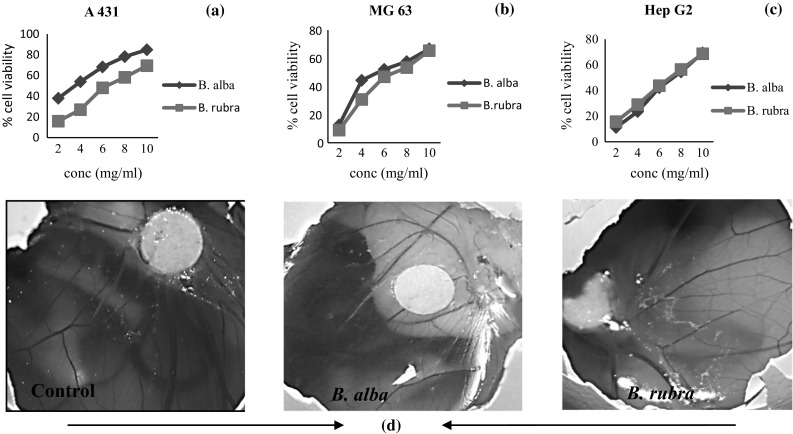
Anti-proliferative activity of B. alba and B. rubra stem extracts against a A431, b HepG2 and c MG63 cell lines and their d anti-angiogenic activity
Conclusion
Stems of B. alba and B. rubra were investigated for its phenolic compounds accumulation, mainly focussing on antioxidant activity, secondary metabolites profiling, anti-proliferative and anti-angiogenic activity. The present study demonstrates good amount of phenolics and flavonoids accumulation through quantitative analysis in both the solvents used for Basella species. Further the antioxidant activity of the aqueous plant samples observed in both the plant species is possibly due to these phenolic compounds. Qualitative analysis revealed the presence of a flavone, hydroxy-benzoic and hydroxy-cinnamic acids. This study further demonstrates anti-proliferative as well as anti-angiogenic activity of Basella sps extracts. Extracts from both the plant species used exhibited anti-proliferative activity against A431, MG 63 and Hep G2 cell lines for the first time. Angiogenesis inhibition suppresses tumor growth, progression and metastasis. The identification of dietary sources of anti-angiogenic molecules is a step towards development of potential nutraceuticals for reducing cancer risk. The biological activity of methylated flavonoids such as antiviral, anti-inflammatory properties and prevention of cell damage have been studied in some plant systems. Further studies on inhibitory activity of the extracts on certain enzymes will reveal further insights into the cancer pathway. The findings in the study provide an additional rationale for the traditional uses of the plants and the potential of identified compounds from Basella species to be used in food and pharmaceutical industries.
Acknowledgements
The authors acknowledge the funding support provided by Ministry of Human Resource Development (MHRD) New Delhi, Government of India. We gratefully acknowledge the support from Department of Chemistry, IIT Kharagpur and School of Medicine Science and Technology, IIT Kharagpur.
Compliance with ethical standards
Conflict of interest
All authors declare no conflict of interest.
References
- Anatharaju PG, Gowda PC, Vimalambike MG, Madhunapantula SV. An overview on the role of dietary phenolics for the treatment of cancers. Nutr J. 2016;15:1–16. doi: 10.1186/s12937-016-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anish K, Adinpunya M. Methoxybenzaldehydes in plants: insight to the natural resources, isolation, application and biosynthesis. Curr Sci. 2016;111:1325–1334. doi: 10.18520/cs/v111/i8/1325-1334. [DOI] [Google Scholar]
- Balasundram N, Sundran K, Samman S. Phenolic Compounds in plants and agri-industrial by-products: antioxidant activity, occurrence and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- Bertin RL, Gonzaga LV, Graciele da Silva CB, Azevedo MS, Maltez HF, Heller M, Micke GA. Nutrient composition and identification/quantification of major phenolic compounds in Sarcocornia ambigua (Amaranthaceae) using HPLC-ESI-MS/MS. Food Res Int. 2014;55:404–411. doi: 10.1016/j.foodres.2013.11.036. [DOI] [Google Scholar]
- Biswas D, Roymon MG. LC/TOF/ESI/MS detection of bioactive compounds present in leaf and bark extract of Acacia Arabica. Recent Res Sci Technol. 2013;5:37–40. [Google Scholar]
- Brand- Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Brooks PC, Montgomery AM, Cheresh DA. Use of the 10-day-old chick embryo model for studying angiogenesis. Methods Mol Bio. 1999;129:257–269. doi: 10.1385/1-59259-249-X:257. [DOI] [PubMed] [Google Scholar]
- Chun-Hua C, Ting-Tsz O, Mon-Yuan Y, Chi-Chou H, Chau-Jong W. Nelumbo nucifera Gaertn leaves extracts inhibits the angiogenesis and metastasis of breast cancer cells by down regulation connective tissue growth factor (CTGF) mediated P13K/AKT/ERK signaling. J Ethnopharmacol. 2016;88:111–122. doi: 10.1016/j.jep.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Erşan S, Ustundag OG, Carle R, Schweiggert RM. Identification of phenolic compounds in red and green pistachio (Pistacia vera L.) Hulls (Exo- and Mesocarp) by HPLC-DAD-ESI-(HR)-MSn. J Agric Food Chem. 2016;64:5334–5344. doi: 10.1021/acs.jafc.6b01745. [DOI] [PubMed] [Google Scholar]
- Gupta S, Prakash J. Studies on Indian green leafy vegetables for their antioxidant activity. Plant Foods Hum Nutr. 2009;64:39–45. doi: 10.1007/s11130-008-0096-6. [DOI] [PubMed] [Google Scholar]
- Habib S, Vahid N, Mehdi T, Hasan E. Ultrasound- assisted extraction process of phenolic antioxidants from Olive leaves: a nutraceutical study using RSM and LC-ESI-DAD-MS. J Food Sci Technol. 2017;54:2361–2371. doi: 10.1007/s13197-017-2676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam MC, Petit J, Zimmer D, Djantou EB, Scher J. Effects of drying and grinding in production of fruit and vegetable powders: a review. J Food Eng. 2016;188:32–49. doi: 10.1016/j.jfoodeng.2016.05.001. [DOI] [Google Scholar]
- Khanam UKS, Oba S, Yanase E, Murakami Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J Funct Foods. 2012;4:979–987. doi: 10.1016/j.jff.2012.07.006. [DOI] [Google Scholar]
- Kilari BP, Kotakadi VS, Penchalaneni J. Anti-proliferative and apoptotic effects of Basella rubra (L.) Against 1, 2-Dimethyl Hydrazine-induced colon carcinogenesis in rats. Asian Pac J Cancer Prev. 2016;17:73–80. doi: 10.7314/APJCP.2016.17.1.73. [DOI] [PubMed] [Google Scholar]
- Kumar BR. Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs) J Pharm Anal. 2017;7:349–364. doi: 10.1016/j.jpha.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Prasad AK, Iyer SV, Vaidya SK. Systematic pharmacognostical, phytochemical and pharmacological review on an ethno medicinal plant, Basella alba L. J Pharmacogn Phytother. 2013;5:53–58. [Google Scholar]
- Kumar SS, Manoj P, Giridhar P, Shrivastava R, Bharadawaj M. Fruit extracts of Basella rubra that are rich in bioactives and betalains exhibit antioxidant activity and cytotoxicity against human cervical carcinoma cells. J Funct Foods. 2015;15:509–515. doi: 10.1016/j.jff.2015.03.052. [DOI] [Google Scholar]
- Kumar SS, Manoj P, Shetty NP, Prakash M, Giridhar P. Characterization of major betalain pigments -gomphrenin, betanin and isobetanin from Basella rubra L. fruit and evaluation of efficacy as a natural colourant in product (ice cream) development. J Food Sci Technol. 2015;52:4994–5002. doi: 10.1007/s13197-014-1527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Manoj P, Giridhar P. A method for red-violet pigments extraction from fruits of Malabar spinach (Basella rubra) with enhanced antioxidant potential under fermentation. J Food Sci Technol. 2015;52:3037–3043. doi: 10.1007/s13197-014-1335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Manoj P, Nimisha G, Giridhar P. Phytoconstituents and stability of betalains in fruit extracts of Malabar spinach (Basella rubra L.) J Food Sci Technol. 2016;53:4014–4022. doi: 10.1007/s13197-016-2404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olajire AA, Azeez L. Total antioxidant activity, phenolic, flavonoids and ascorbic acid contents of Nigerian vegetables. Afr J Food Sci. 2011;2:22–29. [Google Scholar]
- Ostrowski W, Wojakowaska A, Grajzer M, Stobiecki M. Mass spectrometric behavior of phenolic acids standards and their analysis in the plant samples with LC/ESI/MS system. J Chromatogr B. 2014;967:21–27. doi: 10.1016/j.jchromb.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Padmavati M, Reddy AR. Flavonoid pathway and cereal defense response: an emerging trend in crop biotechnology. J Plant Biochem Biotechnol. 1999;8:15–20. doi: 10.1007/BF03263051. [DOI] [Google Scholar]
- Padmavati M, Sakthivel N, Thara KV, Reddy AR. Differential sensitivity of rice pathogens to growth inhibition by flavonoids. Phytochemistry. 1998;46:499–502. doi: 10.1016/S0031-9422(97)00325-7. [DOI] [Google Scholar]
- Piazzon A, Vrhovsek U, Masuero D, Mattivi F, Mandoj F, Nardini M. Antioxidant activity of phenolic acids and their metabolites: synthesis and antioxidant properties of the sulfate derivatives of ferulic and caffeic acids and of the acyl glucuronide of ferulic acid. J Agric Food Chem. 2012;60:12312–12323. doi: 10.1021/jf304076z. [DOI] [PubMed] [Google Scholar]
- Priya K, Gupta A, Mahajan S, Agnihotri RK, Sharma R. Evaluation of antimicrobial properties of Basella rubra methanolic extracts on selected microorganisms. Int J Pharma Sci Drug Res. 2015;6:334–336. [Google Scholar]
- Razzaghi-Asl N, Garride J, Khazraei H, Borges G, Firuzi D. Antioxidant properties of hydroxycinnamic acids: a review of structure activity relationships. Curr Med Chem. 2013;20:4436–4450. doi: 10.2174/09298673113209990141. [DOI] [PubMed] [Google Scholar]
- Santi MM, Dipjyoti C. Mass spectrometric detection of Phenolic acids. In: Ramawat KG, Merillion JM, editors. Natural products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes. Berlin, Heidelberg, New York: Springer; 2013. pp. 2047–2057. [Google Scholar]
- Shenbhagaraman R, Jagadish LK, Premalatha K, Kaviyarasan V. Optimization of extracellular glucan production from Pleuotus eryngii and its impact on angiogenesis. Int J Biol Macromolec. 2012;50:957–964. doi: 10.1016/j.ijbiomac.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agro climatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv Nutr. 2012;3:506–516. doi: 10.3945/an.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzing FC, Kammerer D, Schieber A, Adama H, Nacoulma OG, Carle R. Betacyanins and Phenolic compounds from Amaranthus spinosus L. And Boerhavia erecta L. Zeitschrift fur Naturforschung. 2004;59:1–8. doi: 10.1515/znc-2004-1-201. [DOI] [PubMed] [Google Scholar]
- Tai-Ping F, Ju-Ching Y, Kar-Wah L, Yue PYK, Wong RNS. Angiogenesis: from plants to blood vessels. Trends Pharmacol Sci. 2006;27:297–309. doi: 10.1016/j.tips.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Zhishen J, Mencheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effect on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]