Abstract
Alginate is a non-toxic, renewable, and linear copolymer obtained from the brown algae Laminaria digitata that can be easily shaped into beads. Its good gel forming properties have made it useful for entrapping food and pharmaceutical ingredients. In this study, alginate beads were used in a novel application as a colorimetric sensor in food intelligent packaging. Colorimetric sensor was developed through entrapping red cabbage extract as a pH indicator in alginate beads. The pH indicator beads were used in rainbow trout packaging for monitoring fillets spoilage. Color change of beads during fish storage was measured using the CIELab method. The alginate bead colorimetric sensor is validated by measuring total volatile basic nitrogen (TVB-N) levels and microbial populations in fish samples. Moreover, peroxide value (PV) and thiobarbituric acid reactive substances (TBARS) were evaluated during storage. Results indicated that increasing the bacterial population during storage and production of proteolytic enzymes resulted in protein degradation, accumulation of volatile amine compounds, increase in the pH and finally color change of alginate beads. The values of TVB-N, pH, PV and TBARS increased with time of storage. The results of TVB-N and microbial growth were in accordance with color change of beads and CIELab data. Therefore, the proposed system enjoys a high sensitivity to pH variations and is capable of monitoring the spoilage of fish or other protein-rich products through its wide range of color changes. The alginate beads containing the red cabbage extract can, thus, be used as a low-cost colorimetric sensor for intelligent packaging applications.
Keywords: Alginate beads, Colorimetric sensor, Red cabbage, Fish spoilage, pH indicator
Introduction
The growth of microorganisms, especially that of bacteria, in food products results in food spoilage and reduced shelf life. Consumption of such contaminated foods increases the risk of food-borne diseases (Kuswandi et al. 2012). Consumer safety can be secured by detecting food quality in a simple, rapid, sensitive, and efficient manner (Cristina Silva-Pereira et al. 2015). A major challenge facing the food industry is the early and real-time detection of food spoilage during storage. For this purpose, intelligent packaging with the ability to monitor food conditions in real time offers a good opportunity for developing a simple, low-cost, non-invasive, and non-destructive method of food spoilage detection. One type of intelligent packaging is the one that uses changes in pH to determine food freshness. The simplest intelligent package based on changes in pH is one that enables the consumer to detect visually food spoilage.
Microorganisms growing in many foodstuffs such as fish produce a variety of metabolites that lead to changes in pH. Fresh fish is highly perishable due to its biological composition and high protein content and its spoilage occurs as the result of biological changes and chemical reactions such as lipid oxidation, reactions caused by the activities of fish enzymes, and the metabolic activities of microorganisms that live on fish skin and gill surfaces where they rapidly increase in numbers after fishing and during storage (Ojagh et al. 2010; Kuswandi et al. 2012).
Pseudomonas spp. is the most common microorganism growing on fish that can be successfully used to determine the shelf life of stored fresh fish under aerobic conditions (Gram and Huss 1996). They decompose fish proteins to produce volatile amines such as ammonia, histamine, putrescine (Put), and cadaverine (Cad) (Morsy et al. 2016). Two other volatile amines, including trimethylamine (TMA) and dimethylamine (DMA), are originated from trimethylamine oxide (TMAO), which is naturally present in the living tissue of many marine fish species as important part of the non-protein nitrogen fraction. During storage, TMAO is demethylated to equimolar formaldehyde and DMA by endogenous enzymes and bacterial reduction of TMAO leads to TMA production with the typical ‘fishy’ odor of spoiling seafood. However, the indictor of seafood’s freshness would rely on the total volatile basic nitrogen (TVB-N) instead of just TMA (Chung and Chan 2009).
TVB-N levels essentially composed of TMA, DMA, and ammonia cause increases in stored fish pH, which can be a potential indicator of fish spoilage. Thus, it is essential to develop a simple and sensitive method based on pH to monitor fish freshness.
Many sensors and indicators have been in recent years developed for the detection of fish spoilage. Some of these include colorimetric sensors (Morsy et al. 2016; Huang et al. 2011; Kuswandi et al. 2012; Pacquit et al. 2006, 2007; Cristina Silva-Pereira et al. 2015), potentiometric electrodes (Gil et al. 2008), electronic noses (Natale et al. 2001), and gas sensors (Alimelli et al. 2007). However, a simple, accurate, inexpensive, and reliable pH-based packaging system is still needed to be designed and manufactured that allows for the visual inspection of fish freshness and spoilage.
Alginate is an anionic polymer produced by brown algae and bacteria. It consists of α-L-guluronic acid (G) and β-D-mannuronic acid (M) residues linearly linked by 1,4-glycosidic linkages (Pawar and Edgar 2012). Alginate is non-toxic, biodegradable, low-cost, and readily available (Goulas and Kontominas 2005). This compound is used in the form of biodegradable packaging films and edible coatings in a variety of pharmaceutical applications (Babu et al. 2007) and in the food industry (Da Silva et al. 2009; Azarakhsh et al. 2014). Alginate beads can be prepared by dissolving alginate in water and having it react with polyvalent metal cations such as calcium so that alginate biopolymer chains are cross-linked through the bivalent calcium cations to form an interconnected open pore network (Harper et al. 2014; Klock et al. 1997). This net-like three dimensional structure is suitable for the entrapment of a variety of compounds such cells (plant cells, animal cells, yeast, mold, and bacteria) (Branyik et al. 2005; Verbelen et al. 2006), enzymes (Nguyen et al. 2016; Won et al. 2005), or even synthetic chemicals.
Anthocyanins belong to a group of naturally occurring phenolic compounds called flavonoids. They are water-soluble pigments and responsible for the coloration of flowers, fruits and vegetables that, depending on their pH, may appear red, purple, or blue (Abolghasemi et al. 2016). Anthocyanin-rich plant food include the raspberry, blueberry, black rice and red cabbage. Interest in the anthocyanins has increased because of their potential as natural and non-toxic colorants as well as beneficial health effects.
The present authors hypothesized that alginate beads could be used in the production of intelligent packaging systems in which a porous alginate bead matrix would encapsulate a natural substance, such as the anthocyanin pigment extracted from red cabbage. The system will be sensitive to changes in pH by absorbing total volatile basic nitrogen in order to detect and monitor fish spoilage. To the best of our knowledge, alginate beads were first used as sensor to monitor food quality. Thus, in this study, we report a novel colorimetric sensor based on alginate beads for monitoring rainbow trout spoilage.
It is the objective of this study to develop and optimize the proposed packaging system as a fully natural, non-toxic, inexpensive, biocompatible, and novel colorimetric sensor for easy application in the food industry.
Materials and methods
Chemicals
Sodium alginate was purchased from Sigma-Aldrich (St. Louis, USA). Calcium chloride, hydrochloric acid, boric acid, methyl red, butanol, thiobarbituric acid, and plate count agar (PCA) culture media were purchased from Merck Co. (Darmstadt, Germany). Red cabbage was prepared from the local vegetable market (Shiraz, Iran).
Anthocyanin extraction
A sample of approximately 150.0 g of red cabbage was crushed and macerated in a beaker containing 80 ml of distilled water. The macerated sample was extracted for 5 min via a 200-W microwave irradiation device. The extract was then filtered before it was centrifuged for 15 min at 1000 rpm to remove any impurities. Total anthocyanin content of red cabbage was measured as milligrams of cyanidin-3-glucoside (Cy3G) per 100 g of fresh red cabbage and was 126 mg Cy3G/100 g. The pH of the extract was adjusted to 5.0 using HCl (0.1 M). The prepared sample containing anthocyanin pigment was stored at 4 °C in a dark bottle until use.
Preparation of pH indicator calcium alginate bead
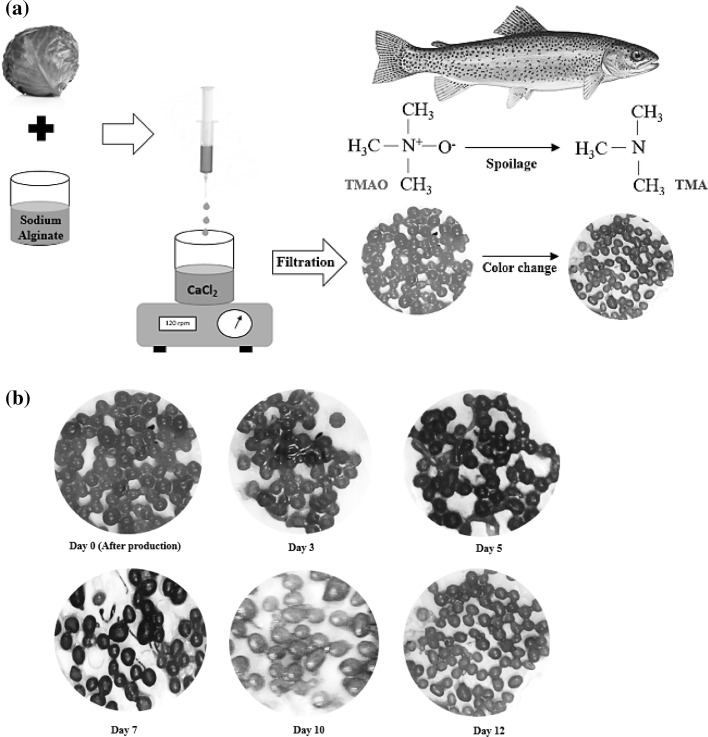
An alginate solution was prepared by dispersing 2% (w/v) sodium alginate in anthocyanin extracted from red cabbage. The solution was stirred for 1 h at 500 rpm using a mechanical stirrer to form a uniform mixture. Calcium alginate beads were then prepared by injecting the sodium alginate solution into a calcium chloride solution to obtain a final concentration of 0.2 M. This converted the sodium alginate from a water-soluble solution to the water-insoluble calcium alginate beads. The beads containing anthocyanin pigments were removed by filtration and washed with deionized water to remove the excess CaCl2. The steps for the production of the colorimetric sensor based on alginate beads are illustrated in Fig. 1a.
Fig. 1.
a Schematic representation of the production process of the colorimetric sensor based on alginate beads and its application in fish spoilage detection; b changes in alginate bead color containing the red cabbage extract during storage at refrigeration temperature
Preparation of fish samples
Fresh water rainbow trout (Oncorhynchus mykiss) with an average weight of 1500 g were purchased from a public market (Shiraz, Iran) and transferred to the laboratory packed in an insulated ice box. TMAO content of the rainbow trout was reported to be 0.31 g kg−1 (wet weight). However, this value can be changed depending on the nutrition and the season (RØrvik et al. 2000). The fishes were aseptically headed and filleted by knife to obtain uniform cubical samples of equal weight (approximately 30 g). All the fish samples were immediately placed in sterilized petri dishes. A number of alginate beads as the pH indicator were fixed on the inner side of the lid of the petri dishes using a liquid stick such that the beads would be facing the headspace standing 3 cm above the fish samples. Closed petri dishes were stored at room temperature for 64 h and at 4 °C for 12 days for spoilage monitoring. At specified intervals during the storage period, the samples were analyzed to detect any chemical or microbial changes. Reference petri dishes contain no fish samples were also maintained over the same experimental period as the ones containing fish samples.
Physico -chemical analyses
pH, total volatile basic nitrogen (TVB-N), and thiobarbituric acid (TBA) were determined in all the samples in triplicate (n = 3).
pH measurement
pH measurements were performed using a digital pH-meter (STARTER 3000, OHAUS Co., Switzerland). Minced fish samples (5 g) were homogenized in 10 ml of distilled water before being subjected to the filtration step. The pH of the filtrate was measured at ambient temperature.
Determination of total volatile basic nitrogen (TVB-N)
Total volatile basic nitrogen (TVB-N) values of rainbow trout fillets were determined according to the method of Goulas and Kontominas (2005). Briefly, 10 g of the fish sample, 2 g of magnesium oxide, 500 ml of water, a few pieces of boiled stones, and some paraffin used as an antifoam agent were added to the Kjedahl distillation flask followed by distillation of the mixture. The distillate was collected in 25 ml of 3% aqueous solution of boric acid and 0.04 ml of a mixed indicator including methyl red and methylene blue (each at a concentration of 0.1% (w/v) in ethanol). Then, the boric acid solution was titrated using a 0.1 N sulfuric acid. The titration was terminated when the color of the distillate turned from yellow to purple upon addition of a further drop of sulfuric acid. The quantity of TVB-N in mg/100 g of fish sample was calculated using the volume (V) of sulfuric acid added and its concentration (C) according to the following relation:
| 1 |
Determination of thiobarbituric acid reactive substance (TBARS)
In this study, the thiobarbituric acid (TBA) content of the fish samples was evaluated by the colorimetric method based on spectrophotometric quantitation of the pink complex formed due to the reaction of one molecule of malondialdehyde (MDA) with two molecules of 2-thiobarbituric acid (Goulas and Kontominas 2005). Briefly, 200 mg of a homogenized fish sample was weighed into a 25-ml volumetric flask where the sample was dissolved in 1 ml of 1-butanol. The mixture was made to volume and mixed well. Afterwards, 5.0 ml of the mixture was poured into a dry stoppered test tube containing 5 ml of thiobarbituric reagent. The stoppered test tubes were vortexed and heated in a boiling water bath for 90 min. After cooling to room temperature in an ice bath, the absorbance (As) at 532 nm was measured against water as the blank using a UV–Vis spectrophotometer (model 2100, UNICO, USA). The absorbance of the reagent blank (Ab) was recorded and the absorbance values were converted to the TBA value (mg of malonaldehyde equivalents/kg of tissue) using the following formula (Cai et al. 2014).
| 2 |
Microbiological analysis
As fish spoilage was being monitored by the pH indicator alginate beads, the samples were subjected to microbiological analysis at regular intervals. In order to determine of bacteriological counts, 10 g of the fillet sample was added to 90 ml of 0.85% NaCl solution under aseptic conditions. The mixture was homogenized with a stomacher. Other decimal dilutions were prepared from this dilution and plated in the appropriate media in Petri dishes. Enumeration of total viable aerobic bacterial counts was performed according to the pour plate method using the plate count agar (PCA purchased from Merck, Darmstadt, Germany). The inoculated Petri dishes were incubated at 37 °C for 2 days to determine the mesophilic counts, and at 10 °C for 7 days to determine the psychrotrophic counts. The colony forming unit (CFU) counts were expressed as log10 CFU/g. All the microbiological experiments were performed in duplicate (Ojagh et al. 2010).
Colorimetric analysis
Images of the pH indicator alginate beads were captured during the fish sample storage using a digital camera installed on top of a light chamber with a white background light under constant lighting conditions. These images were used to extract colorimetric data using Photoshop 7.0 software to isolate CIELab coordinates or L*, a*, b* values to describe the color of the pH indicator alginate beads. L* is lightness, a* is deviation towards green (negative values) or red (positive values), and b* is deviation towards blue (negative values) or yellow (positive values). The color change (ΔE) of alginate beads during storage was determined in comparison to the reference alginate beads (as produced) using the Eq. (3).
| 3 |
where ΔE is color change; L2*, a2* and b2* are the color attributes of the alginate beads at each interval during the storage period; L1*, a1* and b1* are the color parameters of the reference alginate beads.
Statistical analysis
All the experiments were replicated three times and analyzed using one-way analysis of variance by SAS software (ver. 9.1, SAS Institute Inc., Cary, NC). Duncan’s multiple range tests were done to compare the means at a confidence level of 0.05. All the graphs were plotted using Microsoft Excel 2013.
Results and discussion
pH indicator calcium alginate beads
In this study, the best alginate bead in terms of its shape, stability, and sensitivity was selected using four sodium alginate concentrations (1, 1.5, 2, and 2.5%) and four calcium chloride concentrations (0.05, 0.1, 0.2, and 0.3 M). It was found that the beads prepared with 2% sodium alginate and 0.2 M calcium chloride were spherical in shape and recorded the best sensitivity toward volatile amine content as reflected in color changes. The beads became non-spherical (egg-shaped) and their sensitivity declined when sodium alginate concentration was kept constant but that of calcium chloride rose to 0.3 M. At low calcium chloride concentrations (0.05 and 0.1 M), the beads were partially gelled and failed to form a firm and stable network.
The beads produced with low concentrations (1 and 1.5%) of sodium alginate were non-spherical and very soft so that they would be easily broken because of their low mechanical strength. With increasing sodium alginate concentration to above 2%, the beads hardened and their permeability and, thereby, their sensitivity toward volatile amines declined. In fact, higher concentrations of sodium alginate led to increased numbers of the biopolymer molecules, which increased the calcium ion binding sites to form a more densely gelled structure. The dense network resisted the permeation of the volatile amine and, thus, led to reduced sensitivity of the pH indicating alginate beads.
The color changes in the red cabbage extract in response to different pH levels were checked and then, the alginate beads were also investigated for their response to volatile amines. Clearly, the red extract at pH 3 turned to blue at a neutral pH (nearly 7) followed by green and yellow at alkaline pH levels (nearly 8 or higher). These results confirm the excellent capability of the red cabbage extract to detect variation in pH. Figure 1b shows the response of the alginate beads containing the red cabbage extract to variations in volatile amine content during fish storage at the refrigeration temperature. Immediately after production, the alginate beads had a pink color which turned to purple on day 3 in the initial stages of fish spoilage. The color later changed to yellow with rising pH after 12 days, indicating complete fish spoilage. During the first 12 h of storage at room temperature, however, no changes were observed in color. After 16 h, the color of the alginate beads changed to blue, indicating rising pH levels. At the end of the storage period, the alginate beads completely turned yellow, indicating complete spoilage of fish. A good agreement was found between these data and the colorimetric data (Tables 1, 2). It may, therefore, be claimed that alginate beads are capable of detecting pH changes and fish spoilage. Other experiments to determine microbial changes and variation in TVB-N content were conducted to verify the accuracy of the data obtained by the alginate bead colorimetric sensor.
Table 1.
CIElab colour parameters for alginate beads containing red cabbage extract during fish samples storage at room temperature
| Storage time (h) | L* | a* | b* | ΔE* |
|---|---|---|---|---|
| 0 | 29.0 ± 0.4a | 19.0 ± 0.0 | − 3.0 ± 0.1 | –b |
| 12 | 19.0 ± 0.2 | 11.0 ± 0.2 | − 12.0 ± 0.4 | 40.43 |
| 16 | 33.5 ± 0.1 | − 8.0 ± 0.1 | − 16.0 ± 0.0 | 33.06 |
| 36 | 23.0 ± 0.2 | − 3.0 ± 0.4 | − 6.0 ± 0.4 | 34.33 |
| 64 | 30.0 ± 0.0 | 5.0 ± 0.2 | 28.0 ± 0.5 | 31.90 |
a± SD Mean deviation of a triplicate
bReference sample
Table 2.
CIElab colour parameters for alginate beads containing red cabbage extract during fish samples storage at refrigerator temperature
| Storage time (days) | L* | a* | b* | ΔE* |
|---|---|---|---|---|
| 0 | 50.0 ± 0.4a | 31.0 ± 0.4 | − 7.5 ± 0.7 | –b |
| 3 | 51.0 ± 0.4 | 11.5 ± 0.7 | − 12.0 ± 0.3 | 20.64 |
| 5 | 54.5 ± 0.7 | − 5.5 ± 0.7 | 1.0 ± 0.1 | 11.24 |
| 7 | 39.0 ± 0.0 | − 6.5 ± 0.3 | 3.0 ± 0.2 | 18.74 |
| 10 | 58.5 ± 0.7 | 8.0 ± 0.0 | 14.0 ± 0.5 | 9.23 |
| 12 | 53.0 ± 0.4 | 12.0 ± 0.0 | 23.0 ± 0.1 | 17.92 |
a± SD Mean deviation of a triplicate
bReference sample
During fish storage, proteolysis due to microbial proteolytic enzymes and fish autolytic enzymes is responsible for fish spoilage. Small peptides and free amino acids can be produced as a result of protein degradation (Ghaly et al. 2010). Some of the produced peptides and biogenic amines remain in fish tissue and lead to an increase in the pH, while some others accumulate in the headspace of fish or meat packaging and result in a bad odor. So, the odor can confirm the presence of volatile amines. Volatile amines that are involved in causing bad odor of spoiled fish include trimethylamine, dimethylamine, ammonia, histamine, putrescine and cadaverine. Other compounds such as unsaturated aldehydes, N-cyclic compounds, short-chain carbonyls, sulfur compounds and acids can also contribute to the formation of bad odor (Morsy et al. 2016). In this study, the color change of alginate beads was due to these volatile amines in the headspace not pH change of fish fillet. Alginate beads were not directly in contact with fish fillet and fixed on the inner side of the lid of the petri dishes and facing the headspace.
Microbial changes during fish spoilage
Fresh fish is one of the most perishable seafood products. It has been established that fish muscle spoilage occurs as the result of a combination of different spoilage mechanisms, including microbial and endogenous enzyme activities, lipid oxidation, and enzymatic browning (Raeisi et al. 2015). In order to evaluate the proposed alginate bead colorimetric sensor, changes in the mesophilic and psychrotrophic bacterial growths were investigated in the fish samples during the first 3 days of storage at room temperature (25 °C) (Fig. 2a) and during the 12 days of storage at chilled temperature (7 °C) (Fig. 2b). The initial mesophilic and psychrotrophic counts in the trout fillet stored at room temperature were 4.48 and 4.30 log CFU/g, respectively, which gradually increased to values higher than 10 log CFU/g at the end of day 3 (Fig. 2a).
Fig. 2.
Changes in mesophilic and psychrotrophic counts of fish samples during storage: a at room temperature, and b at refrigeration temperature
In the present study, similar bacterial growth patterns were observed during refrigerated storage and storage at room temperature. The mesophilic and psychrotrophic microorganism counts during refrigerated storage increased from 3.29 and 3.30 log CFU/g to 11.17 and 13.35 log CFU/g, respectively, after 12 days (Fig. 2b). These results are in agreement with those reported in other studies of microbial quality of trout fillet (Jouki et al. 2014; Ojagh et al. 2010). The Gram negative psychrotrophic bacteria are the most common group of microorganisms causing spoilage in fresh fish aerobically-stored at chilled temperatures (Ibrahim Sallam 2007). According to a report by International Commission of Microbiological Standards for Foods (ICMSF 1986), the maximum acceptable microbial limit recommended for fresh fish is 7 log CFU/g. The fish samples in this study reached this acceptable limit after 12 h of storage at room temperature and approximately 3 days of storage under refrigeration.
Comparison of the visually (by naked eye) detected (Fig. 1b) or calorimetrically obtained (L*, a*, b* parameters) of the responses of alginate beads realized in color changes (Tables 1, 2) reveals that not only is the colorimetric sensor response correlated with variations in bacterial populations, but the onset of alginate bead color change is also correlated with the rejection level of the product. These results indicate the accurate and reliable responses of alginate bead as a colorimetric sensor to increasing total volatile basic nitrogen (TVB-N) as a result of microbial growths in the headspace of the packages. Moreover, it is observed that the range of color changes in alginate beads corresponds to the permissible bacterial limits so that higher levels represent microbial spoilage of fish samples. This is while the visual inspection of color changes of in-package alginate beads is far simpler and handy than other indicators.
pH changes
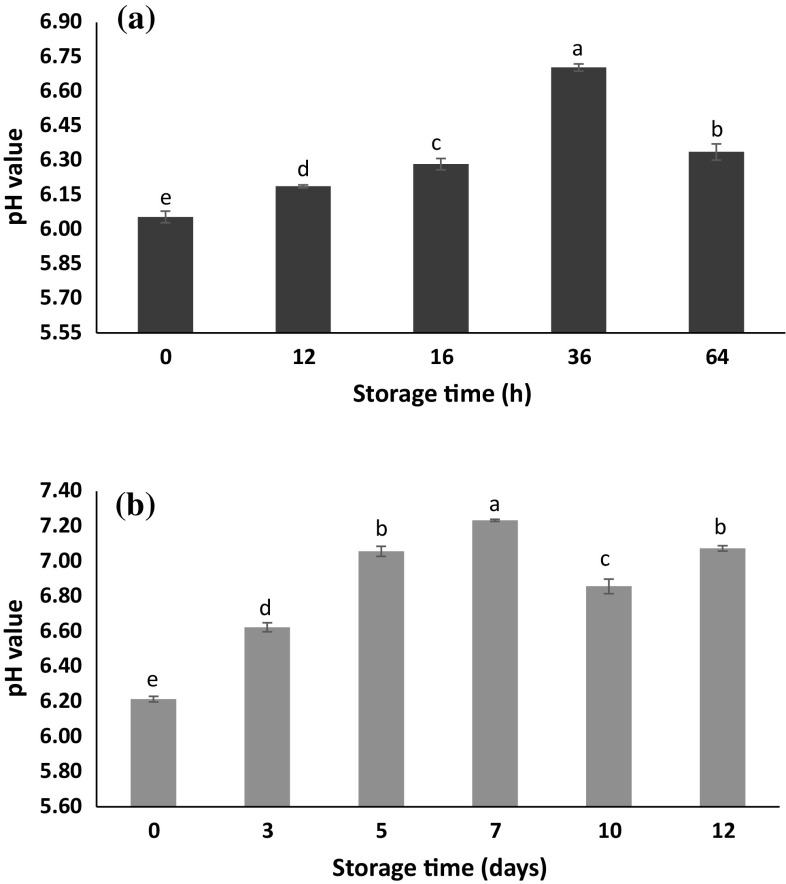
One of the main characteristics of fish is its high pH after death. Furthermore, pH level in fish muscles increases only slightly during the storage period due to the production of amine compounds such as trimethylamine (TMA), dimethylamine (DMA) and ammonia by microorganisms (Goulas and Kontominas 2005). In this study, the initial pH of the fish samples at room temperature was recorded at 6.05, which increased to 6.70 after 36 h but later decreased to 6.34 after 64 h (Fig. 3a). During the refrigerated storage, the pH level of fish fillets rose from 6.21 to 7.07 (Fig. 3b). The sudden decrease observed in pH after day 10 under chilled conditions or that after 36 h in ambient conditions could be attributed to the growth of lactic acid bacteria (LAB) and their production of lactic acid in the samples. Comparison of the pH values with colorimetric data show that the color change of alginate beads is proportional to the pH increase. The pH increase of fish samples due to microbial growth and production of volatile amines had a strong relationship with color change of alginate beads during the storage of both samples at room and refrigerator temperature.
Fig. 3.
Changes in pH values of fish samples during storage: a at room temperature, and b at refrigeration temperature. Different letters at different sampling times indicate significant differences (p < 0.05)
Total volatile basic nitrogen (TVB-N)
TVB-N is one of the most important parameters to determine fish quality and freshness. The TVB-N values obtained for trout fillets during storage are presented in Fig. 4a, b. Clearly, the initial TVB-N values were low and properly matched the low initial microbial counts. However, TVB-N content gradually increased from 3.32 mg/100 g to 20.70 mg/100 g after 36 h of storage at room temperature but suddenly decreased in the 64th hour (Fig. 4a). The same trend was observed in chilled-stored samples such that the TVB-N content increased from 5.37 mg/100 g on day 0 to 22.99 mg/100 g on day 7 but decreased to 18.14 mg/100 g on day 10 before it increased to 24.01 mg/100 g on day 12 (Fig. 4b). Comparison of the TVB-N values with colorimetric analysis shows that the color change of alginate beads is proportional to the TVB-N content. Increasing the amount of TVB-N led to pH increase in the headspace and color change of alginate beads. The first color change of alginate beads for refrigerated samples was observed on the third day of storage along with the significant increase in TVB-N content. We found a similar relationship between alginate beads color change and TVB-N content of fish samples on the first day of storage. However, the TVB-N content of fish samples at room temperature increased insignificantly after 12 h but was able to increase the pH in the headspace and change the color of alginate beads.
Fig. 4.
Changes in TVB-N and TBA values of fish samples during storage. a TVB-N values at room temperature, and b at refrigeration temperature; c TBA values at room temperature, and d at refrigeration temperature. Different letters at different sampling times indicate significant differences (p < 0.05)
Changes in pH values during storage exhibited a similar trend such that a decline was observed in pH value at the 64th hour and on day 10. This can be attributed to the production of some acidic substances. Both fish skin and digestive system host a variety of bacteria (Souza et al. 2010), a group of which are the lactic acid bacteria (LAB) that are facultative anaerobes and grow very well in microaerophilic conditions (Jouki et al. 2014). These bacteria produce lactic acid as a major metabolic end product of carbohydrate consumption. When the LAB population increases during storage, the lactic acid content also increases to neutralize the alkaline amine products and reduce pH. Another reason for the declining pH could be the reaction of amine compounds with aldehydes as secondary products of lipid oxidation. Based on the colorimetric results, the alginate bead responses are found to exhibit a trend similar to TVB-N measurements (Fig. 1b).
Thiobarbituric acid (TBA)
Lipid oxidation development measures are based on the formation of primary (hydroperoxides) and secondary oxidation compounds (aldehydes). TBA index represents the secondary oxidation compound content and is widely used as an indicator for the assessment of fish spoilage (Ibrahim Sallam 2007). Figure 4c and d presents the TBA values obtained at different times during the storage period. Changes in TBA content exhibits a trend similar to that of TVB-N. The initial TBA values (mg of malonaldehyde equivalents/kg of tissue) under the chilled and ambient temperatures were 0.24 and 0.01, respectively (Fig. 4c, d). These values increased with storage time under both conditions; by the end of the storage period, however, significant decreases were observed in TBA on days 5 and 10 in refrigerated storage and at the end of storage at room temperature. These decreases were attributed to the reaction of aldehydes and amine compounds because of the close temporal association between reduced TVB-N content and the declining TBA content. These results were in agreement with those previously reported (Giatrakou et al. 2008; Frangos et al. 2010). Fernandez et al. (1997) claimed that TBA values do not represent actual lipid oxidation rates due to the multitude of interactions between malonaldehyde and amino acids, proteins, glucose, and other fish constituents during the storage period.
According to Ibrahim Sallam (2007), the maximum TBA level indicating good fish quality is 5 mg of malonaldehyde equivalents/kg of tissue. However, fish may still be consumed with TBA values up to 8 mg of malonaldehyde equivalents/kg of tissue (Ojagh et al. 2010). In the present study, TBA values measured in trout fillet samples throughout the storage period were found to be much lower than the proposed limits. Comparison of the TBA values with colorimetric data show that the response of the colorimetric sensor is correlated with TBA increase as an indicator of fish spoilage. The TBA increase of fish samples due to lipid oxidation had a strong relationship with color change of alginate beads during the storage of both samples at room and refrigerator temperature.
Colorimetric analysis
Color parameters were determined for the alginate beads containing the red cabbage extract. Based on CIElab color system, L* indicates lightness with a scaling between 100 (white) to 0 (black), while the scales for a* and b* parameters, respectively, are + a (red) to − a (green), + b (yellow) to − b (blue). According to Tables 1 and 2, the values for a* decreased from 19 (redder) to 5 (greener) after 64 h of storage at room temperature. In the case of refrigerated samples, the a* values decreased from 31 to 12 after 12 days. This means that the green color had a greater intensity at the end of the storage period. The values recorded for b* under both conditions increased from − 3 to 28 (for samples at room temperature) and from − 10 to 16 (for samples in refrigerated storage), indicating that the yellow color emerged only at the end of the storage period. According to Tassanawat et al. (2007), a ΔE* value of larger than 5 can be easily detected by naked eye, while values greater than 12 imply a complete color difference which is detectable even by untrained panelists. Therefore, ΔE* of the alginate beads containing red cabbage anthocyanins are detected by the human eye during fish storage. Overall, the alginate beads used as a colorimetric sensor exhibit a range of good color variation depending on the pH which is affected by TVB-N content during the storage period. This has the obvious advantage that color changes can be inspected by the naked eye to detect the spoilage rate of packed fish.
Conclusion
In this study, a new colorimetric sensor was developed using alginate beads containing the anthocyanin extracted from red cabbage extract for fish spoilage monitoring. The sensor was found to be very sensitive to changes in pH and the volatile amines produced during fish storage. Results of visual inspection and CIELab colorimetric analysis of the sensor were found to agree well with the TVB-N values and microbial growth patterns, which can be used as pH indicators of spoilage in fish and other high protein food products. The advantages of ease of manufacture and application as well as the low-cost of materials such as porous plastic bags are among the advantage of the pH indicating alginate bead sensor developed in this study that makes them especially useful in a variety of food packaging. The disadvantage of this system is instability of anthocyanins when store in room temperature with light incidence. However, red cabbage anthocyanins have a greater stability compared with anthocyanins from other sources due to presence of acylated anthocyanin which create a more stability for the molecule (Prietto et al. 2017). In conclusion, pH indicator system based on anthocyanin is suitable for monitoring the quality of refrigerated foods such as fish and meat.
Acknowledgements
The authors would like to acknowledge Shiraz University for their financial support.
Funding
This study was funded by Shiraz University.
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants performed by any of the authors.
References
- Abolghasemi MM, Sobhi M, Piryaei M. Preparation of a novel green optical pH sensor based onimmobilization of red grape extract on bioorganic agarose membrane. Sens Actuators B Chem. 2016;224:391–395. doi: 10.1016/j.snb.2015.10.038. [DOI] [Google Scholar]
- Alimelli A, Pennazza G, Santonico M, Paolesse R, Filippini D, D’Amico A, Lundström I, Di Natale C. Fish freshness detection by a computer screen photoassisted based gas sensor array. Anal Chim Acta. 2007;582:320–328. doi: 10.1016/j.aca.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Azarakhsh N, Osman A, Ghazali HM, Tan CP, Adzahan NM. Effects of gellanbased edible coating on the quality of fresh-cut pineapple during refrigerated storage. Food Bioprocess Technol. 2014;7:2144–2151. doi: 10.1007/s11947-014-1261-6. [DOI] [Google Scholar]
- Babu VR, Sairam M, Hosamani KM, Aminabhavi TM. Preparation of sodium alginate–methylcellulose blend microspheres for controlled release of nifedipine. Carbohydr Polym. 2007;69:241–250. doi: 10.1016/j.carbpol.2006.09.027. [DOI] [Google Scholar]
- Branyik T, Vicente AA, Dostalek P, Teixeira JA. Continuous beer fermentation using immobilized yeast cell bioreactor systems. Biotechnol Prog. 2005;21:653–663. doi: 10.1021/bp050012u. [DOI] [PubMed] [Google Scholar]
- Cai L, Wu X, Dung Z, Li X, Yi S, Li J. Physicochemical responses and quality changes of red sea bream (Pagrosomus major) to gum arabic coating enriched with ergothioneine treatment during refrigerated storage. Food Chem. 2014;160:82–89. doi: 10.1016/j.foodchem.2014.03.093. [DOI] [PubMed] [Google Scholar]
- Chung SWC, Chan BTP. Trimethylamine oxide, dimethylamine, trimethylamine and formaldehyde levels in main traded fish species in Hong Kong. Food Addit Contam Part B. 2009;2:44–51. doi: 10.1080/02652030902858921. [DOI] [PubMed] [Google Scholar]
- Cristina Silva-Pereira M, Augusto Teixeira J, Aniceto Pereira-Júnior V, Stefani R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT Food Sci Technol. 2015;61:258–262. doi: 10.1016/j.lwt.2014.11.041. [DOI] [Google Scholar]
- Da Silva MA, Bierhalz ACK, Kieckbusch TG. Alginate and pectin composite films cross-linked with Ca2+ ions: effect of the plasticizer concentration. Carbohydr Polym. 2009;77:736–742. doi: 10.1016/j.carbpol.2009.02.014. [DOI] [Google Scholar]
- Fernandez J, Perez-Alvarez IA, Fernandez-Lopez JA. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997;59:345–353. doi: 10.1016/S0308-8146(96)00114-8. [DOI] [Google Scholar]
- Frangos L, Pyrgotou N, Giatrakou V, Ntzimani A, Savvaidis IN. Combined effects of salting, oregano oil and vacuum-packaging on the shelf-life of refrigerated trout fillets. Food Microbiol. 2010;27:115–121. doi: 10.1016/j.fm.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Ghaly AE, Dave D, Budge S, Brooks MS. Fish spoilage mechanisms and preservation techniques: review. Am J Appl Sci. 2010;7:859–877. doi: 10.3844/ajassp.2010.859.877. [DOI] [Google Scholar]
- Giatrakou V, Kykkidou S, Papavergou A, Kontominas MG, Savvaidis IN. Potential of oregano essential oil and MAP to extend the shelf life of fresh swordfish: a comparative study with ice storage. J Food Sci. 2008;73:167–173. doi: 10.1111/j.1750-3841.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- Gil L, Barat JM, Garcia-Breijo E, Ibañez J, Martínez-Máñez R, Soto J, Llobet E, Brezmes J, Aristoy MC, Toldrá F. Fish freshness analysis using metallic potentiometric electrodes. Sens. Actuators B Chem. 2008;131:362–370. doi: 10.1016/j.snb.2007.11.052. [DOI] [Google Scholar]
- Goulas AE, Kontominas MG. Effect of salting and smoking-method on the keeping quality of chub mackerel (Scomber japonicus): biochemical and sensory attributes. Food Chem. 2005;93:511–520. doi: 10.1016/j.foodchem.2004.09.040. [DOI] [Google Scholar]
- Gram L, Huss HH. Microbiological spoilage of fish and fish products. Int J Food Microbiol. 1996;33:121–137. doi: 10.1016/0168-1605(96)01134-8. [DOI] [PubMed] [Google Scholar]
- Harper BA, Barbut S, Lim L, Marcone MF. Effect of various gelling cations on the physical properties of “wet” alginate films. J Food Sci. 2014;79:562–567. doi: 10.1111/1750-3841.12376. [DOI] [PubMed] [Google Scholar]
- Huang X, Xin J, Zhao JJ. A novel technique for rapid evaluation of fish freshness using colorimetric sensor array. J Food Eng. 2011;105:632–637. doi: 10.1016/j.jfoodeng.2011.03.034. [DOI] [Google Scholar]
- Ibrahim Sallam K. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control. 2007;18:566–575. doi: 10.1016/j.foodcont.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Commission on Microbiological Specifications for Foods (ICMSF) (1986) Sampling plans for fish and shellfish, ICMSF, Microorganisms in foods, second ed. Sampling for microbiological analysis: principles and scientific applications, vol 2. University of Toronto Press, Toronto, pp 181–196
- Jouki M, Tabatabaei Yazdi F, Mortazavi SA, Koocheki A, Khazaei N. Effect of quince seed mucilage edible films incorporated with oregano or thyme essential oil on shelf life extension of refrigerated rainbow trout fillets. Int J Food Microbiol. 2014;174:88–97. doi: 10.1016/j.ijfoodmicro.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Klock G, Pfeffrmann A, Ryser C, Grohn P, Kuttler B, Hahn HJ, Zimmermann U. Biocompatibility of mannuronic acid-rich alginates. Biomaterials. 1997;18:707–713. doi: 10.1016/S0142-9612(96)00204-9. [DOI] [PubMed] [Google Scholar]
- Kuswandi B, Restyana A, Abdullah A, Heng LY, Ahmad M. A novel colorimetric food package label for fish spoilage based on polyaniline film. Food Control. 2012;25:184–189. doi: 10.1016/j.foodcont.2011.10.008. [DOI] [Google Scholar]
- Morsy MK, Zor K, Kostesha N, Sonne Alstrøm T, Heiskanen A, El-Tanahi H, Sharoba A, Papkovsky D, Larsen J, Khalaf H, Jakobsen MH, Emnéus J. Development and validation of a colorimetric sensor array for fish spoilage monitoring. Food Control. 2016;60:346–352. doi: 10.1016/j.foodcont.2015.07.038. [DOI] [Google Scholar]
- Natale CD, Olafsdottir G, Einarsson S, Martinelli E, Paolesse R, D’Amico A. Comparison and integration of different electronic noses for freshness evaluation of cod-fish fillets. Sens Actuators B Chem. 2001;77:572–578. doi: 10.1016/S0925-4005(01)00692-X. [DOI] [Google Scholar]
- Nguyen LT, Lau YS, Yang KL. Entrapment of cross-linked cellulase colloids in alginate beads forhydrolysis of cellulose. Colloid Surf B. 2016;145:862–869. doi: 10.1016/j.colsurfb.2016.06.008. [DOI] [PubMed] [Google Scholar]
- Ojagh SM, Rezaei M, Razavi SH, Hosseini SMH. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010;120:193–198. doi: 10.1016/j.foodchem.2009.10.006. [DOI] [Google Scholar]
- Pacquit A, Lau KT, McLaughlin H, Frisby J, Quilty B, Diamond D. Development of a volatile amine sensor for the monitoring of fish spoilage. Talanta. 2006;69:515–520. doi: 10.1016/j.talanta.2005.10.046. [DOI] [PubMed] [Google Scholar]
- Pacquit A, Frisby J, Diamond D, Lau K, Farrell A, Quilty B. Development of a smart packaging for the monitoring of fish spoilage. Food Chem. 2007;102:466–470. doi: 10.1016/j.foodchem.2006.05.052. [DOI] [Google Scholar]
- Pawar SN, Edgar KJ. Alginate derivatization: a review of chemistry, properties and applications. Biomaterials. 2012;33:3279–3305. doi: 10.1016/j.biomaterials.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Prietto L, Mirapalhete TC, Pinto VZ, Hoffmann JF, Vanier NL, Lim LT, Guerra Dias AR, Zavareze EDR. pH-sensitive films containing anthocyanins extracted from black bean seed coat and red cabbage. LWT Food Sci Technol. 2017;80:492–500. doi: 10.1016/j.lwt.2017.03.006. [DOI] [Google Scholar]
- Raeisi M, Tajik H, Aliakbarlu J, Mirhosseini SH, Hosseini SMH. Effect of carboxymethyl cellulose-based coatings incorporated with Zataria multiflora Boiss. essential oil and grape seed extract on the shelf life of rainbow trout fillets. LWT Food Sci Technol. 2015;64:898–904. doi: 10.1016/j.lwt.2015.06.010. [DOI] [Google Scholar]
- RØrvik KA, Steien SH, Saltkjelsvik B, Thomassen MS. Urea and trimethylamine oxide in diets for seawater farmed rainbow trout: effect on fat belching, skin vesicle, winter ulcer and quality grading. Aquacult Nutr. 2000;6:247–254. doi: 10.1046/j.1365-2095.2000.00143.x. [DOI] [Google Scholar]
- Souza BWS, Cerqueira MA, Ruiz HA, Martins JT, Casariego A, Teixeira JA, Vicente AA. Effect of chitosan-based coatings on the shelf life of salmon (Salmo salar) J Agric Food Chem. 2010;58:11456–11462. doi: 10.1021/jf102366k. [DOI] [PubMed] [Google Scholar]
- Tassanawat S, Phandee A, Magaraphan R, Nithitanakul M, Manuspiya H (2007) pH-sensitive PP/clay nanocomposites for beverage smart packaging. In: Proceedings of the 2nd IEEE international, conference on nano/micro engineered and molecular systems
- Verbelen PJ, De Schutter DP, Delvaux F, Verstrepen KJ, Delvaux FR. Immobilized yeast cell systems for continuous fermentation applications. Biotechnol Lett. 2006;28:1515–1525. doi: 10.1007/s10529-006-9132-5. [DOI] [PubMed] [Google Scholar]
- Won K, Kim S, Kim KJ, Park HW, Moon SJ. Optimization of lipase entrapment in Ca-alginate gel beads. Process Biochem. 2005;40:2149–2154. doi: 10.1016/j.procbio.2004.08.014. [DOI] [Google Scholar]