Abstract
Natural foods are used in many folks and household treatments and have immense potential to treat a serious complication and health benefits, in addition to the basic nutritional values. These food products improve health, delay the aging process, increase life expectancy, and possibly prevent chronic diseases. Purple head Brassica oleracea L. var. italica Plenck is one of such foods and in current studies was explored for chemical compounds at different development stages by gas chromatography-mass spectrometry. Antioxidant potential was explored employing different assays like molybdate ion reduction, DPPH, superoxide anion radical scavenging and plasmid nicking assay. Inspired by antioxidant activity results, we further explored these extracts for antiproliferative potential by morphological changes, cell cycle analysis, measurement of intracellular peroxides and mitochondrial membrane potential changes. Current study provides the scientific basis for the use of broccoli as easily affordable potent functional food.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3095-0) contains supplementary material, which is available to authorized users.
Keywords: Broccoli, Antioxidant assay, Cell cycle, ROS, GC–MS, MTT, Apoptosis
Introduction
Natural plant products are preferred world wide as the age old medicinal system like Ayurveda is being used for many years to treat various diseases. In recent years, great attention has been given to plant products to prevent various ailments because of their less ill effects (Manson et al. 2005). Epidemiological studies showed that natural food products strengthen the immune system and act as anti-inflammatory, antioxidant, anti-mutagenic and anti-cancer agents (Miyata 2007; Espín et al. 2007). The mechanism based discovery of drug via cell death has been chosen as a new end point like cell cycle arrest, reactive oxygen species, attenuation in mitochondrial membrane potential, cytochrome C, apoptosis inducing factor, caspases and post-apoptotic necrosis (Qian et al. 2012; Kello et al. 2014). Functional foods are distinct products which are used to improve the health (Moreno et al. 2006; Sharma et al. 2014).
Various number of plant like oats, flaxseed, tomatoes, garlic, broccoli, citrus fruit, cranberry and tea are considered functional foods. A large amount of research data gives an ample support to the assumption that daily intake of broccoli is useful in decreasing the cancer risk (Kim and Milner 2005; Keum et al. 2005). Glucosinolates (GLSs) are a group of thioglycosides, which on hydrolysis give different products having the beneficial effects on human health (Ciska et al. 2000). Isothiocyanates are known to provide defense against cancer caused by a range of toxicants (Talalay and Fahey 2001). These products play a significant role in decreasing the free radical load responsible for causing different chronic ailments by acting as antioxidants (Verkerk et al. 2009).
In continuation of our previous work, green head broccoli (Palam Samridhi) was studied for prostate cancer prevention (Chaudhary et al. 2014). The present study was planned on purple head broccoli to explore its antioxidant potential via molybdate ion reduction, DPPH, superoxide anion radical scavenging, plasmid nicking assay and antiproliferative potential by morphological changes, cell cycle analysis, measurement of intracellular peroxides and mitochondrial membrane potential changes.
Materials and methods
Plant material
The seeds of the Palam Vichitra (PV) were procured from Chaudhary Sarwan Kumar Himachal Pradesh Krishi Vishvavidyalaya, (CSKHPV) Palampur. The Seeds were dipped in 70% ethanol for one minutes and 1.3% sodium hypochlorite for 15 min for surface sterilization and then allowed to germinate in seed germinator (Narang Scientific Work model NSW-191-192) for 3, 5 and 7 days with 16 h light and 8 h dark at 22 °C. The seeds of PV were sown in the Botanical garden, GNDU, Amritsar under irrigated field conditions and grown up to their leaflet and floret stages.
Chemicals and reagents
Roswell park memorial institute medium (RPMI-1640), foetal bovine serum (FBS), penicillin, streptomycin, 2,2′-diphenyl- 1-picryl hydrazyl (DPPH), RNase A and Triton X-100 and the fluorescent probes via Rhodamine 123 (Rh 123), 2′,7′-dichlorofluorescein diacetate (DCFH-DA), were obtained from Sigma–Aldrich Corp. Propidium iodide (PI) was purchased from Invitrogen Life Technologies (Carlsbad, CA). pBR 322 plasmid DNA was obtained from GeNeiTM. Gentamicin was obtained from Abbott Healthcare Pvt. Ltd. Mumbai, India. Ammonium molybdate, EDTA, nitroblue tetrazolium (NBT), phenazine methosulphate (PMS), NADH, Sodium hydrogen carbonate (NaHCO3), ascorbic acid and remaining reagents were of analytical grade. Prostate cancer cells (PC-3) was obtained from Indian Institute of Integrative Medicine (IIIM), Jammu, India.
Preparation of extracts
The extraction of plant material (seeds, sprouts (PV3, PV5, PV7), leaves, and florets) was done by the method (Chaudhary et al. 2014) (Fig. SI-1). The fresh plant material homogenised along with water for 5 min. and autolyzed at room temperature for 30 min. After autolyzing, the meals extracted two times with dichloromethane (DCM), which further combined and salted with 2.5 g anhydrous sodium sulphate and then the fraction dried at 30 °C under vacuum on rotary evaporator.
Gas chromatography-mass spectrometry
The analysis was carried out on a Shimadzu (QP 2010) series AOC-20i auto-sampler coupled, and a DB-5MS capillary column (30 m × 0.25 mM i.d, 0.25 µM). The initial temperature of the column was maintained to 70 °C for 4 min programmed to 230 °C at 4 °C/min then held for 15 min at 230 °C. The sample (extracts) injection volume was 2 µl in GC grade DCM and at a flow rate of 1.1 ml/min. On split mode (1:50) helium used as carrier gas. Ionization voltage of 70 eV, ion source temperature: 200 °C and interface temperature 250 °C was used. The spectrum was scanned from m/z 50 to 600 amu.
Antioxidant potential
Molybdate ion reduction assay
The capacity of various extracts to reduce molybdate ions was analyzed following the process of Prieto et al. (1999). In this method, plant extract was mixed with 3 ml of reagent solution (4 mM ammonium molybdate, 0.6 M sulphuric acid, and 28 mM sodium phosphate). Then the mixture was allowed to maintain at 95 °C for 90 min to complete the reaction and absorbance of the solution was monitored at 695 nm aligned with blank after lowering its temperature to 25 °C. The reduction ability of crude extracts in this assay was evaluated as the amount equivalents of AAE.
Plasmid nicking assay
The protective effect of test samples against hydroxyl radicals was illustrated by DNA nicking assay as performed by Lee et al. (2002). The DNA nicking assay was carried out by using supercoiled pBR322 plasmid DNA with small changes. Plasmid DNA was incubated with Fenton’s reagent containing extracts, and the final amount of the mixture was raised up to 20 µl with double distilled water. The iron (II) in aqueous solution slowly autoxidizes to form O2•, which rapidly dismutate to H2O2 at pH 7.4, and forms •OH radicals by the Fenton reaction in the presence of ascorbic acid as a catalyst. The reaction mixture was then maintained at 37 °C for 30 min in the incubator. After incubation, 2.5 µl of loading buffer (0.25% bromophenol blue, 50% glycerol) was added and centrifuged for 15 s. DNA was then run on 1% agarose gel prepared in 1X TBE buffer. Bands were then analyzed using gel documentation unit. Rutin (100 µg/ml) was used as positive control.
DPPH-radical scavenging assay
DPPH represents both mechanism for hydrogen and electron donating ability of the different extracts (Blois 1958; Prior et al. 2005). Briefly, the resultant mixture contained 100 µl of different test sample concentrations (0.125–2 mg/ml) and 2 ml of DPPH solution. The resultant mixture was then placed in test tubes for 20 min in the oven. Then transferred to microtiter plate and read at 517 nm in a plate reader (Biotek HT Synergy) against the blank, which did not contain test sample. The positive control used in present study was L-ascorbic acid. The percent DPPH decolorisation of the sample was calculated by Radical scavenging activity = [(A0-A1)/A0 × 100] Where, A0 is the absorbance of control (without test sample), A1 is the absorbance of reaction mixture (with test sample).
Superoxide anion radical scavenging assay
The assessing of superoxide anion radical scavenging activity of different extracts was done using protocol by Chaudhary et al. (2014). In this method, one ml of nitroblue tetrazolium, one ml of NADH solution and various concentration of different extracts (0.125–2 mg/ml) were mixed and the reaction was initiated by adding 100 µl of phenazine methosulphate (PMS) solution (60 µM) prepared in phosphate buffer (100 mM, pH 7.4). The reaction mixture was maintained at 25 °C for 5 min, and the absorbance was read at 560 nm.
where A0 is the absorbance of control (without test sample), A1 is the absorbance of the reaction mixture (with test sample).
Statistical analysis
Superoxide anion radical scavenging assay, total antioxidant capacity, and DPPH assay was performed in triplicate and the data is presented as mean ± standard error (SE). To compare the difference in means, two way analysis of variance (ANOVA) was performed with Tukey’s post hoc test both within the treatments and within the varieties at p ≤ 0.05 (95% level of significance) using SigmaStat for Windows® Version 3.5 and Microsoft excel 2007.
Antiproliferative activities
Preparation of test material
The plant extracts were dissolved in DMSO, at a concentration of 5 mg/ml, before being diluted with RPMI 1640 and 10% FBS. The solution was then filtered and sterilized, through a 0.2 µM syringe filter, to prepare the working solution. Since the final concentration of DMSO, used to dissolve extract, was 2% and for compounds, 0.5% a similar amount was added to the control cells.
Preparation of positive controls
A stock solution of 2 × 10−2 M concentration of the positive control (camptothecin) was prepared. The solvent used for camptothecin was DMSO. Aliquots of the stocks were stored at −20 °C.
MTT assay
The cell growth was calculated by using MTT assay method of Chaudhary et al. (2014). The cell density was adjusted to 10,000 cells/100 µl in the cell suspension. The cell suspension of 100 µl was further added to individual well of 96 wells plate. The plates were maintained at 37 °C, in incubator provided with 5% CO2 and Relative Humidity (RH) of 90% for 24 h. After 24 h, 100 µl extracts were added to the 96 wells plates. After that, 100 µl of freshly prepared MTT (5 mg/10 ml in PBS solution, sterile filtered) was supplemented to individual well. Culture plates were gently stirred at 150 rpm for 5 min, to mix thoroughly MTT into the media and incubated further for 4 h at 37 °C, to allow metabolization of MTT. The plates were spinned at 2000 rpm for 10 min and remaining solution was removed. MTT formazan crystals were resuspended in 100 µl of DMSO. After that, plates were stirred for 20 min till the dissolution of formazan crystals and OD was monitored at 570 nm. Cell proliferation as percent viability was intended by comparing the OD of treated sample versus healthy cells as:
Morphological analysis
In this method, a nuclear morphology of the treated cells was studied by staining fixed cells with DAPI dye as per the method given by Bhushan et al. (2007). The fluorescent dye (DAPI) was used to visualize the changes of cell morphological features as well as changes in the nuclei. DAPI is a popular cell-permeable nuclear stain that emits blue fluorescence after binding to dsDNA. The PC-3 cells (1 × 106) in 1 ml of culture medium were transferred into 24 wells plate and grown to confluence at 37ºC with 5% CO2 and RH 90% for 8 h. The cultures were then exposed to different extracts at their IC50 concentration and incubated for another 12 h. After incubation, the medium was provided with 1 ml culture medium without serum and DAPI (10 mM) for 1 h at 37 °C. Then the cells were fixed in 4% paraformaldehyde (PFA). The fluorescent images of the cells treated with extracts were scanned on a Nikon Eclipse TiE inverted fluorescence microscope.
Cell cycle analysis
The measurement of DNA amount in the cells distributed in different phases of cell cycle was determined via following the method given by Hu et al. (2010) with minor changes. The cells (1 × 106 cells/ml/well) in 24 wells plate were maintained to raise in the co-existence with and without extract. After 12 h of treatment, cells were collected from a plate by combining trypsinized cells as well as the cells floating in the medium. For each circumstance, a volume of the cell suspension (1 × 106 cells) was centrifuged, and the final cell pellet was dissolved in chilled PBS (1.0 ml). The cells were permanently fixed in chilled 70% ethanol, stained with PI and analyzed in FL-2 channel by BD Accuri C6 flow cytometer (Chaudhary et al. 2014).
Reactive oxygen species (ROS) generation
The intensity of intracellular peroxides was monitored by using DCFH-DA (Chaudhary et al. 2014). The trypsinized cells were then centrifuged, and the final cell pellet was dissolved in chilled PBS (1.0 ml). A bulk of cellular ROS is generated through mitochondria during stress conditions that lose membrane integrity. The level of intracellular peroxides in PC-3 cells was analyzed in FL-1 channel on BD Accuri C6 flow cytometer.
Spectrofluorometry analysis (MMP potential)
The mitochondrial perturbation causes the changes in mitochondrial membrane potential that were detected via using the procedure described by Deng et al. (2013). The PC-3 cells (1 × 106) were incubated in the with and without plant extracts at different concentrations in 24 wells plates for 12 h at 37 °C in 5% CO2 incubator and RH 90% and then washed with phosphate buffered saline (PBS). Then, Rh-123 (10 µm/ml) was added to individual well for 1 h. Samples were measured directly in a multimode plate reader (Biotek Synergy HT) at the 485/20 nm excitation and 528/20 nm emission wavelengths respectively.
Results
GC–MS
It was observed that PV7 showed the presence of a maximum number of hydrolytic products. 3-butenyl isothiocyanates was found in PV3, PV7 and PVF extracts whereas 3-methyl-1-(methyl sulfinyl) butane was found in PVS, PV3 and PV7 extracts. 4-(methyl thio) butane nitrile was present in PVS, PV3 and PV7 extracts and allyl isothiocyanates was detected in PV7 and PVF extracts. It was further seen that Iberin nitrile was present in PVS extract and 3-phenylpropionitrile was present only in PVL extract (Table 1).
Table 1.
GC–MS Analysis of different extracts of variety Palam Vichitra at different developmental stages
| Extracts | Hydrolytic products of glucosinolates detected | Peak no. | Retention time (in min) | Area (%) |
|---|---|---|---|---|
| Palam Vichitra Seeds (PVS) | 4-(Methylthio)butanenitrile | 2 | 9.71 | 5.17 |
| Iberin nitrile | 5 | 15.55 | 2.41 | |
| 3-Methyl-1-(methylsulfinyl)butane | 9 | 17.43 | 23.64 | |
| Palam Vichitra 3 Day Sprouts (PV3) | 3-Butenyl isothiocyanate | 1 | 7.26 | 7.91 |
| 3-Methyl-1-(methylsulfinyl)butane | 4 | 17.40 | 27.27 | |
| Palam Vichitra 5 Day Sprouts (PV5) | 4-(Methylthio)butanenitrile | 2 | 9.72 | 6.81 |
| 1-Thio-(N-Hydroxy-5-(methylthio)pentanitrile) | 7 | 15.70 | 6.07 | |
| Palam Vichitra 7 Day Sprouts (PV7) | Allylisothiocyanate | 2 | 4.52 | 0.14 |
| 3-butenyl isothiocyanate | 5 | 6.70 | 0.94 | |
| 4-(Methylthio)butanenitrile | 10 | 8.95 | 0.15 | |
| 1-isothiocyanatopropane | 18 | 13.04 | 0.78 | |
| Erucin | 19 | 14.88 | 5.35 | |
| 3-Methyl-1-(Methylsulfinyl)butane | 22 | 16.22 | 0.13 | |
| Palam Vichitra Leaves (PVL) | 3-Phenylpropionitrile | 1 | 12.70 | 47.01 |
| Palam Vichitra Florets (PVF) | Allylisothiocyanate | 2 | 4.47 | 0.55 |
| 3-butenyl isothiocyanate | 5 | 7.19 | 1.14 |
MS was confirmed from NIST Database
Molybdate ion reduction assay
The molybdate ions reduction ability of extracts was measured by taking ascorbic acid as standard. The different concentrations of ascorbic acid ranging from 20 to 200 μg/ml were used to obtain the standard curve. The tendency of different extracts to reduce molybdate ions in phospho-molybdenum complex was expressed in terms of number of Ascorbic Acid Equivalents (AAE) in mg/100 mg weight of extract as calculated from the standard curve obtained for ascorbic acid. The ability of different extracts of PV to reduce molybdate ions in terms of mg AAE/100 mg dry weight of extracts. It was found that leaves (PVL) extract exhibited the reduction ability of 23.85 mg in terms of AAE/100 mg dry weight of extract followed by PV3 (15.01) > PV5 (14.522) > PV7 (14.411) > PVF (14.08) > PVS (13.91) at 125 µg/ml concentration (Fig. 1 A).
Fig. 1.
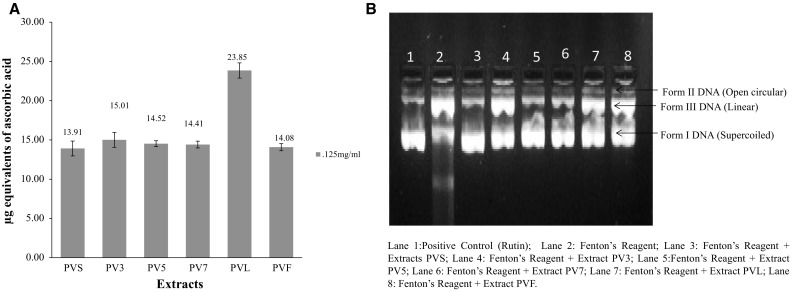
(a, b): a Molybdate ion reduction ability of different extracts of Palam Vichitra in terms of number of Ascorbic Acid Equivalents (AAE) in mg/100 mg weight of extracts at 125 μg/ml concentration as calculated from the standard curve obtained for ascorbic acid. b. Amount of plasmid DNA (in %) after treatment with different extracts of Palam Vichitra Extracts at 200 μg/ml
DNA nicking assay
The diminution effect of hydroxyl radical mediated damage by different extracts of PV at the concentration of 200 µg/ml against the DNA damage were more or less capable of maintaining the integrity of supercoiled DNA. The DNA protective activity of different extracts of PV (PVS, PV3, PV5, PV7, PVL and PVF) and rutin (positive control) at 200 μg/ml concentration. It has been found that the addition of Fenton’s reaction (Fe3+ + H2O2 + ascorbic acid) mixture to plasmid DNA resulted in increase of single stranded (ss) and double stranded (ds) nicked and linear forms of DNA. This is due to the attack of •OH radicals, on the nitrogenous bases or the deoxyribosyl backbone of DNA. In this case, Fenton’s reagent treated DNA (positive control), Form I i.e. supercoiled DNA was completely broken-down to ss nicked i.e. Form II (78.1%) and ds nicked DNA i.e. Form III (21.9%). The addition of various extracts to reaction mixture reduced the hydroxyl radical mediated strand breaking. The amount of supercoiled DNA, in the presence of extracts, was 34.9, 50.9, 64.4, 61.9, 47.1, 49.1 and 69.7% in case of PVS, PV3, PV5, PV7, PVF, PVL and rutin (positive control) respectively (Fig. 1 B).
DPPH free radical scavenging assay
In this assay, the hydrogen donating ability of different extracts and different concentration (0.125–2 mg/ml) was analyzed by the reduction of DPPH radical. It was observed that all the extracts showed dose-dependent response. The IC50 value of PV7 extract was found to be lowest i.e. 3.723 mg/ml and highest in PV5 extract i.e. 5.869 mg/ml. The two-way ANOVA and Tukey’s HSD post hoc test was used for its pairwise multiple comparisons between the concentration and between different extracts of PV. It was seen that pairwise comparison between all the extracts was statistically significant at p ≤ 0.05 except PV5 versus PVS and pairwise comparison between the concentrations of all the extracts was statistically significantly different (Table 2 and Fig. SI-2).
Table 2.
In vitro antioxidant by DPPH and superoxide anion scavenging radical assay and cytotoxicity of different extracts of Palam Vichitra
| S.No. | Extracts | R2-Value | IC50 Value (mg/ml) | ||||
|---|---|---|---|---|---|---|---|
| DPPH | SAO | MTT | DPPH | SAO | MTT | ||
| 1 | PVS | 0.956 | 0.953 | 0.995 | 5.55 | 0.79 | 36.47 |
| 2 | PV3 | 0.989 | 0.922 | 0.999 | 3.96 | 0.07 | 77.31 |
| 3 | PV5 | 0.996 | 0.898 | 0.996 | 4.17 | 0.08 | 80.13 |
| 4 | PV7 | 0.911 | 0.985 | 0.999 | 3.72 | 0.29 | 54.29 |
| 5 | PVL | 0.951 | 0.939 | 0.99 | 5.86 | 0.67 | 48.69 |
| 6 | PVF | 0.973 | 0.944 | 0.998 | 3.74 | 0.85 | 77.07 |
Data shown are % Inhibition Mean of experiment performed in triplicate
PVS Palam Vichitra seeds, PV3 Palam Vichitra 3 day sprout, PV5 Palam Vichitra 5 day sprout, PV7 Palam Vichitra 7 day sprout, PVL Palam Vichitra Leaves, PVF Palam Vichitra Floret
Scavenging of superoxide radical
The percent inhibition at different concentration (0.125–2 mg/ml) was analyzed by the reduction of PMS-NADH-NBT system. The IC50 value obtained for PV was highest in 3 Day sprouts extract (PV3) i.e. 0.0768 mg/ml and lowest in floret (PVF) extract i.e. 0.852 mg/ml. The IC50 value of other extracts PV5, PV7, PVL and PVS is 0.082, 0.290, 0.672 and 0.791 mg/ml respectively. The order of effectiveness of the extracts in terms of IC50 values obtained was PV3 > PV5 > PV7 > PVL > PVS > PVF. It was noticed that all the extracts were capable of scavenging hydrogen peroxide in an dose-dependent manner. The two-way ANOVA and Tukey’s HSD post hoc test was used for its pairwise multiple comparisons between the concentration and between different extracts of PV. It was seen that pairwise comparison between all the extracts was statistically significant at p ≤ 0.05 except PV5 versus PV3 and PVF versus PVS and pairwise comparison between the concentrations for all the extracts was statistically significant different except 1c versus 5c (Table 2 and Fig. SI-2).
MTT assay
In MTT assay, the potency of the extracts to inhibit the growth of PC-3 cells was measured at different concentrations (10–100 µg/ml) and compared with known cell growth inhibitors like camptothecin. The extracts limited the proliferation of PC-3 cells with IC50 values (concentrations of extract leading to 50% inhibition of cell growth) calculated by using best-fit regression model. All extracts of PV were assessed at different concentrations i.e. 10–100 µg/ml. All the extracts showed the dose-dependent cytotoxic effect on PC-3 cell line. It was observed that the PVS extract was highly effective as it inhibited 50% growth at 36.47 µg/ml concentration. The other extracts PVL, PV7, PVF, PV3 and PV5 exhibited IC50 concentration in the order: 48.69, 54.29, 77.07, 77.31 and 80.13 µg/ml (Table 2).
Nuclear morphology changes
The different extracts were also evaluated for their nuclear morphological changes at their respective IC50 values on PC-3 cell line stained with 4′,6-diamidino-2-phenylindole (DAPI) using the fluorescent microscope. The presence of apoptotic bodies in the cells treated with positive control (camptothecin) further points out towards the fact that these triggered cell death via apoptosis. The microscopy revealed that among the different extracts of PV, PVS and PVF showed comparatively more changes in morphology characteristics of apoptosis as compared to other extracts. The treated cells as compared to control were observed to be less adherent, lost polygonal shape, significant shrinkages and a few prominent surface blebs, the characteristics of apoptosis (Fig. 2).
Fig. 2.
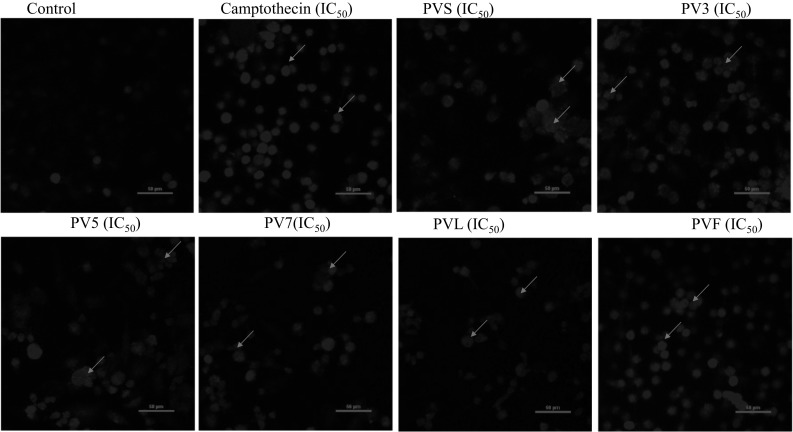
DAPI fluorescence staining of PC-3 cells treated with different extracts of PV, at IC50 concentration and analyzed for nuclear morphology and apoptotic bodies. Picture shown was amplified by NIS element software which indicates apoptotic features like rounding, nuclei condensation and nucleus fragmentation
Cell cycle analysis
It was observed that the PC-3 cells treated with plant extracts at their corresponding IC50 concentration for 12 h restricted the cells in hypo-diploid (sub G0) phase of the cell cycle as compared to the control. The particular characteristics of cell apoptosis are DNA fragmentation that leads to the appearance of hypodiploid cells (sub G0 population) which indicated that extracts could induce cell apoptosis. The cells with DNA content less than the content in G1 phase of the cell cycle are counted as the hypodiploid cells. DNA content is analyzed in the least 10,000 single events after staining of cellular DNA with PI at their respective concentrations. The Sub G0 fraction was 25.7% in control cells (2% DMSO), which increased to 75.0% for standard camptothecin at the IC50 concentration. It was observed that the cells treated with PVS extracts have the highest percentage of DNA content in sub G0 population i.e. 77.0%. The DNA content in sub G0 population treated with other extracts i.e. PVF, PV5, PV7, PVL and PV3 was 63.5, 62.9, 55.2, 55.1 and 51.8%, respectively (Fig. 3).
Fig. 3.
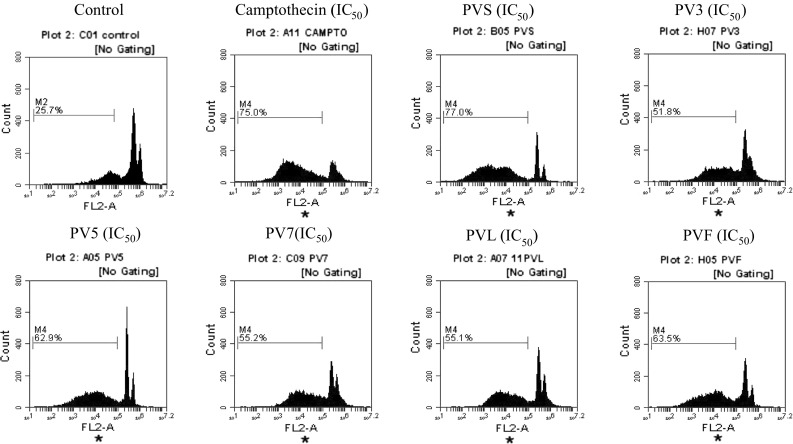
DNA cell cycle analysis in PC-3 cells exposed to different extracts of Palam Vichitra at their respective IC50 concentration. Cells were stained with PI to determine DNA fluorescence and cell cycle phase distribution, 10,000 events acquired and gated population analyzed using flow cytometer
Generation of reactive oxygen species (ROS)
The 2,7 dichloroflurorescein diacetate (DCFDA) a non-fluorescent cell-membrane permeable probe, react with cellular esterases/ROS, and then metabolized into fluorescent DCF which was used to stain the treated PC-3 cells. The cells were treated with extract at different concentration for 12 h and then analyzed in FL-1 channel. Treatment with camptothecin at different concentration was found to enhance ROS generation by 20.1, 27.9 and 36.1% at their respective IC30, IC50 and IC70 concentrations as compared to low DCF fluorescence observed in Control (2% DMSO) in PC-3 cells. It was observed that all the extracts of PV, showed dose dependent increase in DCF fluorescence as analyzed by flow cytometer. In case of PVS, PV5, PVL, PV3, PV7 and PVF extracts, the maximum DCF fluorescence of 18.5, 23.5, 28.6, 33.0, 36.3 and 39.3.0% was observed at IC70 concentration respectively compared to low DCF fluorescence of 7.1% in untreated cells (Fig. 4a and Fig. SI-3, 4, 5).
Fig. 4.
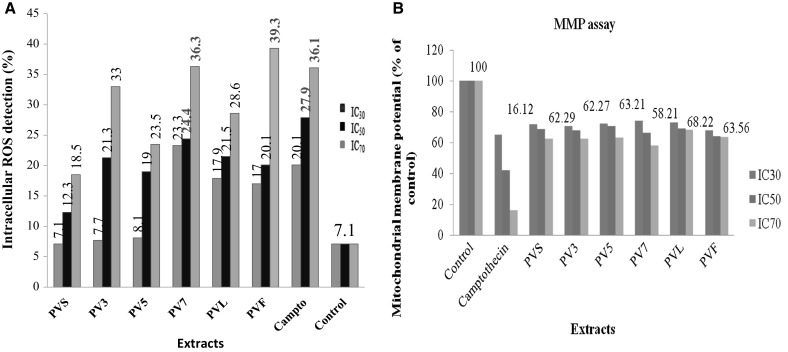
(a, b) Effect of different extracts of Palam Vichitra, a ROS and b mitochondrial membrane potential in prostate cancer (PC-3) cell line at its respective IC30, IC50 and IC70 concentrations
Shift of mitochondrial membrane potential (MMP)
The Rh-123 fluorescence was used to determine shift in mitochondria transmembrane potential in PC-3 cells following treatment with different concentrations of extracts at their respective IC30, IC50 and IC70 concentrations. The shift in mitochondrial transmembrane potential was measured at 485/20 nm excitation and 528/20 nm emission wavelengths. It was observed that all the untreated PC-3 cells were functionally active (100%) with high Rh-123 signals while the camptothecin-induced shift in mitochondrial membrane potential in a concentration-dependent manner. The different extracts PV exhibited a remarkable attenuation of mitochondrial membrane potential after 12 h exposure of PC-3 cells. A shift of mitochondrial membrane potential in all extracts i.e. PV7, PV3, PVS, PV5, PVF and PVL was 58.21, 62.27, 62.29, 63.21, 63.56 and 68.22% respectively at IC70 concentration (Fig. 4 B).
Discussion
Nutraceuticals are inexpensive and readily available and have drawn considerable attention due to their multitargeted potential. Several combination strategies have been tested in preclinical studies by combining ITCs among themselves or with conventional or new anticancer therapies (Gupta, et al. 2014). The seeds, sprouts (3, 5 and 7 days) of PV and Leaves and Floret extracts of PV were explored for the presence of different phytochemical by GC–MS. The analysis of different extracts showed a wide variation in the hydrolytic products of glucosinolates as well as other phytochemicals. The individual component of different plant extracts was identified by comparison of their mass spectra (MS) with NIST database and Adams libraries. A similar study was conducted by Jirovetz et al. (2002) for the aroma compound analysis of Eruca sativa. Likewise, Chiang et al. (1998) found that under split/splitless conditions using a GC–MS system operated revealed that ≈ 80% of sulforaphane was degraded to 3-butenyl isothiocyanate. In molybdate ion reduction assay the activity was found to be highest in leave (PVL), exhibited the highest reduction ability of 23.85 mg in terms of AAE/100 mg dry weight at 125 µg/ml concentration. Gülçin et al. (2004) also studied the water and ethanol extracts of broccoli florets for in vitro anti-oxidant properties: total antioxidant activity, reducing power, free radical scavenging, superoxide anion radical scavenging, hydrogen peroxide scavenging, and metal chelating activities and found that both extracts exhibited strong total antioxidant activity. It was observed that floret (PVF) showed maximum inhibition in DPPH assay at the high concentration of 2 mg/ml. Kaur et al. (2007) analyzed the antioxidant activity of extracts from 34 vegetables and also concluded that broccoli belongs to the group of vegetables with a high antioxidant activity (> 70%). Farag and Motaal (2010) reported that chloroform extracts of red cabbage and chinese kale exhibited good DPPH radical scavenging activity with percentage inhibition values of 73 and 54 respectively at highest dose i.e. 10 mg/ml. Among all PV extracts, superoxide anion scavenging ability of PV5 extract was 81.38% at 2 mg/ml concentration. Bidchol, et al. (2011) reported that Brassica oleracea L. var. italica effectively scavenged superoxide in a concentration-dependent manner with IC50 values for the aqueous and ethanolic extracts was 0.93 and 0.25 mg/ml, respectively. The amount of supercoiled DNA, in the presence of PV extracts, was seems protective. Similarly, Kaur et al. (2013) reported that Lepidium latifolium extracts showed an ability to quench free radicals which is rich in glucosinolates in three in vitro assays namely DPPH, O•2 scavenging activity, OH• scavenging activity and DNA protective activity of leaf. The two antioxidant assays suggest that potent antioxidants are present in the dichloromethane and hexane samples of broccoli sprouts (Jang et al. 2015).
It was seen that all extracts exerted dose-dependent cytotoxicity, but floret (PVF) extract exhibited comparatively more effect with low IC50 concentration. Bachiega et al. 2016 showed antiproliferative activities in different maturation stages of broccoli biofortified with selenium on U251, MCF-7, 786-0, NCI-H460, HT29 and HaCaT. Likewise, Hwang and Lim 2015 studied the antioxidant and anticancer activities of broccoli by-products from different cultivars and maturity stages. Tang et al. (2006) also showed that isothiocyanate rich broccoli sprout extracts inhibited cancer cell growth very effectively. The PVS and PV3 showed more visible features of apoptosis as compared to other extracts in microscopy analysis. Abdulah et al. (2009) observed apparent morphological changes in LNCaP prostate cancer cells when treated with selenium-enriched broccoli sprout extract and induced cell death.
The particular characteristics of cell apoptosis like DNA fragmentation, induced by extracts lead to the appearance of hypo diploid cells (sub G0 population). It was observed that the cells treated with PVS extracts have the highest percentage of DNA content in sub G0 population and other extracts also elevated the DNA content in sub G0 population as compared to control. The floret extract (PVF), showed maximum ROS generation. The different extracts of PV exhibited the highest attenuation of mitochondrial membrane potential, but the maximum was found in PV7 extract. Earlier findings also supports our results that cell cycle arrest is key characteristics of apoptosis which is reported to induce cell death by changes in nuclear morphology and ROS generation. ROS-dependent disruption of mitochondrial membrane integrity lead to induction of cytochrome c from altered mitochondria (Tang et al. 2006; Huang et al. 2012; Park et al. 2014).
Conclusion
The present study, ascertained anticancer and anti-inflammatory potential of broccoli. The study indicates the potential use of broccoli as source of natural, good and cheap antioxidants, which may be industrially exploited. Moreover, an in vitro antiproliferative study against prostate cancer cells (PC-3) supports the florets as well as sprouts to be a good source of anticancer agents. The modes of action of these phytochemicals are still unclear, therefore, exploring the detailed mechanisms will help in providing useful information for their possible application in cancer prevention.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We gratefully acknowledge the financial support received from the Council of Scientific and Industrial Research, New Delhi (38(1193)/08/EMR-П) and CEPEPA (UGC). The authors are also grateful to Director, CSIR-IHBT-Palampur for providing GC–MS facilities.
Abbreviations
- PVS
Palam Vichitra seeds
- PV3
Palam Vichitra 3 day sprout
- PV5
Palam Vichitra 5 day sprout
- PV7
Palam Vichitra 7 day sprout
- PVL
Palam Vichitra leaves
- PVF
Palam Vichitra floret
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3095-0) contains supplementary material, which is available to authorized users.
Contributor Information
Ashun Chaudhary, Email: ashun.chaudhary@gmail.com.
Saroj Arora, Phone: 0183-2501931, Email: jrosh1@rediffmail.com.
References
- Abdulah R, Faried A, Kobayashi K, Yamazaki C, Suradji EW, Ito K, Koyama H. Selenium enrichment of broccoli sprout extract increases chemosensitivity and apoptosis of LNCaP prostate cancer cells. BMC Cancer. 2009;9:414. doi: 10.1186/1471-2407-9-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachiega P, Salgado JM, de Carvalho JE, Ruiz ALT, Schwarz K, Tezotto T, Morzelle MC. Antioxidant and antiproliferative activities in different maturation stages of broccoli (Brassica oleracea Italica) biofortified with selenium. Food Chem. 2016;190:771–776. doi: 10.1016/j.foodchem.2015.06.024. [DOI] [PubMed] [Google Scholar]
- Bhushan S, Kumar A, Malik F, Andotra SS, Sethi VK, Kaur IP, Taneja SC, Qazi GN, Singh J. A triterpenediol from Boswellia serrata induces apoptosis through both the intrinsic and extrinsic apoptotic pathways in human leukemia HL-60 cells. Apoptosis. 2007;12:1911–1926. doi: 10.1007/s10495-007-0105-5. [DOI] [PubMed] [Google Scholar]
- Bidchol AM, Wilfred A, Abhijna P, Harish R. Free radical scavenging activity of aqueous and ethanolic extract of Brassica oleracea L. var. italica. Food Bioprocess Technol. 2011;4:1137–1143. doi: 10.1007/s11947-009-0196-9. [DOI] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Chaudhary A, Sharma U, Vig AP, Singh B, Arora S. Free radical scavenging, antiproliferative activities and profiling of variations in the level of phytochemicals in different parts of broccoli (Brassica oleracea italica) Food Chem. 2014;148:373–380. doi: 10.1016/j.foodchem.2013.10.042. [DOI] [PubMed] [Google Scholar]
- Chiang WC, Pusateri DJ, Leitz RE. Gas chromatography/mass spectrometry method for the determination of sulforaphane and sulforaphane nitrile in broccoli. J Agric Food Chem. 1998;46:1018–1021. doi: 10.1021/jf970572b. [DOI] [Google Scholar]
- Ciska E, Martyniak-Przybyszewska B, Kozlowska H. Content of glucosinolates in cruciferous vegetables grown at the same site for two years under different climatic conditions. J Agric Food Chem. 2000;48:2862–2867. doi: 10.1021/jf981373a. [DOI] [PubMed] [Google Scholar]
- Deng S, Yuan H, Yi J, Lu Y, Wei Q, Guo C, He Z. Gossypol acetic acid induces apoptosis in RAW264.7 cells via a caspase-dependent mitochondrial signaling pathway. J Vet Sci. 2013;14:281–289. doi: 10.4142/jvs.2013.14.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espín JC, García-Conesa MT, Tomás-Barberán FA. Nutraceuticals: facts and fiction. Phytochemistry. 2007;68:2986–3008. doi: 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Farag MA, Motaal AAA. Sulforaphane composition, cytotoxic and antioxidant activity of crucifer vegetables. J Adv Res. 2010;1:65–70. doi: 10.1016/j.jare.2010.02.005. [DOI] [Google Scholar]
- Gülçin I, Küfrevioǧlu Öİ, Oktay M, Büyükokuroǧlu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.) J Ethnopharmacol. 2004;90:205–215. doi: 10.1016/j.jep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Gupta P, Kim B, Kim SH, Srivastava SK. Molecular targets of isothiocyanates in cancer: recent advances. Mol Nutr Food Res. 2014;58:1685–1707. doi: 10.1002/mnfr.201300684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Zhang X, Qiu S, Yu D, Lin S. Salidroside induces cell-cycle arrest and apoptosis in human breast cancer cells. Biochem Biophys Res Commun. 2010;398:62–67. doi: 10.1016/j.bbrc.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Huang SH, Wu LW, Huang AC, Yu CC, Lien JC, Huang YP, Yang JS, Yang JH, Hsiao YP, Wood WG, Yu CS, Chung JG. Benzyl isothiocyanate (BITC) induces G2/M phase arrest and apoptosis in human melanoma A375.S2 cells through reactive oxygen species (ROS) and both mitochondria-dependent and death receptor-mediated multiple signaling pathways. J Agric Food Chem. 2012;60:665–675. doi: 10.1021/jf204193v. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Lim SB. Antioxidant and anticancer activities of broccoli by-products from different cultivars and maturity stages at harvest. Prev Nutr Sci. 2015;20:8–14. doi: 10.3746/pnf.2015.20.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HW, Moon JK, Shibamoto T. Analysis and Antioxidant Activity of Extracts from Broccoli (Brassica oleracea L.) Sprouts. J Agric Food Chem. 2015;63:1169–1174. doi: 10.1021/jf504929m. [DOI] [PubMed] [Google Scholar]
- Jirovetz L, Smith D, Buchbauer G. Aroma compound analysis of Eruca sativa (Brassicaceae) SPME headspace leaf samples using GC, GC–MS, and olfactometry. J Agric Food Chem. 2002;50:4643–4646. doi: 10.1021/jf020129n. [DOI] [PubMed] [Google Scholar]
- Kaur C, Kumar K, Anil D, Kapoor HC. Variations in antioxidant activity in broccoli (Brassica oleracea L.) cultivars. J Food Biochem. 2007;31:621–638. doi: 10.1111/j.1745-4514.2007.00134.x. [DOI] [Google Scholar]
- Kaur T, Bhat HA, Raina A, Koul S, Vyas D. Glutathione regulates enzymatic antioxidant defence with differential thiol content in perennial pepperweed and helps adapting to extreme environment. Acta Physiol Plant. 2013;35:2501–2511. doi: 10.1007/s11738-013-1286-x. [DOI] [Google Scholar]
- Kello M, Drutovic D, Chripkova M, Pilatova M, Budovska M, Kulikova L, Urdzik P, Mojzis J. ROS-dependent antiproliferative effect of brassinin derivative homobrassinin in human colorectal cancer Caco2 cells. Molecules. 2014;19:10877–10897. doi: 10.3390/molecules190810877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keum YS, Jeong WS, Kong AN. Chemopreventive functions of isothiocyanates. Drug News Perspect. 2005;18:445–451. doi: 10.1358/dnp.2005.18.7.939350. [DOI] [PubMed] [Google Scholar]
- Kim YS, Milner JA. Targets for indole-3-carbinol in cancer prevention. J Nutr Biochem. 2005;16:65–73. doi: 10.1016/j.jnutbio.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lee JC, Kim HR, Kim J, Jang YS. Antioxidant property of an Ethanol extract of the stem of Opuntia ficus-indica var. saboten. J Agric Food Chem. 2002;50:6490–6496. doi: 10.1021/jf020388c. [DOI] [PubMed] [Google Scholar]
- Manson MM, Farmer PB, Gescher A, Steward WP (2005) Innovative agents in cancer prevention. In: Senn H-J, Morant R (eds) Tumor prevention and genetics III. Springer, Berlin, pp 257–275 [DOI] [PubMed]
- Miyata T. Pharmacological basis of traditional medicines and health supplements as curatives. J Pharmacol Sci. 2007;103:127–131. doi: 10.1254/jphs.CPJ06016X. [DOI] [PubMed] [Google Scholar]
- Moreno DA, Carvajal M, López-Berenguer C, García-Viguera C. Chemical and biological characterisation of nutraceutical compounds of broccoli. J Pharm Biomed Anal. 2006;41:1508–1522. doi: 10.1016/j.jpba.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Park HS, Han MH, Kim GY, Moon SK, Kim WJ, Hwang HJ, Park KY, Choi YH. Sulforaphane induces reactive oxygen species-mediated mitotic arrest and subsequent apoptosis in human bladder cancer 5637 cells. Food Chem Toxicol. 2014;64:157–165. doi: 10.1016/j.fct.2013.11.034. [DOI] [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Qian Y, Guan T, Huang M, Cao L, Li Y, Cheng H, Jin H, Yu D. Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-κB activation in a cerebral ischemia mouse model. Neurochem Int. 2012;60:759–767. doi: 10.1016/j.neuint.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Sharma G, Prakash D, Gupta C, Prakash D, Sharma G. Phytochemicals of nutraceutical importance: do they defend against diseases? In: Parkash D, Sharma G, editors. Phytochemicals of nutraceutical importance. Uttar Pardesh: Amity University; 2014. pp. 1–19. [Google Scholar]
- Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr. 2001;131:3027–3033. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- Tang L, Zhang Y, Jobson HE, Li J, Stephenson KK, Wade KL, Fahey JW. Potent activation of mitochondria-mediated apoptosis and arrest in S and M phases of cancer cells by a broccoli sprout extract. Mol Cancer Ther. 2006;5:935–944. doi: 10.1158/1535-7163.MCT-05-0476. [DOI] [PubMed] [Google Scholar]
- Verkerk R, Schreiner M, Krumbein A, Ciska E, Holst B, Rowland I, De Schrijver R, Hansen M, Gerhäuser C, Richard Mithen R, Dekker M. Glucosinolates in Brassica vegetables: the influence of the food supply chain on intake, bioavailability and human health. Mol Nutr Food Res. 2009;53:219. doi: 10.1002/mnfr.200800065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


