Abstract
Drip loss of fresh-cut watermelon has become a concern for both producers and consumers. The effect of visible light exposure on the drip loss of fresh-cut watermelon was evaluated. Visible light treatments of 3000 and 10 Lux were applied to fresh-cut watermelon at 4 °C during the shelf life for 5 days, with light exposure of 150 Lux as the control. The drip loss of the fresh-cut watermelon at 3000 Lux was 74.8% of that at 150 Lux on the fifth day, and the moisture evaporation at 3000 Lux was 1.89 times that at 150 Lux. Moreover, the light exposure of 3000 Lux reduced the activity of polygalacturonase, which is a key hydrolase related to cell wall degradation. The cell wall degradation ratio of the fresh-cut watermelon at 3000 Lux was 81.7% of that at 150 Lux on the fifth day. Overall, light exposure of 3000 Lux reduced drip loss by accelerating moisture evaporation in fresh-cut watermelon, as well as by reducing the activity of polygalacturonase and the ratio of cell wall degradation. Hence, exposing the fresh-cut watermelon to visible light of 3000 Lux during the shelf life was a feasible way of reducing drip loss.
Keywords: Fresh-cut watermelon, Visible light exposure, Cell wall degradation, Drip loss, Polygalacturonase
Introduction
Fresh-cut watermelon has a crispy texture and refreshing flavor, and provides both moisture and sucrose (McGlynn et al. 2003; Petrou et al. 2013). The shelf life of fresh-cut watermelon is usually 4–7 days, depending on the cultivars, transportation, and shelf conditions (Artés-Hernández et al. 2010; Cai et al. 2015; Fonseca and Rushing 2006; Mao et al. 2006; Robert et al. 2007). The drip loss of fresh-cut watermelon is usually around 4–10%, during its shelf life (Zhan et al. 2012a). Drip loss deposited at the bottom of the trays reduces sales by reducing the consumers’ purchasing desire, and also increases the chance of microbial contamination. Owing to these problems, the drip loss of fresh-cut watermelon has become a source of concern for both producers and consumers.
The drip loss of the fresh-cut watermelon can be initiated by cutting, microbial contamination, and physiological metabolism. The drip loss initiated by cutting can be reduced by using a sharp knife and/or by draining the drip before packaging. The drip loss initiated by microbial contamination can be reduced by maintaining sanitary conditions, and by implementing good manufacturing practice. As a result, the drip loss initiated by cutting and microbial contamination is generally reduced with the efforts of the producers. However, the drip loss initiated by the physiological metabolism is more complicated. Specifically, the physiological metabolism leads to the breakdown of the cell wall, causing the intracellular inclusions to run out, accumulate, and form the drip (Brummell et al. 2004). In succession, the intracellular inclusions, such as cellulase and pecticase, hydrolyze the adjacent cell walls, leading to their destruction (Karakurt and Huber 2002). These two steps repeat during the whole shelf life, and form the drip loss. Hence, developing a technique to reduce the physiological metabolism would prove beneficial to reducing the drip loss.
Two methods have been applied to reduce the drip loss of fresh-cut fruit. One is passive, by inserting absorbent pads. These pads absorb the drip loss in the trays of fresh-cut melon (Fernández et al. 2010), cantaloupe (Cucumis melo L.) (Bai et al. 2001), kiwifruit, mango, persimmon (Vilas-Boas and Kader 2007), and tomato (Gil et al. 2002). The second method is positive, by reducing the physiological metabolism of fruit. Modified atmosphere packaging and 1-methylcyclopropene treatments have successfully reduced the physiological metabolism of Cucurbitaceae (Mao et al. 2006; Nilsson 2005; Robert et al. 2007; Zhou et al. 2006).
Visible light exposure is a novel, non-toxic, cheap, and residue-free approach for preserving the quality of fresh-cut produce (Manzocco et al. 2009b; Zhan et al. 2012a, b). Visible light exposure retards the physiological metabolism of plants by changing the photosynthesis and physiological activity (Olarte et al. 2009; Sanz et al. 2009, 2007; Zhan et al. 2012a, b). Visible light exposure inactivates cellulase, pectinase, polyphenol oxidase, peroxidase, and superoxide dismutase activity in fruit (Cakmak and Marschner 1992; Manzocco et al. 2009a, b; Zhan et al. 2012b). In succession, the inactivated enzymes reduce the rate of cell wall degradation. Moreover, visible light exposure inhibits the growth of some bacteria (Fonseca and Rushing 2006; Jia et al. 2009), and enhances moisture evaporation (Martínez-Sánchez et al. 2011). Hence, visible light exposure during the shelf life could potentially reduce the physiological metabolism of fresh-cut watermelons, thereby reducing their drip loss.
Fresh-cut watermelon is usually stored in an air curtain cabinet in the supermarket, with light exposure of around 150 Lux. In this trial, light exposure of 3000 and 10 Lux was applied the fresh-cut watermelon during the shelf life for 5 days with light exposure of 150 Lux as the control. The qualities related to drip loss were evaluated during the shelf life, to provide a deeper insight into the underlying mechanisms of drip loss.
Materials and methods
Light exposure treatment of fresh-cut watermelon
The mature and uniform seeded watermelons (Citrullus vulgaris, var. Jingxin No. 3) were bought from a local fruit market. The fruit were round with regular stripes, and weighted about 3–4 kg per fruit. The flesh of the fruits was red and crispy, with a soluble-solid content of 11.5–13.5%.
A batch of fruits was stored at 4 °C for 24 h before cutting. The surface of the fruits was washed in icy sodium hypochlorite solution (100 mg/L) and flushed with icy water, and then the fruits were drained in a bracket. The drained fruits were moved to a sanitary processing room with a temperature of 4 °C. The fruits were peeled until the red flesh was revealed. The flesh was cut into cubes with the dimensions 2.5 cm × 2.5 cm × 2.5 cm. All cubes were mixed, and random cubes weighing approximately 300 g were fitted in a polyethylene tray and sealed with a transparent cover. The trays were stored in a three-shelf air-curtain cabinet, at 4 °C. The cabinet was illuminated from the top with two fluorescent lamps (Philips TLD lamp YZ36RR25, 36W, China), during the entire shelf life. The intensity of the visible light and dosage of the ultraviolet radiation were monitored by a portable light meter (TES-1339R Data Logger, TES Electrical Electronic Corp., Taipei, China) and an ultraviolet radiation meter (LS125, Shenzhen Linshang Technology Co., Ltd., Shenzhen, China), respectively. The light and ultraviolet sensors were sealed in the trays and placed on each shelf. The visible light intensity was adjusted to 3000, 150, and 10 Lux, from the top to the bottom of the cabinet, by adjusting the bracket position or by covering the trays with dark polyethylene film. The temperature of each shelf was monitored by the Onset HOBO data logger U14-001 (Onset Computer Corporation, Bourne, MA, 02532, USA). The average temperature was the arithmetic mean of the temperature during the shelf life. The qualities of the fruit were evaluated on the first, third, and fifth day.
Weight loss
The total fruit weight was measured as the difference between the weight of the tray with the fruits and the weight of the empty tray. At the end of the shelf life study, the drip resulting from the fruit was drained using a Kimberly–Clark tissue. The weight loss was the difference between the total fruit weight and the weight of the drained fruit. The weight loss ratio was measured as the percentage of the weight loss in the total fruit weight.
The weight loss was further divided into moisture evaporation and drip loss. The drip loss included the drip deposited on the inner wall and on the bottom of the tray, as well as the drip attached to the fruit. Consequently, the drip loss was the sum of the drip absorbed in the Kimberly–Clark tissue from the fruits and the tray. The moisture evaporation was the difference between drip loss and weight loss. The drip loss ratio was the ratio of the drip loss in the total weight loss of the fruit.
Evaluation of cell degradation ratio
The cubes were tiled in a flat plate, and photographed with uniform exposure parameters by Canon EOS600D (Canon Group, Japan). The image was calculated by the Image-pro-plus 6.0 software (Media Cybernetics, Inc., Bethesda, MD, USA). A rectangle region was delineated randomly in the picture. The red color index of 140–190 in the RGB system was applied to the rectangle region, to screen for cell degradation. The cell-degradation region was marked in blue, and counted as the number of pixels in the selected rectangle region. The cell degradation ratio was defined as the percentage of the pixel number in the blue region of the pixel number in the selected rectangle region. The rectangle region was randomly selected at least six times for each picture. The cell degradation ratio was an average of the replicates.
Measurement of electrolyte leakage
Electrolyte leakage was measured according to a recently reported method (Jiang et al. 2001) with some modifications. Briefly, the fruits of 100 g was immersed in 0.33 L of distilled water and incubated at 25 °C for 1 h. Initial electrolyte leakage of the fruit was measured by a DJS-1 conductivity immersion electrode (DDB-6200, Shanghai Leici Apparatus, Shanghai, China). Then, the fruit was boiled for 30 min and cooled to 25 °C. Distilled water was added to reach the amount before the boiling. The total electrolyte leakage was measured. Relative conductivity was defined as the ratio of the initial electrolyte leakage in the total electrolyte leakage.
Measurement of pectic lyase, polygalacturonase, and pectin methylesterase activity
The fruit was homogenized in a Philips food mixer (HR1861 mixer, Philips, Dongguan, China) and centrifuged at 7000×g and 4 °C for 10 min (Sigma 3-18K, Sartorius, Gattingen, Germany). The supernatant was evaluated for the activity of pectic lyase (PL), polygalacturonase (PG) and pectin methylesterase (PME).
The PL activity was assayed by a UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan), based on the absorption at 235 nm. The reaction was started by the addition of 75 μL of supernatant to 3 mL of incubation medium containing 0.425 mL 0.1 mol/L phosphate/citrate buffer pH 6.0, 1 mL of 0.1 mol/L calcium chloride solution and 1.5 mL of pectin aqueous solution as substrate. The changes in absorbance at 235 nm were monitored for 10 min. The pectin aqueous solution was prepared by stirring 5.0 g/L apple pectin with a methoxylation degree higher than 75% (Sigma, St. Louis, MO, USA) in 0.1 mol/L phosphate/citrate buffer of pH 6.0, at 40 °C. The activity was expressed as the changes in absorbance per minute, calculated by linear regression.
PG activity was measured by a UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan), based on the release of reducing groups produced by PG. Specifically, 100 μL of the supernatant were mixed with 300 μL of 0.2% polygalacturonic acid, and incubated at 35 °C for 10 min. To stop the reaction, 2 mL of 0.1 mol/L borate buffer (pH 9.0) and 400 μL of 1% cyanoacetamide solution were added to the reaction mixture and boiled for 10 min. The absorbance was measured at 276 nm and 22 °C, with a blank of the same mixture minus the supernatant. The standard curve was built with α-d-galacturonic acid as the reducing sugar. One unit of PG activity was defined as the amount of enzyme that releases 1 μmol/L of galacturonic acid per min.
PME activity was measured based on carboxyl group titration. An aliquot of 0.20 mL of supernatant was mixed with 20 mL of 10 mg/mL pectin (Sigma, USA) containing 0.1 M NaCl, and incubated at 23 °C. An aliquot of 25 μL of 0.1 mol/L NaOH was added to the mixture, and its pH was adjusted to 7.5 using 0.1 mol/L NaOH (TitroLine Easy, Schott, Mainz, Germany). The duration of the solution returning to pH 7.5 was measured. The activity of PME was calculated by Eq. (1).
| 1 |
where A is the PME activity, CNaOH is the concentration of NaOH (0.1 mol/L), VNaOH is the volume of NaOH used (25 μL), Vsample is the volume of sample used (0.20 mL), and t is the time needed for pH to return to 7.5 after the addition of NaOH (min).
The residual activity of each enzyme was calculated as the percentage of the enzymatic activity at time t in that at time zero.
Statistical analysis
The trial was repeated three times. More than 60 trays were used for quality evaluation in each trial. The data were expressed as the average ± SD of at least six repetitions. Duncan’s multiple range test was used to compare differences in the results. All analyses were conducted in SPSS (Window Version 19).
Results and discussion
Shelf life conditions of fresh-cut watermelon
The visible light exposure of cold cabinet in supermarkets, such as Walmart and Carrefour, is around 100–200 Lux, which provides consumers with comfortable illumination to select the desired products. All fresh-cut products are subjected to this level of light exposure during their shelf life, except for the ones in opaque packaging. Hence, light exposure of 150 Lux was set as the control in the following discussion. The visible light intensity of 3000 Lux provided the same illumination used for a jewelry or watch counter in a department store, which enhanced the shopping experience for the consumers. The light exposure of 10 Lux created an almost a dark condition, like being sealed in opaque packaging. The light intensity of the 3000 Lux reached 352 W/m2, which was about 15 times that of 150 Lux (22.2 W/m2) and 234 times that of 10 Lux (1.5 W/m2), respectively.
The ultraviolet B and C radiations reduce the microbial contamination and inactivate the cellulose, pectinase, and hydrolase in fresh-cut products (Artés-Hernández et al. 2010; Fonseca and Rushing 2006; Manzocco et al. 2009a; Tran and Farid 2004). Ultraviolet B radiation was not detected in any treatments, whereas ultraviolet C radiation was detected at low levels. The level of ultraviolet C radiation was 30, 2.8, and 0 W/m2 in the light exposure of 3000, 150, and 10 Lux, respectively.
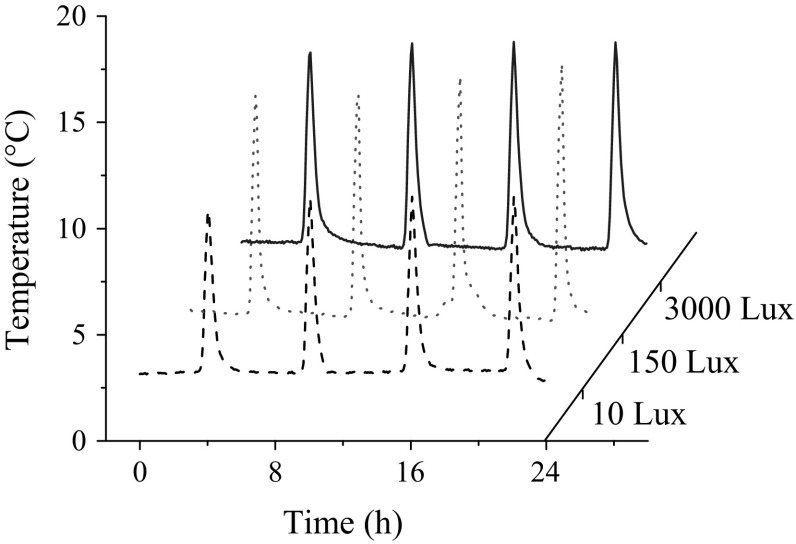
A typical temperature fluctuation with time in the air-curtain cabinet has been shown in Fig. 1. The temperature was (4.01 ± 1.85), (3.84 ± 2.25) and (4.14 ± 2.16) °C at light exposure of 10, 150, and 3000 Lux, respectively, which was statistically similar. Hence, the intensity of the light exposure and level of ultraviolet C radiation were the main differences of the three treatments.
Fig. 1.
A typical temperature cycle of the visible light exposure
Effect of visible light exposure on the cell wall degradation of fresh-cut watermelon
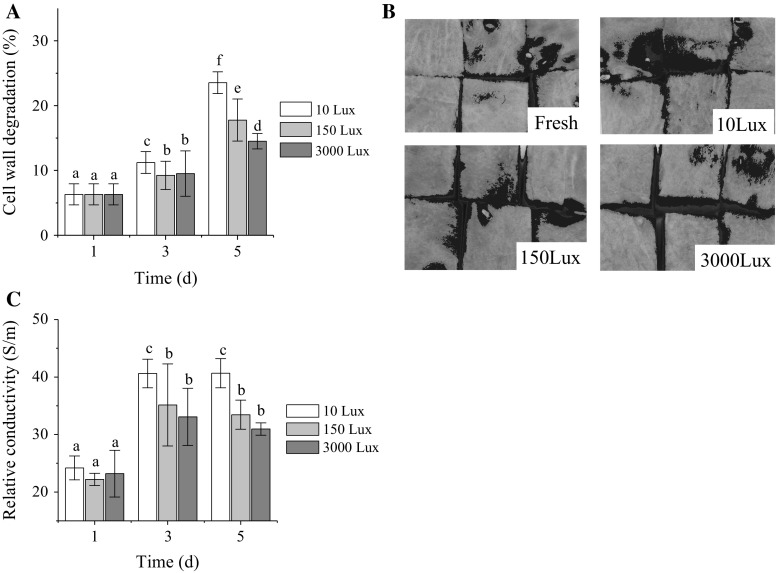
Drip loss mainly results due to the cell wall degradation of fruits (Mao et al. 2004; Marín-Rodríguez et al. 2002). The cell wall degradation of the fresh-cut watermelon caused translucency/dark-red color on the fruit surface. Hence, the appearance and relative conductivity of the fresh-cut watermelon were measured to reflect the degree of cell wall degradation from different views.
The cell wall degradation ratio of the fresh-cut watermelon increased with time, during the shelf life (Fig. 2a). The cell wall degradation ratio at 3000 Lux was 81.7 and 61.7% of that at 150 and 10 Lux on the fifth day, respectively. Consequently, the cell wall degradation ratio at 3000 Lux was significantly lower than that at 150 and 10 Lux on the fifth day. This proves that the intensity of the light exposure was negatively related to the cell wall degradation ratio of the fresh-cut watermelon. In addition, the translucent/dark-red colored region indicates breakage of the cell wall (Mao et al. 2004; Marín-Rodríguez et al. 2002), and was used as a direct indicator of cell wall degradation. Blue regions identified on the surface of the fruit were reduced with the intensity of the light exposure on the fifth day (Fig. 2b). Remarkably, the blue region mainly present around the margins of each cube, which correlated with the onset of the cell wall degradation. Remarkably, visible light exposure at 3000 Lux inhibited the spreading of cell wall degradation.
Fig. 2.
Effect of visible light exposure on cell wall degradation ratio (a), appearance (b), and relative conductivity (c) of fresh-cut watermelon in the fifth day. The blue region represents the degradation of cells. Data are mean ± SD (n ≥ 6). Means with a different letter represent a significant difference (p < 0.05)
Relative conductivity of fruit tissues provides information on cell integrity, being an indirect measurement of cell wall degradation (Martínez-Sánchez et al. 2011). Consequently, cell wall degradation increases the relative conductivity value (Jiang et al. 2001). The relative conductivity of the fresh-cut watermelon was enhanced during the shelf life (Fig. 2c). The relative conductivity of the fruit at 3000 Lux was significantly lower than that at 10 Lux. Therefore, visible light exposure of 3000 Lux reduced the cell wall degradation of fresh-cut watermelon.
Effect of visible light exposure on PL, PG and PME activity in fresh-cut watermelon
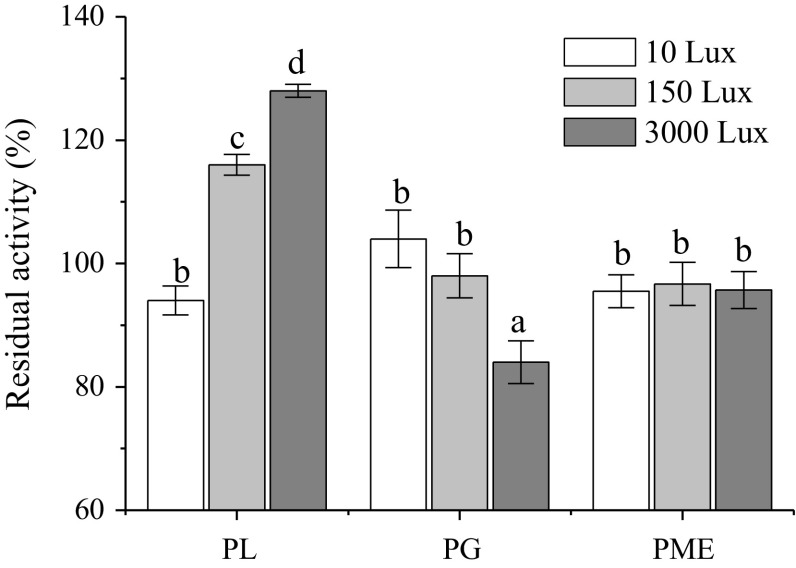
PL, PG, and PME are closely related to the cell wall degradation of fruits (Marín-Rodríguez et al. 2002). Specifically, PL catalyzes the eliminative cleavage of de-esterified pectin in the cell wall (Jia et al. 2009); PG randomly cleaves the glycosidic bonds of pectic acid or polygalacturonates, and produces mono-, di- and oligo-galacturonate (Jia et al. 2009); and PME cleaves the methyl group from esterified galacturonic acid residues in pectin chains, which are hydrolysis products of PL and PG. Consequently, PL and PG are considered key factors in cell wall degradation (Marín-Rodríguez et al. 2002), whereas PME is an auxiliary pectinase in the degradation of the cell wall. The effect of visible light exposure on the activity of PL, PG and PME in fresh-cut watermelon is shown in Fig. 3. The light exposure of 3000 Lux activated PL, reduced PG activity, and showed no significant influence on PME activity. Remarkably, the activity of PG at 3000 Lux was significantly higher than that at 10 Lux. PG activity in fresh-cut watermelon was negatively related to the intensity of the light exposure with correlation factor of − 0.968. The light exposure of 3000 Lux inhibited the determinants of cell wall degradation in fruits. Hence, the reduction in PG activity contributed to the decrease in drip loss of the fresh-cut watermelon.
Fig. 3.
Effect of visible light exposure on the residual activity of the pectic lyase (PL), polygalacturonase (PG) and pectin methylesterase (PME) of fresh-cut watermelon in the fifth day. Data are mean ± SD (n ≥ 6). Means with a different letter represent a significant difference (p < 0.05)
Effect of visible light exposure on drip loss of fresh-cut watermelon
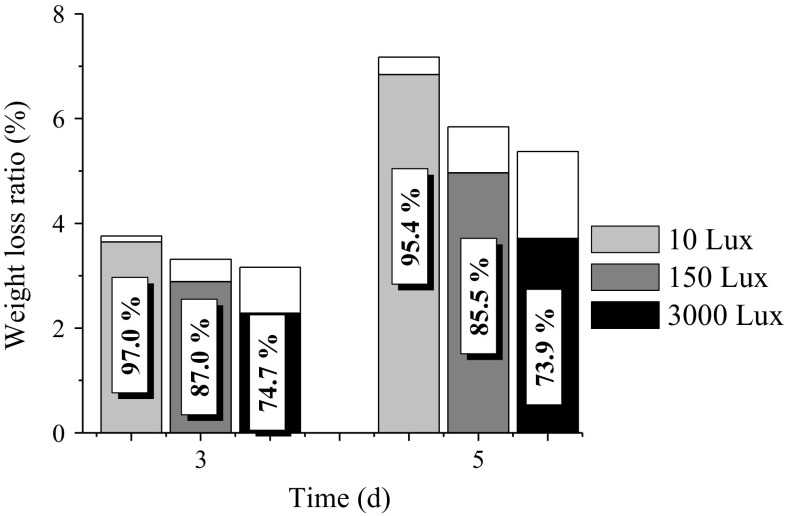
The weight loss of the fresh-cut watermelon was further divided into drip loss and moisture evaporation. The drip loss deposited in the trays was visible, thereby reducing the consumers’ purchase desire and enhancing the chance of microbial contamination. Moreover, the moisture evaporation had to be paid for by the producers or the consumers, although it was invisible. Consequently, weight loss is an important concern for both producers and consumers.
The effect of visible light exposure on the weight loss of fresh-cut watermelon is shown in Fig. 4. The drip loss ratio at each light intensity decreased with the storage time. The drip loss at 3000 Lux was 74.8 and 54.2% of that at 150 and 10 Lux, respectively, on the fifth day. Moreover, the drip loss ratio at 3000 Lux was also significantly lower than those at 10 and 150 Lux. Consequently, a stronger light exposure reduced the drip loss of fresh-cut watermelon. However, moisture evaporation was positively related to light intensity and shelf life. The moisture evaporation at 3000 Lux was 1.89 and 5.01 times of that at 150 and 10 Lux, respectively, on the fifth day. The light exposure of 3000 Lux accelerated moisture evaporation from both the fruit surface and the drip loss fluid in the tray. Hence, the light exposure of 3000 Lux was an effective way to reduce the accumulation of drip loss fluid in the tray. The weight loss ratio at 3000 Lux was 92.0 and 74.9% of that at 150 and 10 Lux, respectively, on the fifth day. This finding is in agreement with the weight loss reported for fresh-cut cauliflower and Chinese kale (Sanz et al. 2007), lettuce (Martínez-Sánchez et al. 2011), and broccoli (Zhan et al. 2012a).
Fig. 4.
Effect of visible light exposure on weight loss of fresh-cut watermelon in the fifth day. The grey and blank regions refer to the drip loss and moisture evaporation, respectively. The data in the suspended frame refers to the drip loss ratio
Hence, the visible light exposure of 3000 Lux reduced the drip loss of fresh-cut watermelon, as well as the weight loss by inhibiting cell wall degradation and accelerating moisture evaporation. The exposure of fresh-cut watermelon to visible light of 3000 Lux is a possible way of reducing drip loss.
Conclusion
Visible light exposure is a novel, non-toxic, cheap, and residue-free approach for preserving the quality of fresh-cut produce. In the current study, the visible light exposure treatment during the shelf life maintained qualities of fresh-cut watermelon by reducing physiological metabolism initiated drip loss. Specifically, the light exposure treatments of 3000, 150, and 10 Lux were applied to the fresh-cut watermelon during the shelf life. The light exposure of 3000 Lux achieved the lowest cell wall degradation ratio. The drip loss ratio at 3000 Lux was significantly lower than those at 10 and 150 Lux on the fifth day, when stored at 4 °C. The moisture evaporation that was a part of the drip loss was positively related to light intensity and shelf life. Consequently, the moisture evaporation at 3000 Lux was significantly higher than that at 150 and 10 Lux. Moreover, the light exposure of 3000 Lux reduced the activity of polygalacturonase, which is a key hydrolase related to cell wall degradation. The cell wall degradation ratio of the fresh-cut watermelon at 3000 Lux was 81.7% of that at 150 Lux on the fifth day. The visible light exposure of 3000 Lux reduced drip loss by accelerating moisture evaporation in fresh-cut watermelon, as well as by reducing the activity of polygalacturonase and the ratio of cell wall degradation. Hence, the visible light exposure treatment during the shelf life would prove beneficial to both producers and consumers.
Acknowledgements
The authors are grateful to financial support of the earmarked fund for China Agricultural Research System (CARS-25), Natural Science Foundation of Beijing Municipality (6172013), National Key Research and Development Program of China (2016YFD0400302-5), and Beijing Key Laboratory of Fruits and Vegetable Storage and Processing (Z141105004414037).
References
- Artés-Hernández F, Robles PA, Gómez PA. Low UV-C illumination for keeping overall quality of fresh-cut watermelon. Postharvest Biol Technol. 2010;55:114–120. doi: 10.1016/j.postharvbio.2009.09.002. [DOI] [Google Scholar]
- Bai JH, Saftner RA, Watada AE, Lee YS. Modified atmosphere maintains quality of fresh-cut cantaloupe (Cucumis melo L.) J Food Sci-Chicago. 2001;66:1207–1211. doi: 10.1111/j.1365-2621.2001.tb16106.x. [DOI] [Google Scholar]
- Brummell DA, Dal Cin V, Crisosto CH, Labavitch JM. Cell wall metabolism during maturation, ripening and senescence of peach fruit. J Exp Bot. 2004;55:2029–2039. doi: 10.1093/jxb/erh227. [DOI] [PubMed] [Google Scholar]
- Cai W, Li W, Li Y, Ma Y, Zhao X, Zhang C (2015) Screening for fresh-cut watermelon from selected cultivars. In: International conference on computer science and environmental engineering, beijing, pp 155–160
- Cakmak I, Marschner H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992;98:1222–1227. doi: 10.1104/pp.98.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández A, Picouet P, Lloret E. Cellulose-silver nanoparticle hybrid materials to control spoilage-related microflora in absorbent pads located in trays of fresh-cut melon. Int J Food Microbiol. 2010;142:222–228. doi: 10.1016/j.ijfoodmicro.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Fonseca JM, Rushing JW. Effect of ultraviolet-C light on quality and microbial population of fresh-cut watermelon. Postharvest Biol Technol. 2006;40:256–261. doi: 10.1016/j.postharvbio.2006.02.003. [DOI] [Google Scholar]
- Gil MI, Conesa MA, Artes F. Quality changes in fresh cut tomato as affected by modified atmosphere packaging. Postharvest Biol Technol. 2002;25:199–207. doi: 10.1016/S0925-5214(01)00166-1. [DOI] [Google Scholar]
- Jia YJ, Feng BZ, Sun WX, Zhang XG. Polygalacturonase, pectate lyase and pectin methylesterase activity in pathogenic strains of phytophthora capsici incubated under different conditions. J Phytopathol. 2009;157:585–591. doi: 10.1111/j.1439-0434.2008.01533.x. [DOI] [Google Scholar]
- Jiang Y, Shina T, Nakamura N, Nakahara A. Electrical conductivity evaluation of postharvest strawberry damage. J Food Sci. 2001;66:1392–1395. doi: 10.1111/j.1365-2621.2001.tb15220.x. [DOI] [Google Scholar]
- Karakurt Y, Huber DJ. Cell wall-degrading enzymes and pectin solubility and depolymerization in immature and ripe watermelon (Citrullus lanatus) fruit in response to exogenous ethylene. Physiol Plantarum. 2002;116:398–405. doi: 10.1034/j.1399-3054.2002.1160316.x. [DOI] [Google Scholar]
- Manzocco L, Dri A, Quarta B. Inactivation of pectic lyases by light exposure in model systems and fresh-cut apple. Innov Food Sci Emerg Technol. 2009;10:500–505. doi: 10.1016/j.ifset.2009.06.002. [DOI] [Google Scholar]
- Manzocco L, Quarta B, Dri A. Polyphenoloxidase inactivation by light exposure in model systems and apple derivatives. Innov Food Sci Emerg Technol. 2009;10:506–511. doi: 10.1016/j.ifset.2009.02.004. [DOI] [Google Scholar]
- Mao L, Karakurt Y, Huber DJ. Incidence of water-soaking and phospholipid catabolism in ripe watermelon (Citrullus lanatus) fruit: induction by ethylene and prophylactic effects of 1-methylcyclopropene. Postharvest Biol Technol. 2004;33:1–9. doi: 10.1016/j.postharvbio.2003.12.007. [DOI] [Google Scholar]
- Mao L, Jeong J, Que F, Huber DJ. Physiological properties of fresh-cut watermelon (Citrullus lanatus) in response to 1-methylcyclopropene and post-processing calcium application. J Sci Food Agric. 2006;86:46–53. doi: 10.1002/jsfa.2297. [DOI] [Google Scholar]
- Marín-Rodríguez MC, Orchard J, Seymour GB. Pectate lyases, cell wall degradation and fruit softening. J Exp Bot. 2002;53:2115–2119. doi: 10.1093/jxb/erf089. [DOI] [PubMed] [Google Scholar]
- Martínez-Sánchez A, Tudela JA, Luna C, Allende A, Gil MI. Low oxygen levels and light exposure affect quality of fresh-cut Romaine lettuce. Postharvest Biol Technol. 2011;59:34–42. doi: 10.1016/j.postharvbio.2010.07.005. [DOI] [Google Scholar]
- McGlynn WG, Bellmer DD, Reilly SS. Effect of precut sanitizing dip and water cutting on quality and shelf-life of fresh-cut watermelon. J Food Quality. 2003;26:489–498. doi: 10.1111/j.1745-4557.2003.tb00263.x. [DOI] [Google Scholar]
- Nilsson T. Effects of ethylene and 1-MCP on ripening and senescence of European seedless cucumbers. Postharvest Biol Technol. 2005;36:113–125. doi: 10.1016/j.postharvbio.2004.11.008. [DOI] [Google Scholar]
- Olarte C, Sanz S, Echávarri JF, Ayala F. Effect of plastic permeability and exposure to light during storage on the quality of minimally processed broccoli and cauliflower. LWT. 2009;42:402–411. doi: 10.1016/j.lwt.2008.07.001. [DOI] [Google Scholar]
- Petrou P, Soteriou G, Schouten RE, Kyriacou MC. Effects of rind removal on physicochemical quality characteristics of fresh-cut watermelon [Citrullus lanatus (Thunb) Matsum & Nakai] during cold storage. Int J Food Sci Technol. 2013;48:357–362. doi: 10.1111/j.1365-2621.2012.03195.x. [DOI] [Google Scholar]
- Robert S, Luo Y, McEvoy JL. Quality characteristics of fresh-cut watermelon slices from non-treated and 1-methylcyclopropene-and/or ethylene-treated whole fruit. Postharvest Biol Technol. 2007;44:71–79. doi: 10.1016/j.postharvbio.2006.11.002. [DOI] [Google Scholar]
- Sanz S, Olarte C, Echávarri JF, Ayala F. Influence of exposure to light on the sensorial quality of minimally processed cauliflower. J Food Sci. 2007;72:S12–S18. doi: 10.1111/j.1750-3841.2006.00228.x. [DOI] [PubMed] [Google Scholar]
- Sanz S, Olarte C, Ayala F, Echávarri JF. Evolution of quality characteristics of minimally processed asparagus during storage in different lighting conditions. J Food Sci. 2009;74:S296–S302. doi: 10.1111/j.1750-3841.2009.01239.x. [DOI] [PubMed] [Google Scholar]
- Tran MTT, Farid M. Ultraviolet treatment of orange juice. Innov Food Sci Emerg Technol. 2004;5:495–502. doi: 10.1016/j.ifset.2004.08.002. [DOI] [Google Scholar]
- Vilas-Boas EVDB, Kader AA. Effect of 1-methylcyclopropene (1-MCP) on softening of fresh-cut kiwifruit, mango and persimmon slices. Postharvest Biol Technol. 2007;43:238–244. doi: 10.1016/j.postharvbio.2006.09.010. [DOI] [Google Scholar]
- Zhan L, Hu J, Li Y, Pang L. Combination of light exposure and low temperature in preserving quality and extending shelf-life of fresh-cut broccoli (Brassica oleracea L.) Postharvest Biol Technol. 2012;72:76–81. doi: 10.1016/j.postharvbio.2012.05.001. [DOI] [Google Scholar]
- Zhan L, Li Y, Hu J, Pang L, Fan H. Browning inhibition and quality preservation of fresh-cut romaine lettuce exposed to high intensity light. Innov Food Sci Emerg Technol. 2012;14:70–76. doi: 10.1016/j.ifset.2012.02.004. [DOI] [Google Scholar]
- Zhou B, McEvoy JL, Luo Y, Saftner RA, Feng H, Beltran T. 1-Methylcyclopropene counteracts ethylene-induced microbial growth on fresh-cut watermelon. J Food Sci. 2006;71:M180–M184. doi: 10.1111/j.1750-3841.2006.00081.x. [DOI] [Google Scholar]