Abstract
In this work, the effects of kaolin silver complex (KAgC) have been evaluated to replace the use of SO2 for the control of spoilage microorganisms in the winemaking process. The results showed that KAgC at a dose of 1 g/L provided effective control against the development of B. bruxellensis and acetic acid bacteria. In wines artificially contaminated with an initial population of B. bruxellensis at 104 CFU/mL, a concentration proven to produce off flavors in wine, only residual populations of the contaminating yeast remained after 24 days of contact with the additive. Populations of acetic bacteria inoculated into wine at concentrations of 102 and 104 CFU/mL were reduced to negligible levels after 72 h of treatment with KAgC. The antimicrobial effect of KAgC against B. bruxellensis and acetic bacteria was also demonstrated in a wine naturally contaminated by these microorganisms, decreasing their population in a similar way to a chitosan treatment. Related to this effect, wines with KAgC showed lower concentrations of acetic acid and 4-ethyl phenol than wines without KAgC. The silver concentration from KAgC that remained in the finished wines was below the legal limits. These results demonstrated the effectiveness of KAgC to reduce spoilage microorganisms in winemaking.
Keywords: Acetic acid bacteria, Brettanomyces bruxellensis, Chitosan, Kaolin silver, Wine
Introduction
Wine quality is greatly influenced by the microorganisms that occur through the winemaking process. Among those that have a negative impact, yeasts belonging to the species Dekkera bruxellensis (anamorph; Brettanomyces bruxellensis) have been associated with ethyl phenols and other off-flavor compounds such as those described as animal, farmyards, horse sweat, medicine and animal leather odorants (Rubio et al. 2015; Schumaker et al. 2017). Traditionally, barrel aging has been considered a source of spoilage. Nevertheless, this problem could occur even in stainless steel tanks (Oelofse et al. 2008).
Brettanomyces bruxellensis-associated issues such as the formation of volatile phenols (4-ethyl phenol and 4-ethylguaiacol) or the production of acetic acid have become more magnificent in recent years because of the reduction of the use of sulfur dioxide (SO2) during winemaking. Additionally, due to climate change, pH of wines tends to be increasingly high and it involves lower SO2 efficiency. This favors the growth of B. bruxellensis during aging. Several authors have shown that controlling the growth of this microorganism is one of the most important challenges of the current winemaking process (Capozzi et al. 2016; Wedral et al. 2010).
On the other hand, acetic acid bacteria (AAB) also play a negative role in winemaking because of their undesirable production of acetic acid, acetaldehyde, ethyl acetate and dihydroxyacetone (Guillamón and Mas 2011). Until now, SO2 addition is the most effective way to reduce spoilages. However, the continuous use of SO2 which causes allergies and some others health concerns, and possible sensory alterations has led winemakers to restrict its use (García-Ruiz et al. 2013). Due to this, there is a growing interest within the scientific community in the development of alternatives to the traditional use of SO2 in winemaking (Izquierdo-Cañas et al. 2012; González-Arenzana et al. 2016).
Among these alternatives, chitosan has received a considerable attention due to the approval of its use as treatment against Brettanomyces by the International Organization of Vine and Wine in OIV Resolution 338A-2009 (OIV 2009a). The effectiveness of chitosan against B. bruxellensis in wines has been examined in mixed culture fermentations (Gómez-Rivas et al. 2004), in vitro conditions (Petrova et al. 2016), a wine-model synthetic medium (Taillandier et al. 2014), real vinifications, and commercially produced wines (Petrova et al. 2016). However, wines treated with chitosan are not completely stable, as populations of this yeast species eventually increase after treatment (Petrova et al. 2016). Furthermore, chitosan can negatively affect some physicochemical characteristics of wine (Ferreira et al. 2013).
An alternative to the addition of SO2, from the point of view of its antimicrobial action, is the use of silver. Recent studies have shown that silver nanomaterials are antimicrobial towards a broad spectrum of Gram-positive and Gram-negative bacteria and exert some antifungal and antiviral activities (García-Ruiz et al. 2015). Despite the great interest in applying these materials in the field of enology, so far, studies on the use of silver as an antimicrobial in winemaking have been very scarce (Monge and Moreno-Arribas 2016).
This study shows the results of two trials that examine the effects of kaolin silver complex (KAgC) on the control of populations of B. bruxellensis and acetic acid bacteria and their metabolites (acetic acid and volatile phenols) in winemaking and provides a comparison with chitosan for B. bruxellensis control. The aim of this work was to demonstrate the possibility of KAgC as an alternative to the use of SO2 to prevent or reduce the presence of the main spoilage yeast and bacteria during the winemaking process.
Materials and methods
Microbial culture
Brettanomyces bruxellensis
Experimental red wine was inoculated with a mixed culture of four B. bruxellensis strains isolated from a naturally contaminated wine that contained 742 μg/L of 4-ethyl phenol. To obtain a sufficient population for inoculation into the wine, these 4 strains were initially grown in YPD (1% yeast extract. 2% Peptone, 2% Dextrose) medium. The experiment was carried out with a culture of B. bruxellensis with an initial concentration of 1.7 × 107 CFU/mL. Different volumes of this culture were added to the wine to get a population of 1.0 × 102 CFU/mL, 1.0 × 104 CFU/mL or 1.0 × 106 CFU/mL.
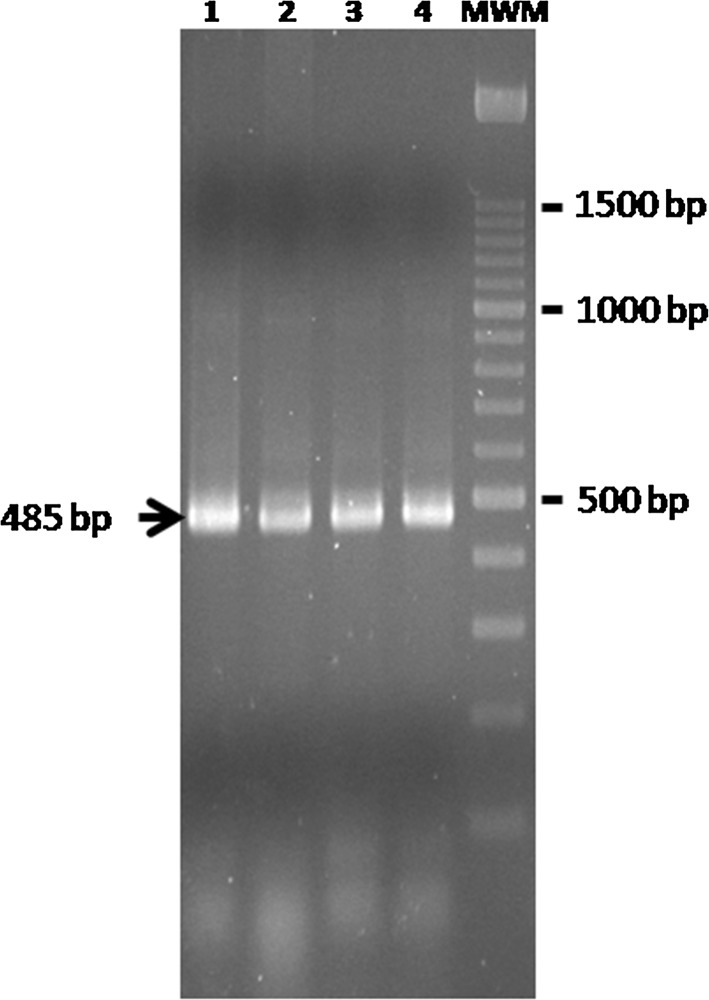
Determination of the internal transcribed spacers (ITS1 and ITS2) region of the 5.8S rRNA was used to identify the four strains at the species level. DNA from each culture was isolated and amplified by PCR following the protocol described by Guillamón et al. 1998 and Esteve-Zarzoso et al. 1999. PCR products were revealed on 1.4% agarose (Roche Diagnostics, Spain), stained with ethidium bromide and visualized with a UV trans-illuminator (GelDoc, Bio-Rad) (Fig. 1). Amplicon size results were compared with those described in the bibliography (Guillamón et al. 1998 and Esteve-Zarzoso et al. 1999) and identified as B. bruxellensis.
Fig. 1.
Amplification of the internal transcribed spacers (ITS1 and ITS2) of the rRNA 5.8S of four B. bruxellensis strains used in the study. Lanes 1–4: strain, 1, strain 2, strain 3, strain 4. MWM: molecular weight marker 100 pb Ladder
Acetic acid bacteria (AAB)
Gluconobacter oxydans (CECT 360) and Acetobacter aceti (CECT 298), obtained from the Spanish Collection of Type Cultures (CECT), were also inoculated to the wine. Bacteria were grown on Mannitol medium (0.5% yeast extract, 0.3% peptone, 2.5% Mannitol) to obtain a sufficient population to be subsequently inoculated into wine to reach a concentration of 1.0 × 102, 1.0 × 104 or 1.0 × 106 CFU/mL.
Kaolin silver complex (KAgC)
KAgC is produced under patent (PCT/ES2015/070532). It is a gray powder with a particle size of around 30 nm, and it is insoluble in ethanol and water, composed of an inorganic inert material (kaolin) used as a support, on the surface of which silver nanoparticles (< 10 nm) are deposited (colloidal silver). KAgC was supplied in permeable bags that contained 1 g of KAgC.
Initial wines
Two experiments were performed: (a) Trial 1: with artificially contaminated wine; and (b) Trial 2: with naturally contaminated wine.
In Trial 1, a filter-sterilised red wine was used (Table 1, Wine 1) which that had been produced without the addition of sulfur dioxide but with natural presence as a secondary metabolite of total SO2 (≤ 4 mg/L). The wine was fined with egg white and filtered through 0.22 microns in order to eliminate any naturally occurring contaminating microorganisms in the wine. The sterile red wine was distributed into 36 aliquots of 1 L in previously sterilized glass bottles, with a magnetic stirrer in their interior to produce gentle agitation (100 rpm).
Table 1.
Physicochemical parameters of initial wines
| Wine 1 | Wine 2 | |
|---|---|---|
| Alcohol content (% v/v) | 11.48 | 14.26 |
| Volatile acidity (g/L of acetic acid) | 0.50 | 0.76 |
| Total acidity (g/L of tartaric acid) | 6.40 | 5.35 |
| pH | 3.48 | 3.66 |
| Glucose + fructose (g/L) | 0.20 | 0.25 |
| Total SO2 (mg/L) | 4 | 85 |
| Free SO2 (mg/L) | n.d. | 7 |
| 4-Ethylphenol (µg/L) | n.d. | 1087 |
| 4-Ethylguaiacol (µg/L) | n.d. | 146 |
n.d. not detected
In Trial 2, a naturally contaminated red wine (Table 1, Wine 2) with a population of B. bruxellensis yeast of 1.0 × 104 CFU/mL and a population of AAB of 1.1 × 105 CFU/mL was used. According to the results of the microbiological analysis, this wine did not contain lactic acid bacteria. The wine had moderately high volatile acidity related to the presence of AAB. Also, the initial wine had high ethyl phenol and ethyl guaiacol content related to the presence of the B. bruxellensis yeast.
Experimental design
In Trial 1, two experimental series were conducted, one with wine artificially contaminated with B. bruxellensis and one with wine artificially contaminated with AAB. Each series consisted of 12 bottles containing Wine 1. Three bottles were inoculated with B. bruxellensis cultures to obtain 1.0 × 102 CFU/mL; the same was done with concentrations of 1.0 × 104 and 1.0 × 106 CFU/mL. To carry out the second series, three bottles were inoculated with AAB cultures to obtain 1.0 × 102 CFU/mL; the same was done with concentrations of 1.0 × 104 and 1.0 × 106 CFU/mL. One bag (1 g) of KAgC was added to these bottles, so the dose of KAgC was 1 g/L. Additionally, in each experimental series (B. bruxellensis or AAB), 3 bottles without KAgC were used as a control.
The bottles were gently stirred (100 rpm) in order to put the bag that contained KAgC in contact with the whole volume of the wine. Samples were taken at different contact times and plate counts, and the acetic acid content and 4-ethyl phenol content were evaluated. The duration of the B. bruxellensis inactivation experiment was 24 days, and it was 3 days for AAB.
In Trial 2, three batches of three bottles (triplicate) were prepared using Wine 2 (Table 1). One batch, without KAgC, was used as a control; 1 g/L of KAgC was added to the bottles of the second batch and 7 g/HL of chitosan (NoBrettInside®, Lallemand, Montreal, Canada) to the last batch. Each bottle was stirred daily, and B. bruxellensis and AAB populations were measured at day 10 of the treatment.
Microbiological counts
CFU counts
In Trial 1, the B. bruxellensis population was assessed at day 0, just after being inoculated, and at 3, 10, 17 and 24 days after the treatment. Serial dilutions (from 10−1 to 10−6) in sterile saline solution were plated onto Sabouraud-chloramphenicol agar plates (Cultimed, Panreac, Barcelona, Spain). Plates were incubated under aerobic conditions at 28 °C for 10 days. After this time, colonies were counted and the results were expressed as colony forming units (CFU) per milliliter of wine.
The AAB population in Trial 1 was counted in each bottle at day 0, just after being inoculated into the wine, and at 1, 2 and 3 days after starting the treatment with KAgC. Samples of 0.1 mL were taken, and serial dilutions (from 10−1 to 10−6) in sterile saline solution were spread onto plates of GYC medium (5% glucose, 1% yeast extract, 0.5% calcic carbonate, 2% agar) to which 50 mg/L nystatin (Sigma-Aldrich) was added. The plates were incubated under aerobic conditions at 30 °C for 5 days.
PCR detection
In Trial 2, cell populations were evaluated by qPCR based on Scorpions (Umiker et al. 2013). qPCR detection was performed using the Scorpions Wine Spoilage Systems module (ETS Laboratories, St. Helena, CA).
After mixing, a 1.5-mL sample was removed and centrifuged (9000×g). The pellet was suspended in 1× wash buffer from the lysis module (LYR-50-01) and centrifuged. The pellet was then suspended in 15 mL of 1× wash buffer prior to transfer to a 15-mL centrifuge tube for recentrifugation. Cell lysis was accomplished by suspending cell pellets in 200 µL of 1× lysis reagent (LYR-50-01). Pellets were then incubated at 37 °C for 30 min, mixed and incubated for an additional 30 min. Next, 20 µL of Proteinase K with 200 µL of PBS and 200 μL of buffer AL (DX Reagent Qiagen Pack for QIAxtractor #950107, Qiagen, Inc., Valencia, CA) were added to the suspension, which was incubated for 30 min at 55 °C, mixed and incubated for an additional 30 min. Cell debris was removed by centrifugation (15,000×g for 6 min) before removal of 420 µL of supernatant for DNA extraction and purification using the QIAxtractor and DX reagent pack according to the manufacturer’s instructions.
For B. bruxellensis, purified nucleic acid (5 µL) was combined with 20 µL Scorpions Yeast Assay Multiplex Master Mix and 5 µL Scorpions Reagent (YDR1-50-01) along with 15 µL Taq Polymerase Master Mix containing dNTPs, MgCl2 and supplied buffer. For acetic acid bacteria, purified nucleic acid (5 µL) was combined with 20 µL Scorpions Bacteria I Assay Multiplex Master Mix and 5 µL Scorpions Reagent. Amplification and detection of DNA were conducted with a Q-Gene thermocycler (Qiagen, Inc.). Samples were quantified, and the efficacy of the assay was determined using standard curves generated by isolating DNA from serial dilutions (106–101) of Brettanomyces and AAB cultures individually grown in wine. The Scorpions Yeast and Bacteria Multiplex assays contain an internal control reaction consisting of primers and a probe to amplify target DNA spiked into the Master Mix. The signal strength of the internal control reaction is monitored to avoid false negatives due to the presence of PCR inhibitors.
For positive controls, samples with known populations of B. bruxellensis and acetic bacteria in wine were lysed, extracted and amplified along with the samples being analyzed. A no template control consisting of 20 µL yeast Scorpions Assay Multiplex Master Mix and 5 µL of molecular biology grade ddH2O was also conducted. Populations of B. bruxellensis were calculated by the analysis software provided with the Q-Gene thermocycler.
Determination of acetic acid and volatile phenols
Acetic acid content was analyzed at each sampling in Trial 1 by enzymatic methods in accordance with Commission Regulation (EC 2676/1990, E.E.C., 1990) and the International Organization of Vine and Wine (OIV 2018).
Ethyl phenol in the wine was analyzed by gas chromatography (Chatonnet et al. 1995) at the end of Trial 1 (day 24) in the Brettanomyces inactivation treatment with KAgC. Dichloromethane was used to extract the analytes of 10 mL of wine.
Analysis of the silver ion content in wines
The content of silver was determined at the end of the experiments in all wines using an inductively coupled plasma mass spectrometer Agilent 7500ce Series, using Argon as the carrier gas and helium as the collision gas, analyzing isotope 107. Prior to analysis, 1 mL of wine was diluted with 3% nitric acid until 20 g. Calibration was carried out with solutions containing silver concentrations between 0.4 and 100 µg/kg using Rh (103) as an internal standard.
Statistical analysis
Data were subjected to Student’s t test and the Student–Newman–Keuls test to identify any statistically significant differences between treatments using SPSS software (version 12.0).
Results and discussion
Inactivation of B. bruxellensis
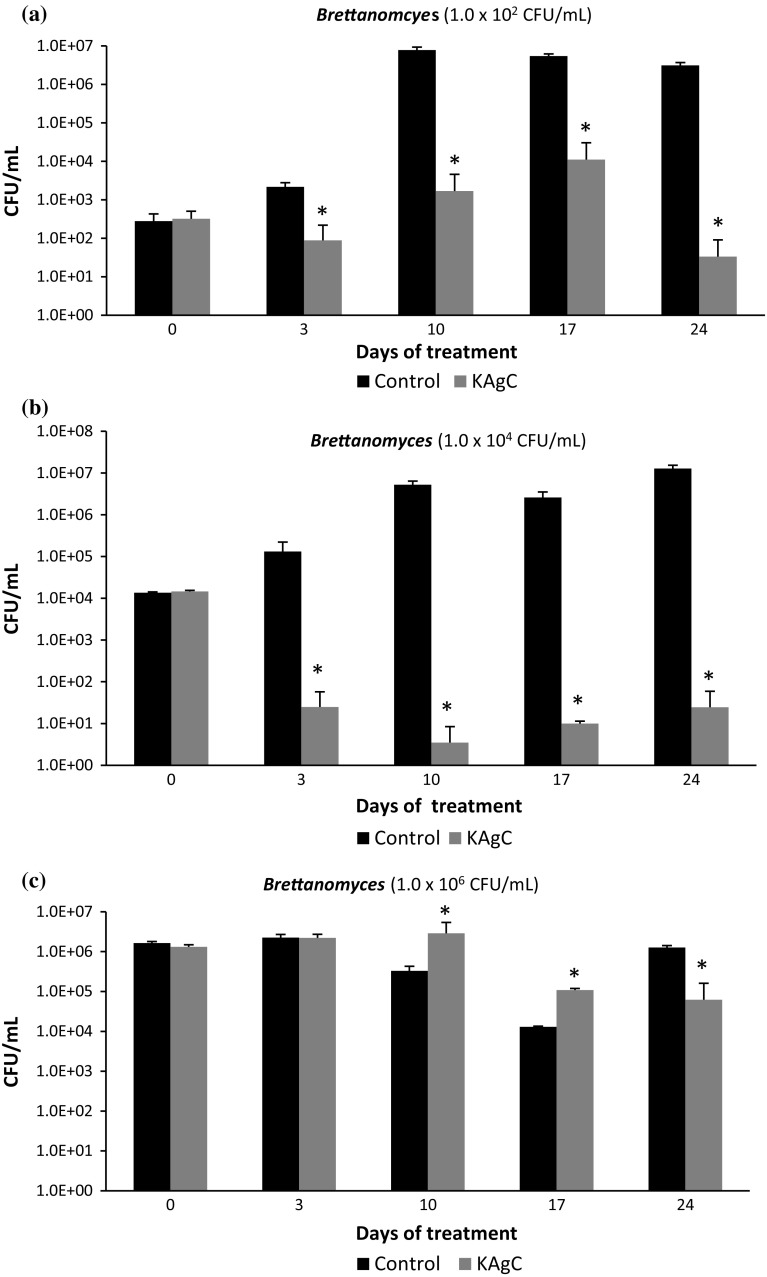
Figure 2 shows the evolution of the viable population of B. Bruxellensis in CFU/mL at 0, 3, 10, 17 and 24 days of Trial 1. When the wine was inoculated with a population of 1.0 × 102 CFU/mL (Fig. 2a), a reduction of almost 1 log unit of the initial population of this yeast species was observed in the wines treated with KAgC 3 days after contact with the product. In the control wine, without KAgC, an increase in the population of B. bruxellensis was detected from day 3, further increasing to 4 log units on day 10. In wines with KAgC, a small increase was also observed on days 10 and 17, probably due to growth inertia of the vegetative cells caused by growth of a culture in synthetic medium (YPD). But this growth ceased, and the population clearly decreased in the sample taken at day 24 after the treatment when the count in the wine with KAgC indicated a reduction of more than 2.5 log units compared to the one conducted on day 17, more than 1.5 log units compared to the baseline population and approximately 5 log units compared to the control wine without KAgC.
Fig. 2.
Populations of B. bruxellensis in wine artificially contaminated along 24 days after KAgC treatment [*statistically significant differences (p < 0.05)]
Figure 2b shows the behavior of B. bruxellensis when the initial population was about 1.0 × 104 CFU/mL. The KAgC showed strong action at day 3, reducing almost 3 log units with respect to the initial population. This inactivation was virtually total at day 10 from the start of treatment. After this sampling, the population of B. bruxellensis remained well below the initial concentration of cells added to the wine: from 14,000 CFU/mL at day 0 to only 49 CFU/mL at day 24 (reduction of 2.45 log units).
Holding B. bruxellensis populations from 1.0 × 102 to 1.0 × 104 CFU/mL, the treatment with KAgC resulted in significantly smaller populations of these microorganisms. These results are in agreement with those obtained by Pires et al. (2016), who verified that the addition of silver nanoparticles to the Ti/TiO2 electrodes used in photoelectrocatalysis caused an increase in disinfection of aqueous environments.
In wines with a population of 1.0 × 106 CFU/mL (Fig. 2c), the level of the B. bruxellensis population inactivation in the previous experiments was not achieved, a result that is in agreement with reports from Franci et al. (2015) and Vazquez-Muñoz et al. (2017) demonstrating that when the microbial population is very complex, silver nanoparticles are not so efficient at inactivating the microorganisms. Nonetheless, a smaller population (between 1 and 1.5 log units) was found on day 24 in treated wines, while the control maintained the same initial population at 24 days. It should be mentioned that it is very difficult to find such high populations of B. bruxellensis in real conditions, in naturally contaminated wines (Puig et al. 2011). In these cases, the results indicate that perhaps it would be necessary to treat the wines with doses of KAgC over 1 g/L.
Acetic acid and 4-ethyl phenol are parameters that could be indicative of contamination of wine with Brettanomyces (Garijo et al. 2017). Table 2 shows the values of these parameters in wines from Trial 1 with different initial concentrations of B. bruxellensis cells, with or without KAgC (control). Control wines were those in which acetic acid had a considerable increase over the 24 days of the trial, reaching values between 1.20 and 1.56 g/L.
Table 2.
Acetic acid and 4-ethyl phenol concentration in wines artificially contaminated with increasing amounts of B. bruxellensis
| Initial (CFU/mL) | 1.0 × 102 | 1.0 × 104 | 1.0 × 106 | |||
|---|---|---|---|---|---|---|
| Time (days) | Control | KAgC | Control | KAgC | Control | KAgC |
| Acetic acid (g/L) | ||||||
| 0 | 0.51 ± 0.01a | 0.51 ± 0.01a | 0.50 ± 0.01a | 0.50 ± 0.01a | 0.49 ± 0.01a | 0.50 ± 0.01a |
| 24 | 1.20 ± 0.30b | 0.53 ± 0.01a | 1.56 ± 0.15b | 0.61 ± 0.20a | 1.50 ± 0.09b | 1.39 ± 0.03b |
| 4-Ethyl phenol (µg/L) | ||||||
| 0 | 32.5 ± 5.2a | 29.1 ± 6.4a | 34.2 ± 4.3a | 30.7 ± 6.1a | 33.8 ± 4.6a | 33.8 ± 4.6a |
| 24 | 143.4 ± 10.6b | 30.1 ± 5.8a | 516 ± 19.1b | 26.3 ± 7.8a | 785 ± 50.8b | 352 ± 31.8b |
Different superscripts (a,b,c) in the same line for each populations the B. bruxellensis indicate significant differences for α = 0.05 according to the Student–Newman–Keuls test. Values are the mean of triplicates
In the case of wines inoculated with B. bruxellensis populations of 1.0 × 102 and 1.0 × 104 CFU/mL where KAgC was added, no significant increases in acetic acid were detected during the 24 days of the study. In the KAgC wines initially inoculated with populations of 1.0 × 106 CFU/mL where high numbers of B. bruxellensis cells were detected, the acetic acid also increased, which demonstrates that the strains used in the trial produced acetic acid. In this case, higher concentrations of KAgC (above 1 g/L) or a combination of KAgC with other antimicrobial substances or techniques may be required to stop B. bruxellensis growth.
Data about 4-ethyl phenol concentrations at the end of the trial (24 days) are shown in Table 2. The content of 4-ethyl phenol was higher in control wines than in those with 1 g/L of KAgC added, whatever the initial inoculum of B. bruxellensis. Values of this metabolite in control wines with 1.0 × 104 or 1.0 × 106 B. bruxellensis cells initially exceeded the perception threshold of 425 µg/L (Chatonnet et al. 1992). In wines treated with KAgC, although 4-ethyl phenol was produced by Brettanomyces cells, it never exceeded this threshold. The concentration of this metabolite in the samples with KAgC was 79% lower than in the control wine starting from an initial B. bruxellensis inoculate of 1.0 × 102 CFU/mL, 95% lower in the case of 1.0 × 104 CFU/mL and 55% less starting from a population of 1.0 × 106 CFU/mL. These results show that KAgC was able to slow the growth and viability of B. bruxellensis and, consequently, decrease the possibility of unpleasant odors being produced due to this contaminating yeast, which would have a negative effect on the sensory profile of the wine.
In Trial 2, both KAgC and chitosan treatments allowed a significant reduction of the Brettanomyces population (Table 3). Thus, when 1 g/L of KAgC was added to the Tempranillo wine naturally contaminated with 1.0 × 104 GU/mL (Genomic Units) B. bruxellensis, populations declined to 1.2 × 102 GU/mL 10 days after addition; however, in the control wines at the same period, B. bruxellensis increased 0.57 log units. When 7 g/HL of fungal chitosan was added to the same initial wine, populations of B. bruxellensis declined to 3.0 × 102 GU/mL 10 days after addition. There were no significant differences between samples treated with KAgC or chitosan. Hence, according to these data, both KAgC and chitosan would reduce, but not eliminate, this spoilage yeast. Regarding chitosan, similar results were obtained by Petrova et al. (2016) when they inoculated 8.8 × 105 CFU/mL of B. bruxellensis into a Merlot wine. In that trial, populations of B. bruxellensis declined to 102 CFU/mL 11 days after addition of 4 or 10 g/HL fungal chitosan. Blateyron-Pic et al. (2012), in wines naturally contaminated with 105 CFU/mL B. bruxellensis, found a residual population 10 days after treatment of nearly 100 CFU/mL with 4 g/HL chitosan. Ferreira et al. (2013) found that the anti-yeast activity of chitosan was strain dependent because when they inoculated two B. bruxellensis strains at 7 log units CFU/mL into a red wine from the Alentejo region of Portugal, one yeast strain was inactivated, while the other yeast strain was more resistant (3 log units reduction).
Table 3.
Populations of B. bruxellensis and acetic acid bacteria (GU/mL) in wine naturally contaminated before and after 10 days of treatments with KAgC and chitosan
| B. bruxellensis | Acetic acid bacteria | |||
|---|---|---|---|---|
| Initial wine | 10 days after treatment | Initial wine | 10 days after treatment | |
| Control | 1.0 × 104 | 3.7 × 104(b) | 1.1 × 105 | 8.3 × 105(b) |
| KAgC | 1.0 × 104 | 1.2 × 102(a) | 1.1 × 105 | 1.7 × 103(a) |
| Chitosan | 1.0 × 104 | 3.0 × 102(a) | 1.1 × 105 | 3.5 × 103(a) |
Different superscripts (a,b) indicate significant differences in the same column for α = 0.05 according to the Student–Newman–Keuls test. Values are the mean of triplicates
Therefore, according to the data obtained by Q-PCR, both treatments would give the impression of being effective at reducing populations of B. bruxellensis in a naturally contaminated wine but would not obtain their elimination completely. It is therefore of interest to check the status of the B. bruxellensis residual population after treatment with KAgC or chitosan.
Inactivation of acetic acid bacteria
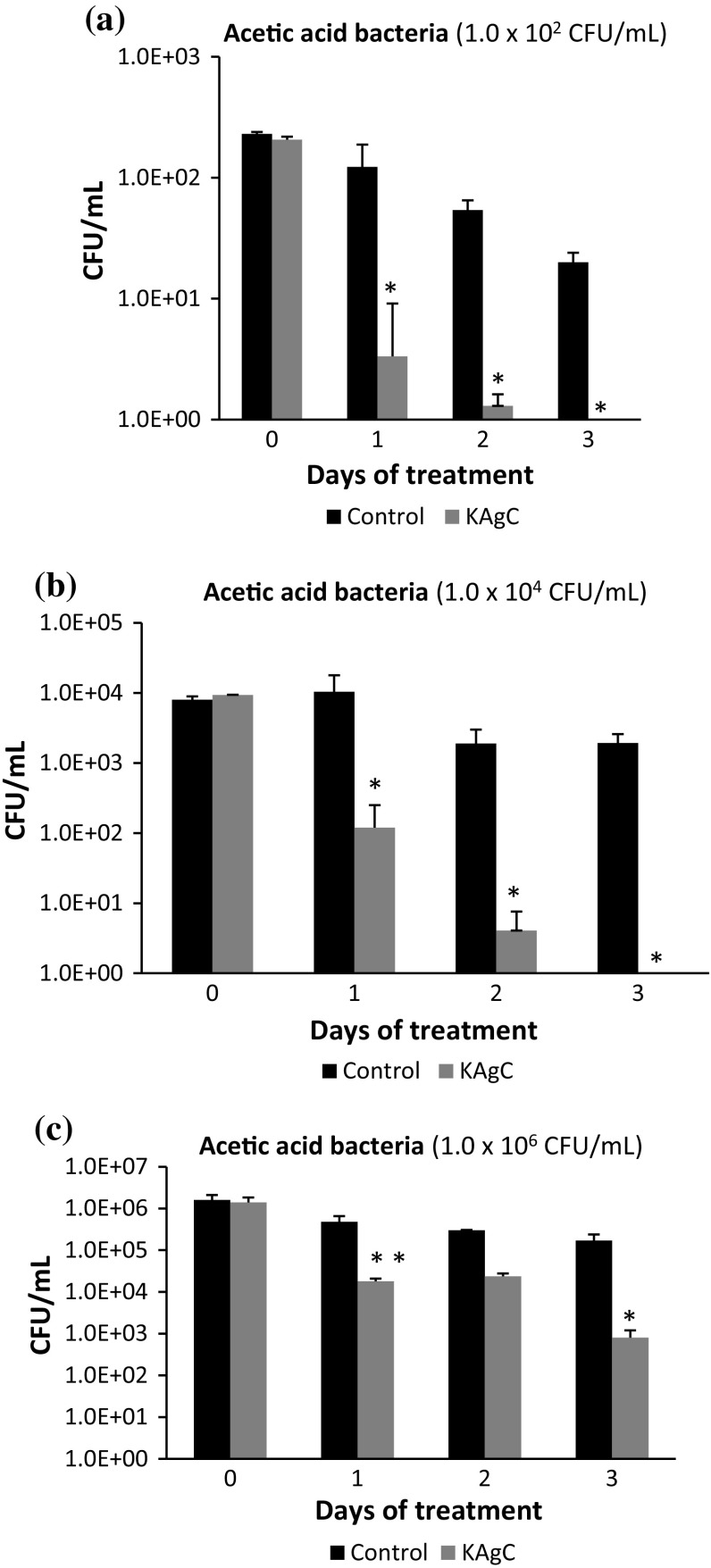
Figure 3 shows the evolution of the population of acetic acid bacteria in CFU/mL at 0, 1, 2 and 3 days of Trial 1. When the wine was inoculated with a population of 1.0 × 102 CFU/mL (Fig. 3a), the population of AAB fell 2 log units during the first day of treatment with KAgC, and no culturable cells were detected by 3 days after the commencement of the experiment. Although there was also a decrease in the control wines on the third day (1 log unit) due to the inhibitory effect that the wine itself has on AAB, this drop was not as great as that in the samples with KAgC.
Fig. 3.
Populations of acetic acid bacteria in wine artificially contaminated along 3 days after KAgC treatment [*statistically significant differences (p < 0.05)]
The evolution of the population with a baseline AAB concentration of 1.0 × 104 CFU/mL (Fig. 3b) was similar to the previous case. There was a reduction in the population of 1.89 log units during the first 24 h of contact with the KAgC complex and 3.37 log units after 48 h. After the third day, the inactivation of the AAB was complete in KAgC wines. In the control wine, the population also fell slightly, 0.62 log units in 3 days.
In wines with a population of 1.0 × 106 CFU/mL (Fig. 3c), the anti-bacterial effect of KAgC was detected only 1 day after its addition with a reduction of 1.9 log units, the same as with the initial concentration of 1.0 × 104 CFU/mL and very similar to that with 1.0 × 102 CFU/mL. In the control wine, over the same period, the loss of viability was only 0.52 log units. After 3 days of contact with KAgC, a decrease in AAB of 2.9 log units was achieved, while in the control batch, the reduction was 0.97 log units.
Similar results were obtained by Izquierdo-Cañas et al. (2012) when a colloidal silver complex at the dose of 1 g/kg was applied to Merseguera and Monastrell musts, achieving a decrease between one and two orders of magnitude in AAB populations at the end of alcoholic fermentation. Garde-Cerdán et al. (2013) compared the action of colloidal silver particles (KAgC) and SO2 on viable AAB counts in the Tempranillo winemaking process in must at 24 h after treatment with SO2 or KAgC and concluded that the addition of SO2 did not affect the AAB population, whereas the presence of KAgC reduced it by 2 log CFU/mL. Finally, García-Ruiz et al. (2015), evaluating silver-based biocompatible nanoparticles for their antimicrobial activity against enological AAB among other microorganisms, also demonstrated the efficiency of ionic Ag in controlling microbial processes in winemaking. In this sense, Ðolic et al. (2015) found that the antimicrobial activity of the sorbent zeolite activated by silver ions caused cell removal against S. aureus and E. coli of 98.8 and 93.5%, respectively.
In our study, the data showed that KAgC had a rapid antimicrobial effect on a group of wine spoilage microorganisms such as the AAB, achieving irrespective of the initial population a fall of almost 2 log units during the first day of contact with KAgC and a reduction between 2.3 and 3.97 log units by the third day.
Moreover, the action of KAgC on these spoilage bacteria reveals an additional advantage compared to other alternatives to SO2 in microbiological control, such as lysozyme, which only acts against gram-positive bacteria and not against gram-negative ones such as AAB.
As in the case of B. bruxellensis, if the initial AAB concentration was around 1.0 × 106 CFU/mL, it would be necessary to test the effect of a KAgC treatment at a concentration above 1 g/L.
In trial 2, both KAgC and chitosan treatments allowed a significant reduction of the acetic acid bacteria population (Table 3). Thus, when 1 g/L of KAgC was added to a Tempranillo red wine naturally contaminated with an acetic acid bacteria population of 1.1 × 105 GU/mL, the population declined to 1.72 × 103 GU/mL. Thus, as occurred with Brettanomyces, KAgC reduced acetic acid bacteria by 2 log units, although it did not completely eliminate these spoilage bacteria. When fungal chitosan at 7 g/HL was added to the same initial wine, the population of acetic acid bacteria declined to 3.5 × 103 GU/mL 10 days after addition. In this sense, when Valera et al. (2017) compared the effects of chitosan and SO2 on wines artificially contaminated with two strains of the acetic acid bacteria species Acetobacter, they detected that their viability decreased with the application of chitosan. In our study, there were no significant differences between samples treated with KAgC or chitosan. Hence, according to these data, both KAgC and chitosan would reduce, but not eliminate, these spoilage bacteria.
Concentration of ionic Ag in the final wines
Regarding the silver content in the final wines, it was far below the legal limit of 100 µg/L (0.1 mg/L) established by the OIV-OENO 145-2009, (OIV 2009b). This corroborates the results of Izquierdo-Cañas et al. (2012) who studied the application of colloidal silver complex in winemaking.
Conclusion
According to these results, the viability of the yeast B. bruxellensis and AAB, the main microorganisms that can affect wine’s organoleptic features, were reduced by the presence of KAgC. In the case of B. bruxellensis and AAB populations of 1.0 × 106 CFU/mL, the effect was less marked and it would be necessary to test whether a greater concentration of KAgC would have the desired effect.
In the case of acetic acid produced by strains of Brettanomyces, it has been shown that the presence of KAgC decreased the risk of their production, although in the wine, there may be small residual populations of this yeast. In the same way, the risk of producing 4-ethyl phenol is decreased in the presence of KAgC in correlation with the inactivation of the strains of Brettanomyces that produce this metabolite.
The effectiveness of KAgC at reducing populations of B. bruxellensis and AAB has been demonstrated by two methods of microbiological analysis: plate counts and Q-PCR. Its action on Brettanomyces cells in naturally contaminated wines was very similar to that achieved with chitosan.
References
- Blateyron-Pic L, Bornet A, Brandam C, Jentzer JB, Granes D, Heras JM, Joannis-Cassan C, Pillet O, Sieczkowski N, Tailandier P. Le chitosane d’origine fongique, un nouvel outil de choix pour lutter contre Brettanomyces dans les vins. Rév Oenol. 2012;143:27–28. [Google Scholar]
- Capozzi V, Di Toro MR, Grieco F, Michelotti V, Salma M, Lamontanara A, Russo P, Orrù L, Alexandre H, Spano G. Viable but not culturable (VBNC) state of Brettanomyces bruxellensis in wine: new insights on molecular basis of VBNC behaviour using a transcriptomic approach. Food Microbiol. 2016;59:196–204. doi: 10.1016/j.fm.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Chatonnet P, Dubourdieu D, Boidron J, Pons M. The origin of ethylphenols in wines. J Sci Food Agric. 1992;60:165–178. doi: 10.1002/jsfa.2740600205. [DOI] [Google Scholar]
- Chatonnet P, Dubourdieu D, Boidron JN. The influence of Brettanomyces/Dekkera sp. yeasts and lactic acid bacteria on the ethylphenol content of red wines. Am J Enol Vitic. 1995;46:463–468. [Google Scholar]
- Ðolic MB, Rajakovic-Ognjanovic VN, Strbac SB, Rakocevic ZLJ, Veljovic DN, Dimitrijevic SI, Rajakovic LV. The antimicrobial efficiency of silver activated sorbents. Appl Surf Sci. 2015;357:819–831. doi: 10.1016/j.apsusc.2015.09.032. [DOI] [Google Scholar]
- Esteve-Zarzoso B, Belloch C, Uruburu F, Querol A. Identification of yeasts by RFLP analysis of the 5,8S rRNA gene and the two ribosomal internal transcriber spacers. Int J Syst Bacteriol. 1999;49:329–337. doi: 10.1099/00207713-49-1-329. [DOI] [PubMed] [Google Scholar]
- Ferreira D, Moreira D, Costa EM, Silva S, Pintado MM, Couto JA. The antimicrobial action of chitosan against the wine spoilage yeast Brettanomyces/Dekkera. J Citin Chitosan Sci. 2013;1:240–245. doi: 10.1166/jcc.2013.1037. [DOI] [Google Scholar]
- Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G, Galdiero M. Silver nanoparticles as potential antibacterial agents. Molecules. 2015;20:8856–8874. doi: 10.3390/molecules20058856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ruiz A, Requena T, Peláez C, Bartolomé B, Moreno-Arribas MV, Martínez-Cuesta MC. Antimicrobial activity of lacticin 3147 against oenological lactic acid bacteria. Combined effect with other antimicrobial agents. Food Control. 2013;32(2):477–483. doi: 10.1016/j.foodcont.2013.01.027. [DOI] [Google Scholar]
- García-Ruiz A, Cespo J, López-de-Luzuriaga JM, Olmos ME, Monge M, Rodríguez-Alfaro MP, Martín- Álvarez PJ, Bartolome B, Moreno-Arribas MV. Novel biocompatible silver nanoparticles for controlling the growth of lactic acid bacteria and acetic acid bacteria in wines. Food Control. 2015;50:613–619. doi: 10.1016/j.foodcont.2014.09.035. [DOI] [Google Scholar]
- Garde-Cerdán T, González-Arenzana L, López N, López R, Santamaría P, López-Alfaro I. Effect of different pulsed electric field treatments on the volatile composition of Graciano, Tempranillo and Grenache grape varieties. Innov Food Sci Emerg Technol. 2013;20:91–99. doi: 10.1016/j.ifset.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Garijo P, Gutiérrez AR, López R, Santamaría P, González-Arenzana L, López-Alfaro I, Garde-Cerdán T, Olarte C, Sanz S. Comparison of Brettanomyces yeast presence in young red wines in two consecutive vintages. Eur Food Res Technol. 2017;243:827–834. doi: 10.1007/s00217-016-2796-8. [DOI] [Google Scholar]
- Gómez-Rivas L, Escudero-Abarca BI, Aguilar-Uscanga MG, Hayward-Jones PM, Mendoza P, Ramírez M. Selective antimicrobial action of chitosan against spoilage yeasts in mixed culture fermentations. J Ind Microbiol Biotechnol. 2004;31:16–22. doi: 10.1007/s10295-004-0112-2. [DOI] [PubMed] [Google Scholar]
- González-Arenzana L, Sevenich R, Rauh C, López R, Knorr D, López-Alfaro I. Inactivation of Brettanomyces bruxellensis by high hydrostatic pressure technology. Food Control. 2016;59:188–195. doi: 10.1016/j.foodcont.2015.04.038. [DOI] [Google Scholar]
- Guillamón JM, Mas A. Acetic Acid Bacteria. In: Carrascosa AV, Muñoz R, González R, editors. Molecular wine microbiology. San Diego: Academic Press; 2011. pp. 227–255. [Google Scholar]
- Guillamón JM, Sabaté J, Barrio E, Cano J, Querol A. Rapid identification of wine yeast species based on RFLP analysis of the ribosomal internal transcribed spacer (ITS) region. Arch Microbiol. 1998;169:387–392. doi: 10.1007/s002030050587. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Cañas PM, García Romero E, Huertas-Nebreda B, Gómez Alonso S. Colloidal silver complex as alternative to sulphur dioxide in winemaking. Food Control. 2012;23:73–81. doi: 10.1016/j.foodcont.2011.06.014. [DOI] [Google Scholar]
- Monge M, Moreno-Arribas MV. Applications of nanotechnology in wine production and quality and safety control. In: Moreno-Arribas MV, Sualdea BB, editors. Wine safety, consumer preference, and human health. Berlin: Springer; 2016. pp. 51–69. [Google Scholar]
- Oelofse A, Pretorius IS, Du Toit M. Significance of Brettanomyces and Dekkera during winemaking: a synoptic review. S Afr J Enol Vitic. 2008;29(2):129–144. [Google Scholar]
- OIV International Organization of Vine and Wine (2009a) OIV/OENO 338A/2009: International code of oenological practices. Wines-treatment using chitosan. OIV, Paris. http://www.oiv.int/public/medias/1082/oiv-oeno-338a-2009-en.pdf
- OIV International Organization of Vine and Wine (2009b) OIV/OENO 145/2009: International Code of oenological Practices. Treatment with silver chloride. OIV, Paris. http://www.oiv.int/public/medias/1071/oiv-oeno-145-2009-en.pdf
- OIV International Organization of Vine and Wine (2018) Compedium of international methods of wine and must analysis, vol 1–2. OIV, Paris. http://oiv.int/en/technical-standards-and-documents/methods-of-analysis
- Petrova B, Cartwright ZM, Edwards ChG. Efectiveness of chitosan preparations aginst Brettanomyces bruxellensis grown in culture media and red wines. J Int Sci Vigne Vin. 2016;50(1):49–56. [Google Scholar]
- Pires RH, Bruguera MF, Zanoni MVB, Giannini MJSM. Effectiveness of photoelectrocatalysis treatment for the inactivation of Candida parapsilosis sensu stricto in planktonic cultures an biofilms. Appl Catal A Gen. 2016;511:149–155. doi: 10.1016/j.apcata.2015.11.036. [DOI] [Google Scholar]
- Puig A, Bertran A, Franquet R, Garcia J, Mínguez S. Brettanomyces bruxellensis prevalence in wines produced and marketed in Spain. Ann Microbiol. 2011;61:145–151. doi: 10.1007/s13213-010-0075-7. [DOI] [Google Scholar]
- Rubio P, Garijo P, Santamaría P, López R, Martínez J, Gutiérrez AR. Influence of oak origin and ageing conditions on wine spoilage by Brettanomyces yeasts. Food Control. 2015;54:176–180. doi: 10.1016/j.foodcont.2015.01.034. [DOI] [Google Scholar]
- Schumaker MR, Chandra M, Malfeito-Ferreira M, Ross CF. Influence of Brettanomyces ethylphenols on red wine aroma evaluated by consumers in the United States and Portugal. Food Res Int. 2017;100:161–167. doi: 10.1016/j.foodres.2017.06.057. [DOI] [PubMed] [Google Scholar]
- Taillandier P, Joannis-Cassan C, Jentzer JB, Gautier S, Sieczkowski N, Granes D, Brandam C. Effect of fungal chitosan preparation on Brettanomyces bruxellensis, a wine contaminant. J Appl Microbiol. 2014;118:123–131. doi: 10.1111/jam.12682. [DOI] [PubMed] [Google Scholar]
- Umiker NL, Descenzo RA, Lee J, Edwards CG. Removal of Brettanomyces bruxellensis from red wine using membrane filtration. J Food Process Preserv. 2013;37:799–805. doi: 10.1111/j.1745-4549.2012.00702.x. [DOI] [Google Scholar]
- Valera MJ, Sainz F, Mas A, Torija MJ. Effect of chitosan on SO2 viability of Acetobacter strains in wine. Int J Food Microbiol. 2017;246:1–4. doi: 10.1016/j.ijfoodmicro.2017.01.022. [DOI] [PubMed] [Google Scholar]
- Vazquez-Muñoz R, Borrego B, Juárez-Moreno K, García-García M, Mota Morales JD, Bogdanchikova N, Huerta-Saquero A. Toxicity of silver nanoparticles in biological systems: does the complexity of biological systems matter? Toxicol Lett. 2017;276:11–20. doi: 10.1016/j.toxlet.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Wedral D, Shewfelt R, Frank J. The challenge of Brettanomyces in wine. LWT Food Sci Technol. 2010;43:1474–1479. doi: 10.1016/j.lwt.2010.06.010. [DOI] [Google Scholar]