Abstract
Adverse health effects of synthetic anti-oxidants have necessitated the use of natural anti-oxidants in food products. However, their incorporation may result into undesirable changes in physico-chemical and sensory attributes of the product. Hence, the present investigation was undertaken to prepare anti-oxidant rich ghee using curcumin (yellow pigment found in turmeric) as the natural anti-oxidant. Effects of varying curcumin levels (160–350 ppm), heating temperature (110–120 °C) and duration (16–22 min) on anti-oxidant, chemical and sensory attributes of ghee were studied. Increasing level of curcumin significantly increased the DPPH free radical scavenging activity and decreased the amount of conjugated dienes formation. Increasing heating time and temperature significantly decreased the anti-oxidant activity in ghee, but their combination significantly increased the activity. Increase in curcumin level and heating temperature improved the sensory attributes of ghee, but longer duration of heating decreased the same. Optimization using Central Composite Rotatable Design yielded 350 ppm of curcumin and heat treatment of 115 °C for 17.89 min for most acceptable, anti-oxidant rich ghee with a desirability value of 0.966. The model developed was found to predict the product characteristics adequately.
Keywords: Anti-oxidant activity, Sensory analysis, CCRD
Introduction
Milk and dairy products represent one of the main diets of Indian people. Among them, ghee is the second largest consumed dairy product after liquid milk in India (Bandyopadhyay and Khamrui 2007). Oxidative deterioration has been recognized as one of the major factors that limit the shelf life of fatty food like ghee resulting in the decrease in consumer acceptability by changing its organoleptic properties, destroying essential nutrients and producing toxic free radicals (Patel et al. 2013). The onset of oxidative rancidity in ghee is mainly due to the oxidation of unsaturated glycerides leading to the development of peroxides (Muir 1996). Scientific evidences revealed that the excessive intake of free radicals due to lipid oxidation may enhance aging (Calabrese and Maines 2006) and cause diseases e.g., cancer (Mantovani et al. 2003), atherosclerosis (Siekmeier et al. 2007), stroke (Spence 2006), rheumatoid arthritis (Firuzi et al. 2006), neurodegeneration (Droge and Schipper 2007), and diabetes (Rahimi et al. 2005).
Antioxidants significantly inhibit or delay oxidative processes of lipids in foods by getting oxidized (Thorat et al. 2013). Antioxidants have been widely used in the food industries to prevent oxidative degradation (Vaya and Aviram 1999). Synthetic antioxidants such as butylated hydroxyanisole (BHA), propyl gallate and tertiary butyl hydroquinone (TBHQ) are often used in ghee to prevent oxidative deterioration (Pawar et al. 2012). However, scientific studies have shown that application of synthetic antioxidants in foods may cause liver damage and cancer (Yeh et al. 2011). It is, therefore necessary to explore the possibility of using naturally occurring antioxidants to safeguard the health of the consumer from prolonging ingestion of chemical antioxidants (Prasad et al. 2017).
One such natural antioxidant is curcumin (diferuloylmethane), a fat-soluble bioactive, yellow pigment present in Indian spice turmeric (Curcuma longa L.), known for its numerous functional attributes e.g., anti-inflammatory, anti-oxidant, hypotensive, hypocholesteremic, antidiabetic, anti-bacterial, anti-viral, etc. (Naik et al. 2010).
Monitoring diene conjugation emerged as a useful technique for the study of lipid oxidation (Antolovich et al. 2002). Conjugated dienes are typically produced during the formation of hydroperoxides from unsaturated fatty acids due to the rearrangement of the double bonds, show an intense absorption at 232 nm and reflects the formation of primary oxidation products in fats and oils (Patel et al. 2013). 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity is a rapid, simple, eminent method to measure the antioxidant capacity of food (Prasad et al. 2018). DPPH is widely used to test the ability of compounds to act as free radical scavengers or hydrogen donors and to evaluate antioxidant activity of foods. It helps to understand the functional attributes of foods, by measuring its total antioxidant capacity (Prasad et al. 2017).
Turmeric has been used in Ayurvedic medicine system for numerous biological attributes like antiinflammatory, antioxidant, anticarcinogenic, antimutagenic, anticoagulant, antidiabetic, antibacterial, antifungal, antiprotozoal, antiviral, hypotensive, hypocholesteremic etc. However, no information is available concerning the application of curcumin, the natural pigment found in turmeric as natural antioxidants in ghee for preventing auto-oxidation and prolonging its shelf life. Thus, the present study was conducted with the objectives of preparing curcumin fortified buffalo ghee and to evaluate the effect of process variables i.e., curcumin content, heat clarification temperature and duration of heat clarification on overall acceptability, the extent of oxidative changes such as conjugated dienes and radical scavenging activity by DPPH method using RSM.
Materials and methods
Raw materials
Buffalo milk procured from Experimental Dairy (ICAR-National Dairy Research Institute, Karnal, Haryana, India), was used for the manufacture of curcumin fortified ghee during this investigation. Curcumin (97% w/w) was procured from M/S Hi-Media Chemicals Pvt. Ltd., A-516, Swastik Disha Business Park, Vadhani Industrial Estate, L.B.S. Marg, Mumbai—400 086, India.
Preparation of curcumin fortified buffalo ghee
Ghee was prepared using the method prescribed by Aneja et al. (2002) with minor modifications. While butter from fermented buffalo cream was melted properly by heating to 65 °C and curcumin was added according to the RSM run being conducted followed by heat clarification for converting into ghee. Curcumin content, heat clarification time and temperature were optimized through RSM.
Sensory evaluation
Samples obtained during the course of investigation were analyzed by trained panellists from the scientific faculty of NDRI, Karnal using the scorecard prescribed by Bureau of Indian Standards (BIS 1976).
Anti-oxidative activity
Conjugated dienes were determined following AOAC (1971) (method 930.27) method and the results are represented as values at 233 nm (wavelength) absorbance. Free radical (DPPH) scavenging activity was evaluated using the method prescribed by Espin et al. (2000).
Experimental design
A three-factor five level Central Composite Rotatable Design (CCRD) of response surface methodology (RSM) was employed using the quadratic model to set up the experimental design. Development of curcumin fortified buffalo ghee involved three independent variables i.e., curcumin content (160–350 ppm), heating temperature (110–120 °C) and heating duration (16–22 min) designated as three factors together with interactions with them. The range of these variables was decided based on literature review for functional attributes of curcumin (EFSA 2010). A total of 20 experiments (runs) were carried out in randomized order that included 8 factorial, 6 axial and 6 central points (Table 1). It was assumed that the response (y) is a function of the experimental factors (A, B, C) or y = f (A, B, C). A second order polynomial equation was fitted to the experimental data for predicting the optimal point and to describe each response. The quadratic model for three variable experiments can be expressed as follow:
where y is the measured response variable; β0 is the intercept; β1, β2, β3 are the first-order coefficients, β12, β13, β23 are the interactions (cross product) coefficients; and β11, β22, β33 are the second order coefficients; and ε is random error. The adequacy of model was tested using F-value and coefficient of determination (R2) (Henika 1982). The ‘Lack of fit test’ was used to compare the residual error from replicated design points. The model with significant lack of fit was selected as suggested by Lee et al. (2000). For each response, analysis of variance (ANOVA) was conducted to determine significant differences among various ingredient combinations. While optimization, conjugated diene value was minimized whereas, overall acceptability and radical-scavenging activity were maximized in order to obtain the most desirable optimized condition for process variables.
Table 1.
Experimental variables for curcumin fortified buffalo ghee (coded and actual values)
| Independent variables (factors) | Levels | ||||
|---|---|---|---|---|---|
| Axial point | Factorial point | Centre point | Factorial point | Axial point | |
| Coded levels | + 1.68 | − 1 | 0 | + 1 | − 1.68 |
| A: Curcumin content (ppm) | 414.77 | 160 | 255 | 350 | 95.22 |
| B: Temperature of heating (°C) | 123.41 | 110 | 115 | 120 | 106.59 |
| C: Duration of heating (min) | 24.05 | 16 | 19 | 22 | 13.95 |
Results and discussion
The three factor, five-level experimental design matrix of CCRD with actual values of the factors with corresponding values of responses (overall acceptability, conjugated dienes and radical scavenging activity) of curcumin fortified ghee is shown in Table 2. ANOVA and model statistics of overall acceptability, conjugated dienes and radical scavenging activity of curcumin fortified buffalo ghee are presented in Table 3. Further, the optimization of variable levels was achieved by desirable minimization or maximization of the necessary response along the fitted quadratic models by numerical optimization procedure of Design Expert software. The effect of change in the levels of selected process variables on the response parameters i.e., overall acceptability, conjugated dienes and radical scavenging activity are represented in Figs. 1 and 2.
Table 2.
Experimental design matrix of CCRD with actual values of the three factors with corresponding overall acceptability, conjugated dienes and radical scavenging activity of curcumin fortified buffalo ghee
| Run | A—Curcumin content (ppm) | B—Heating/clarification temp (°C) | C—Duration of heating/clarification (min) | Overall acceptability | Conjugated dienes value (%) | Radical scavenging activity (%) |
|---|---|---|---|---|---|---|
| 1 | 160.00 | 110 | 16 | 80.2 | 0.597 | 37.83 |
| 2 | 350.00 | 110 | 16 | 81.3 | 0.405 | 77.68 |
| 3 | 160.00 | 120 | 16 | 84 | 0.638 | 39.06 |
| 4 | 350.00 | 120 | 16 | 87.4 | 0.386 | 77.32 |
| 5 | 160.00 | 110 | 22 | 82.3 | 0.619 | 38.36 |
| 6 | 350.00 | 110 | 22 | 83.2 | 0.437 | 76.42 |
| 7 | 160.00 | 120 | 22 | 77 | 0.667 | 35.46 |
| 8 | 350.00 | 120 | 22 | 84.5 | 0.416 | 76.81 |
| 9 | 255.00 | 115 | 19 | 86.4 | 0.485 | 65.98 |
| 10 | 255.00 | 115 | 19 | 86 | 0.484 | 65.07 |
| 11 | 255.00 | 115 | 19 | 85.5 | 0.49 | 66.46 |
| 12 | 255.00 | 115 | 19 | 85.3 | 0.487 | 66.26 |
| 13 | 95.23 | 115 | 19 | 85.1 | 0.687 | 27.17 |
| 14 | 414.77 | 115 | 19 | 87.8 | 0.343 | 80.04 |
| 15 | 255.00 | 106.59 | 19 | 74 | 0.514 | 58.66 |
| 16 | 255.00 | 123.41 | 19 | 77.8 | 0.537 | 53.34 |
| 17 | 255.00 | 115 | 13.95 | 78.4 | 0.478 | 57.05 |
| 18 | 255.00 | 115 | 24.05 | 75 | 0.533 | 54.08 |
| 19 | 255.00 | 115 | 19 | 85 | 0.485 | 63.26 |
| 20 | 255.00 | 115 | 19 | 86.7 | 0.489 | 67.11 |
Table 3.
Regression coefficients and ANOVA of fitted quadratic models for overall acceptability, conjugated dienes and radical scavenging activity of curcumin fortified buffalo ghee
| Factor | Overall acceptability | Conjugated dienes value | Radical scavenging activity |
|---|---|---|---|
| Intercept | 85.42 | 0.49 | 65.29 |
| A—Curcumin content (ppm) | 1.28** | − 0.11** | 18.04** |
| B—Heating temp (°C) | 0.90* | 6.420E − 003** | − 0.78 |
| C—Duration of heating (min) | − 0.85* | 0.015** | − 0.72 |
| AB | 1.11* | − 0.016** | 0.21 |
| AC | 0.49 | 1.375E − 003 | 0.16 |
| BC | − 1.74** | 6.250E − 004 | − 0.42 |
| A2 | 0.93* | 0.011** | − 3.61** |
| B2 | − 2.80** | 0.015** | − 2.77** |
| C2 | − 2.52** | 7.446E − 003** | − 2.92** |
| R2 | 0.9463 | 0.9985 | 0.9848** |
| APV (Adq. Pre value) | 14.072 | 91.505 | 28.445 |
| PRESS | 99.44 | 0.002 | 682.97 |
| Model F-value | 17.63** | 659.27** | 64.65** |
| Lack of fit | NS | NS | NS |
NS non-significant
**Significant at 1% level; *significant at 5% level
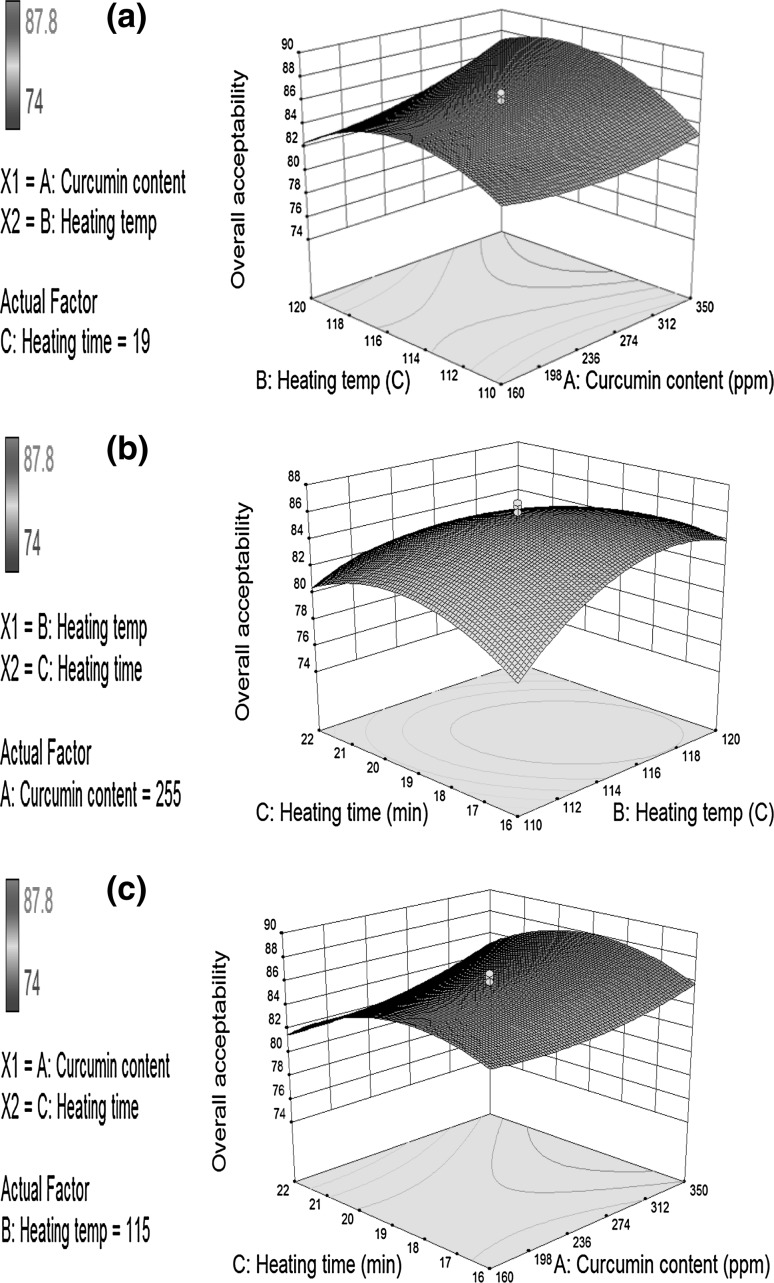
Fig. 1.
Response surface graphs for overall acceptability (OA) of curcumin fortified buffalo ghee influenced by the level of curcumin content, heating temperature and duration of heating
Fig. 2.
Response surface graphs for conjugated dienes value and DPPH activity of curcumin fortified buffalo ghee influenced by the level of curcumin content, heating temperature and duration of heating
Effect of selected process variables on overall acceptability of curcumin fortified buffalo ghee
The acceptability of any product and its market potential is greatly attributed to the sensory profile of the product. Usually, good quality buffalo ghee should have white or without a yellowish or greenish tinge with a pleasant enjoyable flavour that lingers on the mouth and possesses medium to large size grains that uniformly distributed throughout the product (Khamrui et al. 2016). The overall acceptability score of curcumin fortified ghee varied between 74.0 and 87.7. The minimum overall acceptability was obtained from the curcumin fortified buffalo ghee sample prepared using the combination with 255 ppm curcumin, heated at 106.59 °C for 19 min whereas, the maximum value was obtained for the combination 414.77 ppm curcumin, heated at 115 °C for 19 min. The partial coefficient of regression presented in Table 2 showed that at linear level, curcumin content had significant (p ≤ 0.01) positive effect on overall acceptability scores of curcumin fortified buffalo ghee, implying that increasing level of curcumin content showed an increase in overall acceptability score (Fig. 1). Ghee prepared at a relatively higher clarification temperature possessed higher levels of low molecular flavour producing ketones than ghee prepared at a lower temperature (Jain et al. 1971). Thus, the overall acceptability score of curcumin fortified buffalo ghee samples was also increased significantly (p ≤ 0.05) with increasing heating temperature at linear level up to 120 °C (Table 2). This is in agreement with the findings of Ganguli and Jain (1972), who reported that ghee clarified at a lower temperature (110 °C) produced ‘curdy’ flavoured ghee which consequently reducing the overall acceptability score of the product. Whereas, the reverse trend was observed in case of the duration of heating which showed significant (p ≤ 0.05) negative effect on overall acceptability score as long duration used for clarification produced burnt flavour as well as loss of volatile flavour ketones which eventually decreased the score.
The significant (p ≤ 0.05) positive coefficient of interaction between curcumin content and heating temperature suggested an increase in overall acceptability score of curcumin fortified buffalo ghee with an increase in curcumin content. The observed effect was greater at higher levels of heating temperature and smaller at lower levels (Fig. 1a). The significant (p ≤ 0.01) negative coefficient of interaction between curcumin content and heating temperature suggested that the overall acceptability score of curcumin fortified buffalo ghee increased with increasing level of curcumin content at shorter heating duration. However, the scores decreased with increasing level of curcumin content, when heated for a longer duration of time (Fig. 1c).
The significant (p < 0.01) positive quadratic terms of curcumin content indicated that higher overall acceptability scores were obtained at higher and lower levels of curcumin content, producing a response surface graph that was convex downward. Opposite (p < 0.01) trend was found for quadratic terms of heating temperature and heating duration on overall acceptability scores of curcumin fortified buffalo ghee. Effect of various process variables on the overall acceptability of curcumin fortified buffalo ghee could be predicted by the Eq. (1), having a R2 of 0.946 revealed that more than 94% of the variability in overall acceptability of curcumin fortified buffalo ghee could be explained by the model.
| 1 |
Effect of selected process variables on conjugated dienes value of curcumin fortified buffalo ghee
Monitoring diene conjugation has emerged as a useful technique for the study of lipid oxidation (Antolovich et al. 2002). The conjugated dienes value of curcumin fortified buffalo ghee samples varied from 0.342 and 0.687 (Table 2). The minimum conjugated dienes value was obtained from the curcumin fortified buffalo ghee sample prepared in the combinations with 414.77 ppm curcumin, heating at 115 °C for 19 min whereas, the maximum value was obtained for the combinations with 95.23 ppm curcumin, heating at 115 °C for 19 min.
The partial coefficient of regression model presented in Table 3 indicated that curcumin content had significant (p ≤ 0.01) negative effect on conjugated dienes value of curcumin fortified buffalo ghee which revealed that increasing level of curcumin content shows a lower conjugated dienes value at the linear level. Conjugated dienes are typically produced during the formation of hydroperoxides from unsaturated fatty acids due to the rearrangement of the double bonds and reflect the formation of primary oxidation products in fats and oils. This provides an important parameter for evaluation of oxidation level. In addition, the inhibition of formation or action of these unstable species by antioxidants can be used as a means of assessing antioxidant activity. Expectedly, conjugated dienes value of curcumin fortified buffalo ghee samples was also increased significantly (p ≤ 0.05) with increasing temperature as well as the duration of heating levels presented in Table 3. With an increase in temperature and exposure time of heating, ghee containing methylene interrupted dienes show a shift in their double bond position during oxidation as a result of isomerization and conjugate formation which eventually increase the absorption at 232 nm. Mahmoud et al. (2009) reported that the content of conjugated dienoic bonds was shown increased gradually with the increase in the microwave and conventional heating time for extra virgin and refined olive oils. Zuta et al. (2007) also reported that the diene conjugation was increased in mackerel oil with increasing temperature from − 40 to 30 °C. In addition to this, the authors also found that increasing concentration of antioxidant like α-tocopherol decreased conjugated diene (CD) value of mackerel oil. The significant (p ≤ 0.01) negative coefficient of interaction between curcumin content and heating temperature suggested that decrease in conjugated dienes value of curcumin fortified buffalo ghee was observed with increasing level of curcumin content at lower heating temperatures (Fig. 2a). The significant (p ≤ 0.01) positive quadratic term of curcumin content revealed that higher conjugated dienes value was obtained at higher and lower levels of curcumin addition. Similar trend was obtained in case of heating temperature as well as the duration of heating on conjugated dienes value of curcumin fortified buffalo ghee. The conjugated dienes value of curcumin fortified buffalo ghee could be predicted by the Eq. (2) having R2 of 0.998 (for actual values of variables) indicating that more than 99% of the variability in conjugated dienes value could be explained by the model.
| 2 |
Effect of selected process variables on DPPH radical scavenging activity of curcumin fortified buffalo ghee
Inhibition process of the auto-oxidation in lipids by antioxidants is linked to the ability of antioxidants to break the radical formation reaction. Consequently, the antioxidant potential can also be estimated in systems in which the radicals are generated by chemical means (Pankaj et al. 2013). The DPPH scavenging assay is a widely used method to primarily evaluate free radical scavenging activity.
The ability to trap the DPPH radicals of curcumin fortified buffalo ghee samples ranged from 0.343 and 0.687 (Table 2). The maximum radical scavenging ability in the curcumin fortified buffalo ghee sample prepared in combinations with 414.77 ppm curcumin, heating at 115 °C for 19 min whereas the minimum value was obtained for combinations with 95.23 ppm curcumin, heating at 115 °C for 19 min. The coefficients of partial regression model presented in Table 3 indicates that at the linear level, the curcumin contents had a significant (p ≤ 0.01) positive effect on radical scavenging activity of curcumin fortified buffalo ghee which revealed that increasing level of curcumin content showed a higher radical scavenging activity up to 350 ppm. The ability of curcumin to trap DPPH stable radical is thought to be due to its phenolic hydrogen or central methylenic hydrogen in the heptadienone moiety (Foti et al. 2016). Jovanovic et al. (1999) studied the antioxidant mechanism of curcumin with laser flash photolysis and pulse radiolysis, and reported that in acidic and neutral conditions (pH 3–7) curcumin is an outstanding H-atom donor, donating the H-atom from the central methylenic group rather than from phenolic group. Formation of an intramolecular hydrogen bond between ortho-methoxy groups with the phenolic hydrogen of curcumin making it easier to donate H-atom to the DPPH free radical. The higher percentage of DPPH scavenging activity may be attributed to the high reducing power and higher total phenolic contents present in curcumin (Borra et al. 2013). The observations are in agreement with Asouri et al. (2013) reported that the scavenging of DPPH radical is dependent on the concentration of antioxidant. He compared the DPPH scavenging potency of curcumin with ascorbic acid and concluded that the concentration-antioxidant activity profile was similar for curcumin and ascorbic acid. Eshghi et al. (2014) evaluated the antioxidant effect of different concentrations of curcumin (120, 160 and 200 ppm) at two different temperatures (25 and 55 °C) under dark and light conditions during 90 days in soybean oil. Results indicated that increasing concentration of curcumin leads to significantly decreased oxidation rates. In contrast, heating temperature and duration of heating do not have any significant effect on DPPH radical scavenging activity of the curcumin fortified buffalo ghee. Zebib et al. (2010) reported that curcumin is thermally stable up to 160 °C. Hence, increasing the temperature and residence time of heating had a negligible effect on the antioxidant potential of curcumin fortified buffalo ghee. Hence, acidic nature of ghee might also have helped in retention of curcumin induced antioxidant activity (Kumavat et al. 2013). The significant (p ≤ 0.01) negative quadratic terms of curcumin content, heating temperature and duration of heating on the DPPH scavenging activity indicated that the lower value of this response was observed at higher and lower levels of these variables (Fig. 2). The radical scavenging activity (DPPH activity) of curcumin fortified buffalo ghee could be predicted by the Eq. (3) given below:
| 3 |
Regression analysis of data presented in Table 3 revealed that the coefficient of determination (R2) for the quadratic model was 0.984 indicating that more than 98% of the variability in DPPH radical scavenging activity could be explained by the model.
Numerical optimization of the Ingredients
The main aim of the optimization technique was to determine the best possible combination of the three factors that would result in a highly acceptable product with respect to overall acceptability score with lower conjugated dienes value and higher radical scavenging activity (DPPH activity). In order to optimize the process variables level, curcumin content was kept at maximum level, as it is a potential antioxidant. Clarification temperature and duration of clarification were kept in range. The solution containing 350 ppm curcumin with clarification temperature at 115 °C for 17.89 (18) min yielded the highest desirability (0.966). Optimized solution was compared with t test (Table 4), revealed that the actual and predicted value did not differ significantly (p ≤ 0.05) for the studied responses. Hence, the solution was found to be optimum for preparing curcumin fortified buffalo ghee.
Table 4.
Comparison of predicted and actual values of responses
| Responses | Predicted score | Actual scorea | t value@ | Remarks |
|---|---|---|---|---|
| Overall acceptability | 87.8 | 87.67 ± 0.16 | 0.80 | ns |
| Conjugated dienes | 0.384 | 0.385 ± 0.004 | 0.20 | ns |
| DPPH radical scavenging activity (% inhibition) | 79.37 | 79.35 ± 0.16 | 0.18 | ns |
Predicted values obtained from Design Expert™ 9.0.5 Package
@t values found non-significant at 5% level of significance; ns non significant (p < 0.05)
aActual mean ± SE (average of 3 trials) of optimized curcumin fortified buffalo ghee
Conclusion
Results of this study revealed that ghee with enhanced antioxidant property and overall sensorial acceptability could be prepared by incorporation of curcumin using the optimized process variables determined by use of response surface methodology. The overall acceptability, DPPH radical scavenging activity and conjugated dienes content of curcumin fortified ghee could be accurately predicted by the three factors viz., curcumin content, heat clarification temperature and duration of heat clarification. In vivo study of curcumin fortified ghee would be required to establish the health effects of curcumin fortified ghee.
References
- Aneja RP, Mathur BN, Chandan RC, Banerjee AK. Technology of Indian milk products: handbook on process technology modernization for professionals, entrepreneurs and scientists. New Delhi: Dairy India Yearbook; 2002. pp. 183–190. [Google Scholar]
- Antolovich M, Prenzler PD, Patsalide E, Mcdonald S, Robards K. Methods for testing antioxidant activity. Analyst. 2002;127:183–198. doi: 10.1039/b009171p. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. 16. Washington: Association of Official Agricultural and Chemists; 1971. pp. 877–988. [Google Scholar]
- Asouri M, Ataee R, Ahmadi AA, Amini A, Moshaei MR. Antioxidant and free radical scavenging activities of curcumin. Asian J Chem. 2013;25:7593–7595. [Google Scholar]
- Bandyopadhyay P, Khamrui K. Technological advancement on traditional Indian desiccated and heat-acid coagulated dairy products. Bull Int Dairy Fed. 2007;415:4–10. [Google Scholar]
- BIS . IS: 1485, Specification for ghee. New Delhi: Bureau of Indian Standards; 1976. [Google Scholar]
- Borra SK, Gurumurthy P, Mahendra J, Jayamathi KM, Cherian CN, Chand R. Antioxidant and free radical scavenging activity of curcumin determined by using different in vitro and ex vivo models. J Med Plant Res. 2013;72:680–2690. [Google Scholar]
- Calabrese V, Maines M. Antiaging medicine: antioxidants and aging. Antioxid Redox Signal. 2006;8:362–364. doi: 10.1089/ars.2006.8.362. [DOI] [PubMed] [Google Scholar]
- Droge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific opinion on the re-evaluation of curcumin (E100) as a food additive. EFSA J. 2010;8(9):1679. [Google Scholar]
- Eshghi N, Asnaashari M, Haddad KMH, Hosseini F. Evaluating the potential of natural curcumin for oxidative stability of soybean oil. Nat Prod Res. 2014;28:1375–1378. doi: 10.1080/14786419.2014.901319. [DOI] [PubMed] [Google Scholar]
- Espin JC, Soler-Rivas C, Wichers HJ. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2, 2-diphenyl-1-picrylhydrazyl radical. J Agri Food Chem. 2000;48:648–656. doi: 10.1021/jf9908188. [DOI] [PubMed] [Google Scholar]
- Firuzi O, Fuksa L, Spadaro C, Bousova I, Riccieri V, Spadaro A, Saso L. Oxidative stress parameters in different systemic rheumatic diseases. J Pharm Pharmacol. 2006;58:951–957. doi: 10.1211/jpp.58.7.0010. [DOI] [PubMed] [Google Scholar]
- Foti MC, Slavova-Kazakova A, Rocco C, Kancheva VD. Kinetics of curcumin oxidation by 2, 2-diphenyl-1-picrylhydrazyl (DPPH˙): an interesting case of separated coupled proton–electron transfer. Org Biomol Chem. 2016;14(35):8331–8337. doi: 10.1039/C6OB01439A. [DOI] [PubMed] [Google Scholar]
- Ganguli NC, Jain MK. Ghee: its chemistry, processing and technology. J Dairy Sci. 1972;6:19–25. [Google Scholar]
- Henika RG. Use of response surface methodology in sensory evaluation. Food Technol. 1982;36:96–101. [Google Scholar]
- Jovanovic SV, Steenken S, Boone CW, Simic MG. H-atom transfer is a preferred antioxidant mechanism of curcumin. J Am Chem Soc. 1999;121:9677–9681. doi: 10.1021/ja991446m. [DOI] [Google Scholar]
- Khamrui K, Prasad WG, Khetra Y. Practical manual on sensory evaluation of milk and milk products. Karnal: NDRI Publication; 2016. p. 76. [Google Scholar]
- Kumavat SD, Chaudhari YS, Borole P, Mishra P, Shenghani K, Duvvuri P. Degradation studies of curcumin. Int. J Pharm Sci Rev Res. 2013;3:50–55. [Google Scholar]
- Lee Y, Ye L, Landen WO, Eitenmiller RR. Optimization of an extraction procedure for the quantification of vitamin Ein tomato and broccoli using response surface methodology. J Food Compos Anal. 2000;13:45–57. doi: 10.1006/jfca.1999.0845. [DOI] [Google Scholar]
- Mahmoud EAEM, El-Moneim A, Dostalova J, Pokorny J, Lukesova D, Dolezal M. Oxidation of olive oils during microwave and conventional heating for fast food preparation. Czech J Food Sci. 2009;27:S173–S177. doi: 10.17221/963-CJFS. [DOI] [Google Scholar]
- Mantovani G, Maccio A, Madeddu C, Mura L, Gramignano G, Lusso M, Massa E. The impact of different antioxidant agents alone or in combination on reactive oxygen species, antioxidant enzymes and cytokines in a series of advanced cancer patients at different sites: correlation with disease progression. Free Radic Res. 2003;37:213–223. doi: 10.1080/10715760303849. [DOI] [PubMed] [Google Scholar]
- Muir DD. The shelf-life of dairy products: 3 Factors influencing intermediate and long life dairy products. Int J Dairy Technol. 1996;49:67–72. doi: 10.1111/j.1471-0307.1996.tb02493.x. [DOI] [Google Scholar]
- Naik SR, Thakare VN, Patil SR. Protective effect of Curcumin on experimentally induced inflammation, hepatotoxicity and cardiotoxicity in rats: evidence of its antioxidant property. Exp Toxicol Pathol. 2010;63:419–431. doi: 10.1016/j.etp.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Pankaj P, Khamrui K, Devaraja HC, Singh RRB. The effects of alcoholic extract of Arjuna (Terminalia arjuna) bark on stability of clarified butterfat. J Med Plants Res. 2013;72:545–2550. [Google Scholar]
- Patel S, Shende S, Arora S, Singh AK. An assessment of the antioxidant potential of coriander extracts in ghee when stored at high temperature and during deep fat frying. Int J Dairy Technol. 2013;66(2):207–213. doi: 10.1111/1471-0307.12023. [DOI] [Google Scholar]
- Pawar N, Arora S, Singh RRB, Wadhwa BK. The effects of Asparagus racemosus (shatavari) extract on oxidative stability of ghee, in relation to added natural and synthetic antioxidant. Int J Dairy Technol. 2012;65:293–299. doi: 10.1111/j.1471-0307.2011.00816.x. [DOI] [Google Scholar]
- Prasad W, Khamrui K, Mandal S, Badola R. Anti-oxidative, physico-chemical and sensory attributes of burfi affected by incorporation of different herbs and its comparison with synthetic anti-oxidant (BHA) J Food Sci Technol. 2017;54(12):3802–3809. doi: 10.1007/s13197-017-2778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad W, Khamrui K, Mandal S, Badola R. Effect of combination of essential oils on physicochemical and sensorial attributes of burfi in 1 comparison with individual essential oil and BHA. Int J Dairy Technol. 2018 [Google Scholar]
- Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005;59:365–373. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Siekmeier R, Steffen C, Maerz W. Role of oxidants and antioxidants in atherosclerosis: results of in vitro and in vivo investigations. J Cardiovasc Pharmacol Ther. 2007;12:265–282. doi: 10.1177/1074248407299519. [DOI] [PubMed] [Google Scholar]
- Spence JD. Nutrition and stroke prevention. Stroke. 2006;37:2430–2435. doi: 10.1161/01.STR.0000236633.40160.ee. [DOI] [PubMed] [Google Scholar]
- Thorat ID, Jagtap DD, Mohapatrac D, Joshib DC, Sutarb RF, Kapdib SS. Antioxidants, their properties, uses in food products and their legal implications. Int J Food Stud. 2013;28:4–14. [Google Scholar]
- Vaya J, Aviram M. Nutritional antioxidants: mechanism of action, analyses of activities and medical application. Nutrition. 1999;49:1–7. [Google Scholar]
- Yeh JY, Hsieh LH, Wu KT, Tsai CF. Antioxidant properties and antioxidant compounds of various extracts from the edible basidiomycete Grifola Frondosa (Maitake) Molecules. 2011;16:3197–3211. doi: 10.3390/molecules16043197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebib B, Mouloungui Z, Noirot V. Stabilization of curcumin by complexation with divalent cations in glycerol/water system. Bioinorg Chem Appl. 2010 doi: 10.1155/2010/292760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuta PC, Simpson BK, Zhao X, Leclerc L. The effect of α-tocopherol on the oxidation of mackerel oil. Food Chem. 2007;100:800–880. doi: 10.1016/j.foodchem.2005.11.003. [DOI] [Google Scholar]