Abstract
Alginate oligosaccharides (AOs) are linear oligosaccharides with alternating sequences of mannuronic acid (M) and guluronic acid (G) residues. AOs can be used as a safe elicitor to induce glyceollins, which have many human health benefits, in soybean seeds. In this research, four AO fractions with different chemical structures and molecular weights were separated, purified, and then characterized by NMR spectroscopy and ESI–MS. With a 4,5-unsaturated hexuronic acid residue (△) at the non-reducing terminus, the structures of these four AO fractions were △G, △MG, △GMG and △MGGG, which exhibited glyceollin-inducing activities of 1.2339, 0.3472, 0.6494 and 1.0611 (mg/g dry weight) in soybean seeds, respectively. The results demonstrated that a larger molecular weight or a higher G/M ratio might correlate with a higher glyceollin-inducing activity. Moreover, the alginate disaccharide △G could be introduced as relatively safe and efficient elicitor of high glyceollin content in soybeans.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3101-6) contains supplementary material, which is available to authorized users.
Keywords: Alginate oligosaccharides, Glyceollin, Soybean, Molecular weight, Ratio of guluronic to mannuronic acid (G/M)
Introduction
Soybeans are a major part of the Chinese diet and have become popular in other parts of the world as well. The popularity of soybeans is associated with their health-promoting properties, which include reduced risks of cancer and cardiovascular disease (Jenkins et al. 2010). These health benefits result from the presence of bioactive compounds such as isoflavones in soybeans (Boue et al. 2009). Glyceollins, a major family of phytoalexins, are important isoflavones in soybeans (Bhattacharyya and Ward 1986). They have many human health benefits, such as preventing breast and ovarian carcinoma (Salvo et al. 2006), preventing hyperglycemia and insulin resistance (Yoon et al. 2013), and regulating lipid and carbohydrate metabolism (Wood et al. 2012). Glyceollins accumulate in soybeans in response to various elicitors, including substances of pathogen origin (biotic elicitors), such as cell walls and spores (Fett and Zacharius 1982; Moesta and Grisebach 1980; Yoshikawa and Yoshikawa 1978), and abiotic elicitors, such as heavy metal salts and detergents (Pitta Alvarez et al. 2000; Stintzi et al. 2001; Stössel 1984). However, those elicitors are either complex microorganic mixtures or poisonous compounds, which may impact the consumption of induced soybean products because of food biosafety considerations and restrict their application in the food industry (Angelova et al. 2006).
Alginate oligosaccharides (AOs) are marine oligosaccharides generated from alginate. They contain guluronic acid (α-l-guluronate, G) and mannuronic acid (β-d-mannuronate, M) units linked by 1 → 4 O-glycoside bonds; these units are arranged in homopolymeric G blocks, M blocks, and random heteropolymeric G and M blocks (Nagasawa et al. 2000; Zhang et al. 2013). To date, the structures of AOs have been elucidated using ESI–MS, NMR spectroscopy and ESI–MS techniques (Holtan et al. 2006; Jochum et al. 2002; Scherz et al. 2005). AOs have multiple roles and can be used as growth factors to stimulate the VEGF-mediated growth and migration of human endothelial cells (Kawada et al. 1997, 1999) and to regulate plant developmental and defensive processes (Akimoto et al. 2000; Chandía et al. 2004; Iwasaki and Matsubara 2000; Ma et al. 2010; Natsume et al. 1994). However, there still have been very few studies on the induction capacity of AOs for glyceollin accumulation in plants, especially soybeans (Zhang et al. 2015).
In our previous research, we reported the glyceollin-inducing activity of AOs in soybeans; however, the AOs used as elicitors were a mixture of sodium alginate oligomers (Jia et al. 2012). The identification of AOs derived from the M-, G-, or MG-blocks and their sequence determination are known to be important for better understanding the structure–function relationships of alginates at the molecular level (Schürks et al. 2002). Therefore, in this study, the structures of four AO fractions were completely determined, and then each fraction was used to induce glyceollin accumulation in soybeans in order to investigate the relationship between the structure and glyceollin-inducing activity of AOs. The final aim of this research is to find a suitable structure for use as an efficient elicitor that is safe for humans to increase the amount of glyceollins in soybeans in order to improve their health benefits.
Materials and methods
Materials and chemicals
The mixture of alginate oligosaccharides (AOs, enzymatically hydrolyzed) used was donated by the Dalian Institute of Chemical Physics (Dalian, China). The glyceollin standard was prepared in our lab by preparative HPLC (Eromosele et al. 2013). Soybean was purchased from Kefeng Group Co. (Haerbin, China). Deuterium oxide (99.9 atom %D) was purchased from Sigma-Aldrich (St. Louis, Missouri, USA). HPLC-grade methanol and acetonitrile were purchased from Fisher Scientific Company (Fair Lawn, NJ, USA). Only HPLC-grade water was used to prepare reagent solutions. All other analytical-grade reagents were purchased from Beijing Chemicals Reagent Company (Beijing, China).
Methods
Separation of alginate oligosaccharides
The AO powder (1 g) was mixed with 100 ml of water for 2 h in room temperature. The mixture was centrifuged at 15,000 g for 5 min, and the supernatant was filtered through a 0.22 μm sterile syringe filter. The filtered extract was applied as a crude AO solution. A 1 ml sample was fractionated and collected by a Sykam S1125 preparative HPLC system equipped with an S3245 UV detector operating at a wavelength of 230 nm, Clarity ver. 7.1 software and a TSKgel DEAE-2SW preparation column (20.0 mm I.D. × 25 cm, Tosoh Corp., Tokyo, Japan) using a 180 min linear gradient of 0–0.25 M NaCl and a flow rate of 7.0 ml/min.
Alginate oligosaccharides desalination
The separated fraction samples were desalinated by a solid phase extraction (SPE) cartridge with a porous graphitic carbon column (Varian Bond Elut Carbon, 500 mg, 6 ml). Each sample was totally evaporated and then redissolved in 10 ml of 0.1% (v/v) trifluoroacetic acid (TFA). The small column was previously conditioned with 5 ml of acetonitrile and 5 ml of 0.1% (v/v) TFA. In each procedure, a 500 µl sample was loaded into column, the column was washed with 6 ml 0.1% (v/v) TFA, and then the oligosaccharide was eluted with 5 ml of 0.1% (v/v) TFA containing 25% (v/v) acetonitrile. The desalination procedures described above were repeated several times until NaCl was completely removed, which was determined by NaCl detection with 1% (w/v) AgNO3. Finally, the desalinated oligosaccharide samples were freeze-dried and stored at − 20 °C.
Purity test on AO fractions
The collected AO fractions were checked on an analytical HPLC Waters 2695 system equipped with a 2996 UV detector and Empower Pro software under previously described conditions (Chaki et al. 2006): Column, TSKgel DEAE-2SW 4.6 mm I.D. × 25 cm (Tosoh Corp., Tokyo, Japan); column temperature, 30 °C; monitor wavelength, 230 nm; mobile phase, water-0.25 M NaCl with a linear gradient (0–60 min); flow rate, 1.0 ml/min; injection amount, 20 µl; analytical time, 60 min. The purity of each fraction was calculated via the HPLC area normalization method.
NMR spectroscopy for structural analysis
Each AO fraction sample (20 mg) was evaporated by lyophilization twice with 0.5 ml of deuterium oxide to remove exchangeable protons before final dissolution in 0.5 ml of deuterium oxide for 1D and 2D NMR analysis on a Bruker Avance 500 spectrometer. 1H-1D, 1H-1H COSY-2D, 1H-13C HSQC-2D and 1H-13C HMBC-2D NMR spectra were recorded at 500 MHz at 25 °C. 13C-1D NMR spectra were obtained at 125 MHz at 25 °C. The water peak in 1H NMR spectra was 4.70. DMSO was used as an internal standard; the DMSO peak in 13C NMR spectra was 37.83.
ESI–MS analysis
ESI–MS was conducted in negative-ion mode with a Waters Xevo TQ-s instrument (Waters Corp.). Each sample was dissolved in water and diluted in 50% aqueous methanol. The ESI–MS conditions were as follows: injection volume, 2 μl; ESI voltage, 4 kV; capillary temperature, 275 °C; capillary voltage, 350 V; tube lens, 250 V; scan range, 400–2000 m/z. Nitrogen was used as the sheath gas and auxiliary gas at a flow rate of 30 and 5 arb, respectively. The mobile phase (methanol/water = 1:1, v/v) was delivered at a flow rate of 200 μl/min.
Glyceollin induction
Glyceollin induction in soybean cotyledons was performed according to the method described by Boué with some modifications (Boué et al. 2000). The AO mixture and the AO single compound samples were dissolved in water to provide 1% (w/v) solutions. Each solution (60 µl) was applied to the cut surface of a soybean cotyledon. In the control group, water (60 µl) was applied. All chambers were sealed with parafilm and incubated for 4 days at 25 °C in the dark.
Glyceollin determination
Soybean cotyledon extraction and sample preparation were conducted according the methods described by Eromosele in our previous research (Eromosele et al. 2013). The accumulation of glyceollins was calculated by the following formula: y = 1.0 × 107x + 754.32 (R2 = 0.9999).
Results
Separation of alginate oligosaccharides
AOs are a kind of polyanionic oligosaccharides and thus were separated on an anion-exchange column by preparative HPLC (Fig. 1a). This method yielded a series of distinct and well-resolved peaks, which were defined as F1 to F12. All the fractions were collected and analyzed further via analytical HPLC (Fig. 1b–n). The retention time and purity of each faction are shown in Table 1. The relative purities of these fractions mainly reached a high level of ≥ 97%; however, F6 and F10 were observed to be divided into 2 peaks upon further analytical HPLC analysis (Fig. 1h, l), which indicated that one-step separation was not sufficient to distinguish every oligomer of the AO mixture, as oligomers with similar structures could have had the same retention time.
Fig. 1.
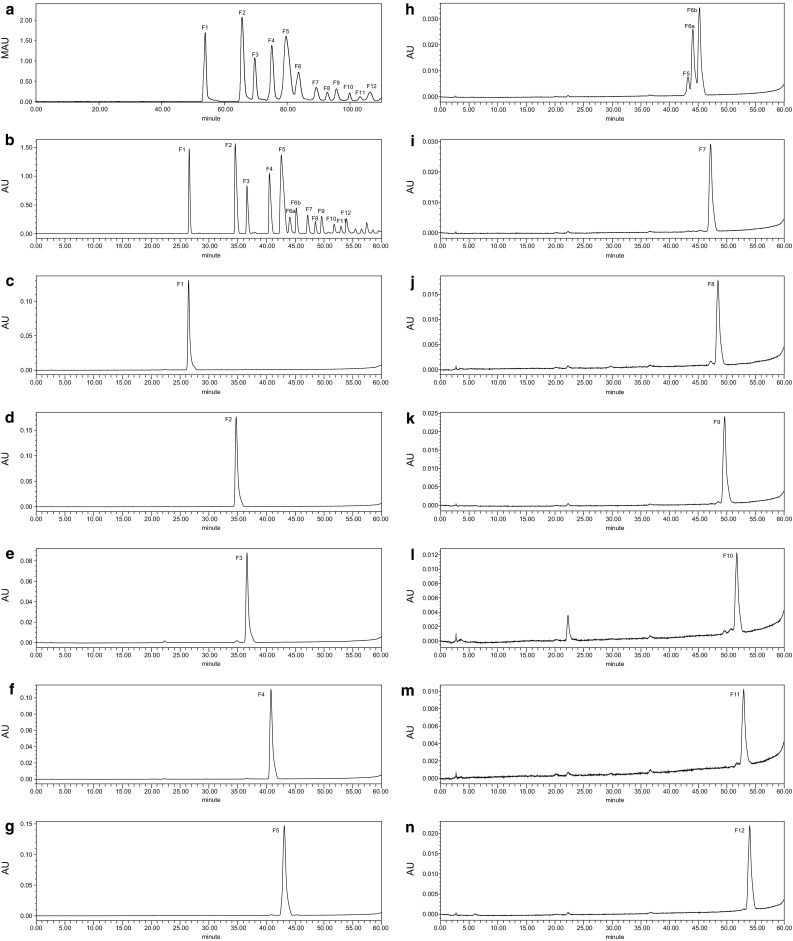
HPLC profiles of AO separation. a Preparative HPLC profile of the AO mixture. A total of 12 fractions collected in 110 min are shown. b Analytical HPLC profile of the AO mixture. Fraction F6, which was defined based on the preparative HPLC chromatogram, was divided into 2 fractions for analytical HPLC. c–n Analytical HPLC profiles of each oligosaccharide fraction collected by preparative HPLC. The purity of each fraction was calculated via the HPLC area normalization method
Table 1.
The retention time and the relative purity of AO fraction F1–F12
| Fraction | Preparative HPLC (min) | Analytical HPLC (min) | Relative purity (%) | Fraction | Preparative HPLC (min) | Analytical HPLC (min) | Relative purity (%) |
|---|---|---|---|---|---|---|---|
| F1 | 53.9 | 26.476 | 98.08 | F7 | 89.4 | 47.158 | 99.86 |
| F2 | 65.6 | 34.740 | 99.67 | F8 | 92.9 | 48.471 | 99.34 |
| F3 | 69.7 | 36.618 | 97.66 | F9 | 95.8 | 49.623 | 99.78 |
| F4 | 75.1 | 40.801 | 98.19 | F10 | 100.0 | 51.741 | – |
| F5 | 79.6 | 43.120 | 99.39 | F11 | 103.3 | 52.929 | 99.16 |
| F6a | 83.7 | 44.076 | – | F12 | 106.5 | 53.983 | 99.76 |
| F6b | 83.7 | 45.231 | – |
Structural confirmation of the separated alginate oligosaccharide fractions
High-resolution NMR spectroscopy was performed to confirm the structure of the AO fractions. Finally, the structures of 4 fractions (F1, F3, F5 and F7) were completely determined, as they displayed clear signals in their NMR spectra. In our study, because the AOs were obtained by lyase cleavage, a 4,5-unsaturated hexuronic acid residue (△) was located at the non-reducing terminus (Zhang et al. 2006). The chemical shift values of 1H and 13C peaks are given in Table 2. Peaks in the 1D NMR spectra were assigned based on 1D 1H and 13C NMR experiments and 2D NMR experiments, including 1H-1H COSY, 1H-13C HSQC and 1H-13C HMBC (see Supplemental Fig. 1-6). According to the previous data of NMR spectroscopy of alginate oligomers (Holtan et al. 2006; Zhang et al. 2004), the structures of F1, F3, F5 and F7 in our research were established as follows: F1: △G, O-(4-deoxy-α-l-erythro-hex-4-enopyranosyluronic acid)-(1 → 4)-O-β-l-gulopyranuronic acid; F3: △MG, O-(4-deoxy-α-l-erythro-hex-4-enopyranosyluronic acid)-(1 → 4)-O-(β-d-mannopyranuronic acid)-(1 → 4)-O-β-l-gulopyranuronic acid; F5: △GMG, O-(4-deoxy-α-l-erythro-hex-4-enopyranosyluronic acid)-(1 → 4)-O-(α-l-gulopyranosyluronic acid)-(1 → 4)-O-(β-d-mannopyranuronic acid)-(1 → 4)-O-β-l-gulopyranuronic acid; and F7: △MGGG, O-(4-deoxy-α-l-erythro-hex-4-enopyranosyluronic acid)-(1 → 4)-O-(β-d-mannopyranuronic acid)-(1 → 4)-O-(α-l-gulopyranosyluronic acid)-(1 → 4)-O-(α-l-gulopyranosyluronic acid)-(1 → 4)-O-β-l-gulopyranuronic acid.
Table 2.
1H and 13C NMR chemical shifts of oligomers of F1, F3, F5 and F7
| Proton chemical shifts (ppm) | Carbon chemical shifts (ppm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H-1 | H-2 | H-3 | H-4 | H-5 | C-1 | C-2 | C-3 | C-4 | C-5 | |
| F1 | ||||||||||
| △G dimer | ||||||||||
| △Gredα | 5.20 | 3.88 | 4.31 | 6.08 | – | 100.73 | 66.51 | 62.24 | 111.97 | 141.16 |
| △Gredβ | 4.86 | 3.50 | 4.21 | 4.16 | 4.63 | 93.47 | 68.56 | 69.59 | 79.54 | 72.12 |
| F3 | ||||||||||
| △MG trimer | ||||||||||
| △MGredα | 5.17 | 3.92 | 4.39 | 6.02 | – | 99.99 | 66.40 | 63.07 | 112.17 | 140.92 |
| △MGredβ | 4.72 | 3.89 | 3.73 | 3.95 | 3.95 | 101.65 | 70.17 | 70.68 | 77.27 | 73.63 |
| △MGredβ | 4.86 | 3.62 | 4.25 | 4.15 | 4.63 | 93.45 | 68.49 | 69.90 | 79.61 | 72.25 |
| F5 | ||||||||||
| △GMG tetramer | ||||||||||
| △GMGredα | 5.17 | 3.84 | 4.32 | 6.07 | – | 100.78 | 66.31 | 62.33 | 112.90 | 140.34 |
| △GMGredα | 4.88 | 3.76 | 4.08 | 4.20 | 4.97 | 99.44 | 64.29 | 68.58 | 79.73 | 66.16 |
| △GMGredβ | 4.69 | 3.80 | 3.70 | 3.79 | 3.97 | 101.72 | 70.54 | 70.74 | 76.50 | 73.64 |
| △GMGredβ | 4.83 | 3.57 | 4.20 | 4.12 | 4.61 | 93.43 | 68.45 | 69.86 | 79.50 | 72.11 |
| F7 | ||||||||||
| △MGGG pentamer | ||||||||||
| △MGGGredα | 5.17 | 3.91 | 4.40 | 6.02 | – | 99.99 | 66.40 | 63.06 | 112.17 | 140.93 |
| △MGGGredβ | 4.74 | 3.89 | 3.73 | 3.94 | 3.96 | 101.54 | 70.14 | 70.71 | 77.24 | 73.60 |
| △MGGGredα | 5.05 | 3.89 | 4.14 | 4.24 | 4.65 | 100.97 | 64.41 | 68.66 | 79.47 | 66.53 |
| △MGGGredα | 5.04 | 3.83 | 3.98 | 4.15 | 4.69 | 100.92 | 64.86 | 68.66 | 79.59 | 66.40 |
| △MGGGredβ | 4.88 | 3.56 | 4.09 | 4.07 | 4.65 | 93.46 | 68.76 | 69.72 | 79.81 | 71.96 |
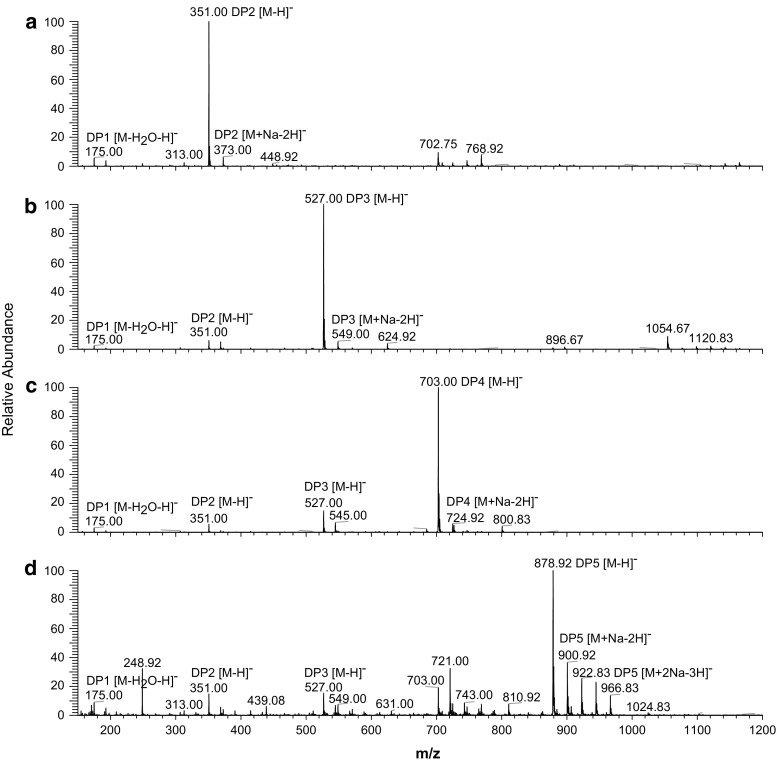
As shown in Fig. 2, the negative-ion mode mass spectra of F1, F3, F5 and F7 displayed signals corresponding to the main deprotonated oligomers [M–H]− (M is the molecular weight of the main solute) and a series of ions with Na, i.e., [M + Na–2H]−, [M + 2Na–3H]−, [M + 4Na–5H]−, etc. The most abundant oligomer in the fractions was consistent with the most abundant deprotonated ion, which indicated that the molecular weights of F1, F3, F5 and F7 were 351.00 (DP2), 527.00 (DP3), 703.00 (DP4) and 878.92 (DP5), respectively (Chaki et al. 2006).
Fig. 2.
Negative-ion ESI mass spectra of oligouronic acid fractions F1, F3, F5 and F7, which were collected by preparative HPLC and then subjected to desalination. DP1-5 refers to the degree of oligomer polymerization. The ESI–MS conditions were as follows: injection volume, 2 F7; ESI voltage, 4 kV; capillary temperature, 275 °C; capillary voltage, 350 V; tube lens, 250 V; scan range, 400–2000 m/z. Nitrogen was used as the sheath gas and auxiliary gas at a flow rate of 30 and 5 arb, respectively. The mobile phase (methanol/water = 1:1, v/v) was delivered at a flow rate of 200 μl/min
Glyceollin induction and determination
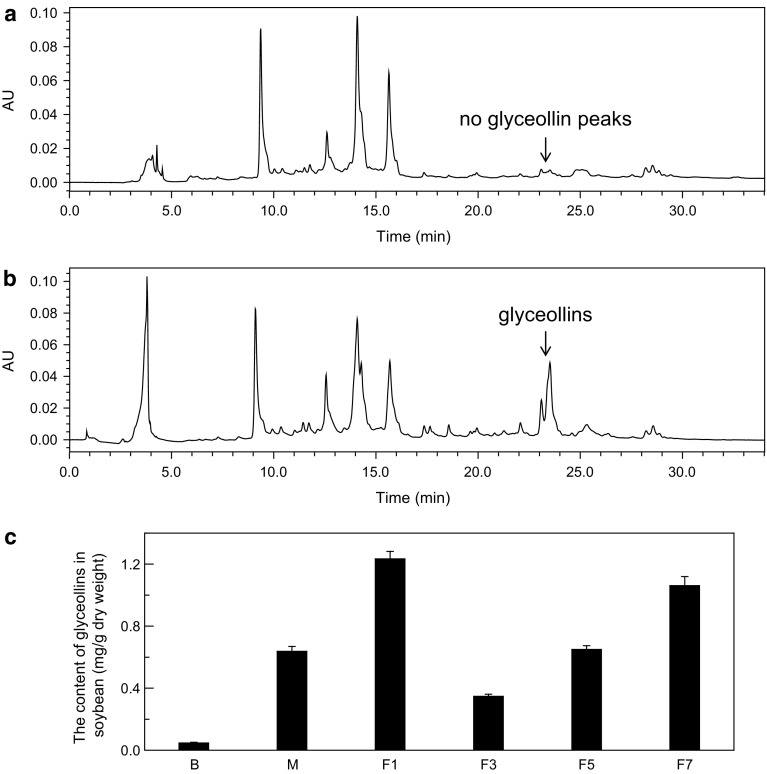
To determine the glyceollin-inducing activities of AOs with different molecular weights and G/M ratios, the glyceollin concentration in soybean cotyledons was calculated by HPLC analysis. As shown in Fig. 3a, b, glyceollins were synthesized after induction by the AO mixture. Glyceollins showed peaks with retention times of 23–24 min, which corresponded to glyceollin I, II and III (Boué et al. 2000). The concentration of glyceollins was calculated according to the method described by Eromosele (Eromosele et al. 2013). Figure 3c shows the glyceollin-inducing activities of the AO mixture and purified AO fractions with different molecular weights and G/M ratios. The glyceollin accumulation levels (mg/g dry weight) induced using the AO mixture, F1 (△G), F3 (△MG), F5 (△GMG) and F7 (△MGGG) were 0.6373, 1.2339, 0.3472, 0.6494 and 1.0611, respectively. Moreover, in our research, the disaccharide △G had outstanding glyceollin-inducing activity.
Fig. 3.
Glyceollin induction by the AO mixtures and purified AO fractions with different molecular weights and G/M ratios. a HPLC chromatogram of 4-day-old uninduced cotyledons showing no glyceollin peaks. b HPLC chromatogram of 4-day-old cotyledons induced by the AO mixture showing glyceollin isomer peaks with retention times of 23–24 min. The chromatograms were obtained by recording the absorbance at 285 nm. c The glyceollin-inducing activities of the AO mixture and purified AO fractions with different molecular weights and G/M ratios. B represents the blank, and M represents the AO mixture. The glyceollin accumulation levels (mg/g dry weight) induced using the AO mixture, F1 (△G), F3 (△MG), F5 (△GMG) and F7 (△MGGG) were 0.6373, 1.2339, 0.3472, 0.6494 and 1.0611, respectively
Discussion
In this research, we characterized 4 AO fractions with degrees of polymerization of 2–5: △G, △MG and △GMG, and △MGGG. These purified fractions were used for the first time as elicitors for glyceollin induction to determine the structure–activity relationship of AOs at the molecular level.
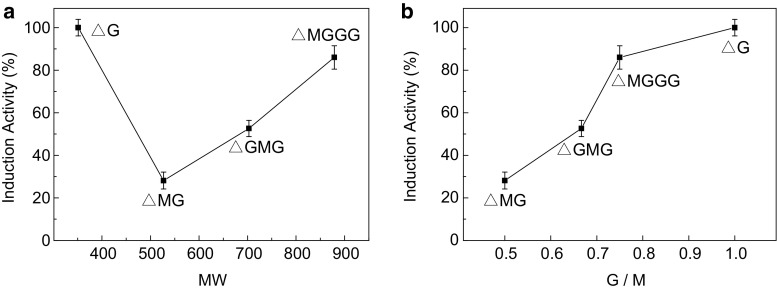
We found that as the molecular weight increased, the tri-, tetra-, and pentasaccharides separated from the AOs mixture had increasing glyceollin-inducing activities in soybeans (Fig. 4a). Such a molecular weight-activity relationship was also observed for the antimicrobial activity of chitosan (Zheng and Zhu 2003) and the growth promotion activity in lettuce seedling roots of pectate oligosaccharide mixtures (Iwasaki and Matsubara 2000).
Fig. 4.
Glyceollin-inducing activities of F1 (△G), F3 (△MG), F5 (△GMG) and F7 (△MGGG). a Glyceollin-inducing activities arranged by molecular weight. b Glyceollin-inducing activities arranged by G/M ratio
Moreover, as shown in Fig. 4b, we found that AOs with a higher G/M ratio induced a higher glyceollin concentration in soybeans, indicating that the glyceollin-inducing activities are related not only to their molecular weight but also to their chemical structures, such as the G/M ratio. This finding is not unique: Küpper found that alginate oligomers with a higher G/M ratio could elicit a higher reactive oxygen species level in the sporophytes of the kelp Laminaria digitata (Küpper et al. 2002). In plants, reactive oxygen species are important in signaling cascades and are continuously produced as byproducts of various metabolic pathways involved in the plant immune system (Apel and Hirt 2004). Glyceollins are the main type of secondary metabolites in soybeans and play crucial roles in plant–microbe interactions (Hai et al. 2010; Landini et al. 2003). Therefore, we propose that the AOs with a higher G/M ratio induce a higher reactive oxygen species level in soybean cotyledons, which may ultimately correspond to a stronger signal for the glyceollin synthesis pathway. To confirm this hypothesis, further studies, as well as large amounts of AOs with different structures, are necessary.
Conclusion
To the best of our knowledge, this is the first report of the different induction capacities of alginate oligosaccharides with different molecular weights and G/M ratios for glyceollin accumulation. Among the 4 AO fractions tested in this research, the alginate disaccharide △G was found to exert the best induction effect. AOs are better elicitors to induce glyceollin biosynthesis in soybeans than metal ions, microorganisms and chemicals because they do not affect the edibility of soybeans and have many human health benefits. Moreover, soybean products with high glyceollin contents can be consumed to confer these health benefits. It is therefore suggested that AOs, especially the alginate disaccharide △G, could be introduced as relatively safe and efficient elicitors to produce soybeans with high glyceollin contents.
Electronic supplementary material
Below is the link to the electronic supplementary material.
1H NMR (500 MHz) spectra of (a) F1, (b) F3, (c) F5 and (d) F7. The experimental temperature was 25 °C. The water peak was 4.70 (EPS 804 kb)
13C NMR (125 MHz) spectra of (a) F1, (b) F3, (c) F5 and (d) F7. The experimental temperature was 25 °C (EPS 704 kb)
2D NMR spectra of F1 (△G) obtained at 500 MHz at 25 °C. (a) COSY, (b) HSQC, and (c) HMBC (EPS 2867 kb)
2D NMR spectra of F3 (△MG) obtained at 500 MHz at 25 °C. (a) COSY, (b) HSQC, and (c) HMBC (EPS 1645 kb)
2D NMR spectra of F5 (△GMG) obtained at 500 MHz at 25 °C. (a) COSY, (b) HSQC, and (c) HMBC (EPS 2008 kb)
2D NMR spectra of F7 (△MGGG) obtained at 500 MHz at 25 °C. (a) COSY, (b) HSQC, and (c) HMBC (EPS 3585 kb)
Acknowledgements
We gratefully acknowledge the moral and financial support received from the National Natural Science Foundation of China (No. 31171628).
Abbreviations
- AO
Alginate oligosaccharide
- G
Guluronic acid
- M
Mannuronic acid
- HPLC
High-performance liquid chromatography
- NMR
Nuclear magnetic resonance
- ESI–MS
Electrospray ionization–mass spectrometry
- SPE
Solid phase extraction
- TFA
Trifluoroacetic acid
- △
O-(4-deoxy-α-l-erythro-hex-4-enopyranosyluronic acid)-(1→
- -G-
→4)-O-(α-l-gulopyranosyluronic acid)-(1→
- -G
→4)-O-β-l-gulopyranuronic acid
- -M-
→4)-O-(β-d-mannopyranuronic acid)-(1→
Compliance with ethical standards
Conflict of interest
There are no conflicts of interest. The author and co-authors alone are responsible for the content and writing of this paper.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3101-6) contains supplementary material, which is available to authorized users.
References
- Akimoto C, Aoyagi H, Dicosmo F, Tanaka H. Synergistic effect of active oxygen species and alginate on chitinase production by Wasabia japonica cells and its application. J Biosci Bioeng. 2000;89:131–137. doi: 10.1016/S1389-1723(00)88726-5. [DOI] [PubMed] [Google Scholar]
- Angelova Z, Georgiev S, Roos W. Elicitation of plants. Biotechnol Biotechnol Equip. 2006;20:72–83. doi: 10.1080/13102818.2006.10817345. [DOI] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya MK, Ward EWB. Resistance, susceptibility and accumulation of glyceollins I–III in soybean organs inoculated with Phytophthora megasperma f. sp. glycinea. Physiol Mol Plant Pathol. 1986;29:227–237. doi: 10.1016/S0048-4059(86)80023-6. [DOI] [Google Scholar]
- Boue SM, Cleveland TE, Carter-Wientjes C, Shih BY, Bhatnagar D, McLachlan JM, Burow ME. Phytoalexin-enriched functional foods. J Agric Food Chem. 2009;57:2614–2622. doi: 10.1021/jf8040403. [DOI] [PubMed] [Google Scholar]
- Boué SM, Carter CH, Ehrlich KC, Cleveland TE. Induction of the soybean phytoalexins coumestrol and glyceollin by Aspergillus. J Agric Food Chem. 2000;48:2167–2172. doi: 10.1021/jf9912809. [DOI] [PubMed] [Google Scholar]
- Chaki T, Kakimi H, Shibata A, Baba T. Detection of alginate oligosaccharides from mollusks. Biosci Biotechnol Biochem. 2006;70:2793–2796. doi: 10.1271/bbb.60313. [DOI] [PubMed] [Google Scholar]
- Chandía NP, Matsuhiro B, Mejías E, Moenne A. Alginic acids in Lessonia vadosa: partial hydrolysis and elicitor properties of the polymannuronic acid fraction. J Appl Phycol. 2004;16:127–133. doi: 10.1023/B:JAPH.0000044778.44193.a8. [DOI] [Google Scholar]
- Eromosele O, Shi B, Liang P. Induction of phytochemical glyceollins accumulation in soybean following treatment with biotic elicitor (Aspergillus oryzae) J Funct Foods. 2013;5:1039–1048. doi: 10.1016/j.jff.2013.02.010. [DOI] [Google Scholar]
- Fett WF, Zacharius RM. Bacterially-induced glyceollin production in soybean cell suspension cultures. Plant Sci Lett. 1982;24:303–309. doi: 10.1016/0304-4211(82)90026-8. [DOI] [Google Scholar]
- Hai D, Huang Y, Tang Y. Genetic and metabolic engineering of isoflavonoid biosynthesis. Appl Microbiol Biotechnol. 2010;86:1293–1312. doi: 10.1007/s00253-010-2512-8. [DOI] [PubMed] [Google Scholar]
- Holtan S, Zhang Q, Strand WI, Skjåk-Braek G. Characterization of the hydrolysis mechanism of polyalternating alginate in weak acid and assignment of the resulting MG-oligosaccharides by NMR spectroscopy and ESI–mass spectrometry. Biomacromolecules. 2006;7:2108–2121. doi: 10.1021/bm050984q. [DOI] [PubMed] [Google Scholar]
- Iwasaki KI, Matsubara Y. Purification of pectate oligosaccharides showing root-growth-promoting activity in lettuce using ultrafiltration and nanofiltration membranes. J Biosci Bioeng. 2000;89:495–497. doi: 10.1016/S1389-1723(00)89104-5. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Mirrahimi A, Srichaikul K, Berryman CE, Wang L, Carleton A, Abdulnour S, Sievenpiper JL, Kendall CW, Kris-Etherton PM. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J Nutr. 2010;140:2302–2311. doi: 10.3945/jn.110.124958. [DOI] [PubMed] [Google Scholar]
- Jia H, Shi B, Eromosele O, Liang P, Li J. Effects of alginate oligosaccharides on the accumulation of glyceollins in soybean. China Agric Sci. 2012;45:1576–1586. [Google Scholar]
- Jochum M, Bakry R, Wartusch I, Huck CW, Engelhardt H, Bonn GK. Analysis of carbohydrates using different quaternized polystyrene-divinylbenzene particles and pulsed amperometric detection. Chromatographia. 2002;56:263–268. doi: 10.1007/BF02491930. [DOI] [Google Scholar]
- Kawada A, Hiura N, Shiraiwa M, Tajima S, Hiruma M, Hara K, Ishibashi A, Takahara H. Stimulation of human keratinocyte growth by alginate oligosaccharides, a possible co-factor for epidermal growth factor in cell culture. FEBS Lett. 1997;408:43–46. doi: 10.1016/S0014-5793(97)00386-4. [DOI] [PubMed] [Google Scholar]
- Kawada A, Hiura N, Tajima S, Takahara H. Alginate oligosaccharides stimulate VEGF-mediated growth and migration of human endothelial cells. Arch Dermatol Res. 1999;291:542–547. doi: 10.1007/s004030050451. [DOI] [PubMed] [Google Scholar]
- Küpper FC, Müller DG, Peters AF, Kloareg B, Potin P. Oligoalginate recognition and oxidative burst play a key role in natural and induced resistance of sporophytes of Laminariales. J Chem Ecol. 2002;28:2057–2081. doi: 10.1023/A:1020706129624. [DOI] [PubMed] [Google Scholar]
- Landini S, Graham MY, Graham TL. Lactofen induces isoflavone accumulation and glyceollin elicitation competency in soybean. Phytochemistry. 2003;62:865–874. doi: 10.1016/S0031-9422(02)00709-4. [DOI] [PubMed] [Google Scholar]
- Ma LJ, Zhang Y, Bu N, Wang SH. Alleviation Effect of alginate-derived oligosaccharides on vicia faba root tip cells damaged by cadmium. Bull Environ Contam Toxicol. 2010;84:161–164. doi: 10.1007/s00128-009-9914-2. [DOI] [PubMed] [Google Scholar]
- Moesta P, Grisebach H. Effects of biotic and abiotic elicitors on phytoalexin metabolism in soybean. Nature. 1980;49:710–711. doi: 10.1038/286710a0. [DOI] [Google Scholar]
- Nagasawa N, Mitomo H, Yoshii F, Kume T. Radiation-induced degradation of sodium alginate. Polym Degrad Stabil. 2000;69:279–285. doi: 10.1016/S0141-3910(00)00070-7. [DOI] [Google Scholar]
- Natsume M, Kamo Y, Hirayama M, Adachi T. Isolation and characterization of alginate-derived oligosaccharides with root growth-promoting activities. Carbohydr Res. 1994;258:187–197. doi: 10.1016/0008-6215(94)84085-7. [DOI] [PubMed] [Google Scholar]
- Pitta Alvarez SI, Spollansky TC, Giulietti AM. The influence of different biotic and abiotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida. Enzyme Microb Technol. 2000;26:252–258. doi: 10.1016/S0141-0229(99)00137-4. [DOI] [PubMed] [Google Scholar]
- Salvo VA, Boué SM, Fonseca JP, Elliott S, Corbitt C, Collins-Burow BM, Curiel TJ, Srivastav SK, Shih BY, Carterwientjes C. Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin Cancer Res. 2006;12:7159–7164. doi: 10.1158/1078-0432.CCR-06-1426. [DOI] [PubMed] [Google Scholar]
- Scherz H, Huck CW, Bonn GK. Electrophoresis carbohydrates. encyclopedia of analytical. Science. 2005;28:433–445. [Google Scholar]
- Schürks N, Wingender J, Flemming HC, Mayer C. Monomer composition and sequence of alginates from Pseudomonas aeruginosa. Int J Biol Macromol. 2002;30:105–111. doi: 10.1016/S0141-8130(02)00002-8. [DOI] [PubMed] [Google Scholar]
- Stintzi A, Weber H, Reymond P, Browse J, Farmer EE. Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc Natl Acad Sci USA. 2001;98:12837–12842. doi: 10.1073/pnas.211311098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stössel P. Regulation by sulfhydryl groups of glyceollin accumulation in soybean hypocotyls. Planta. 1984;160:314–319. doi: 10.1007/BF00393412. [DOI] [PubMed] [Google Scholar]
- Wood CE, Boue SM, Collins-Burow BM, Rhodes LV, Register TC, Cline JM, Dewi FN, Burow ME. Glyceollin-elicited soy protein consumption induces distinct transcriptional effects as compared to standard soy protein. J Agric Food Chem. 2012;60:81–86. doi: 10.1021/jf2034863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon EK, Jeong YT, Li X, Song-Cui PD, Kim YH, Yong DK, Chang HW, Lee SH, Hwang SL. Glyceollin improves endoplasmic reticulum stress-induced insulin resistance through CaMKK-AMPK pathway in L6 myotubes. J Nutr Biochem. 2013;24:1053–1061. doi: 10.1016/j.jnutbio.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Yoshikawa M. Divers mode of action of biotic and abiotic phytoalexin elicitors. Nature. 1978;275:546–547. doi: 10.1038/275546a0. [DOI] [Google Scholar]
- Zhang Z, Yu G, Guan H, Zhao X, Du Y, Jiang X. Preparation and structure elucidation of alginate oligosaccharides degraded by alginate lyase from Vibro sp. 510. Carbohydr Res. 2004;339:1475–1481. doi: 10.1016/j.carres.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yu G, Zhao X, Liu H, Guan H, Lawson AM, Chai W. Sequence analysis of alginate-derived oligosaccharides by negative-ion electrospray tandem mass spectrometry. J Am Soc Mass Spectrom. 2006;17:621–630. doi: 10.1016/j.jasms.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu H, Yin H, Wang W, Zhao X, Du Y. Nitric oxide mediates alginate oligosaccharides-induced root development in wheat (Triticum aestivum L.) Plant Physiol Biochem. 2013;71:49–56. doi: 10.1016/j.plaphy.2013.06.023. [DOI] [PubMed] [Google Scholar]
- Zhang S, Tang W, Jiang L, Hou Y, Yang F, Chen W, Li X. Elicitor activity of algino-oligosaccharide and its potential application in protection of rice plant (Oryza saliva L.) against Magnaporthe grisea. Biotechnol Biotechnol Equip. 2015;29:1–7. doi: 10.1080/13102818.2014.981368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng LY, Zhu JF. Study on antimicrobial activity of chitosan with different molecular weights. Carbohyd Polym. 2003;54:527–530. doi: 10.1016/j.carbpol.2003.07.009. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1H NMR (500 MHz) spectra of (a) F1, (b) F3, (c) F5 and (d) F7. The experimental temperature was 25 °C. The water peak was 4.70 (EPS 804 kb)
13C NMR (125 MHz) spectra of (a) F1, (b) F3, (c) F5 and (d) F7. The experimental temperature was 25 °C (EPS 704 kb)
2D NMR spectra of F1 (△G) obtained at 500 MHz at 25 °C. (a) COSY, (b) HSQC, and (c) HMBC (EPS 2867 kb)
2D NMR spectra of F3 (△MG) obtained at 500 MHz at 25 °C. (a) COSY, (b) HSQC, and (c) HMBC (EPS 1645 kb)
2D NMR spectra of F5 (△GMG) obtained at 500 MHz at 25 °C. (a) COSY, (b) HSQC, and (c) HMBC (EPS 2008 kb)
2D NMR spectra of F7 (△MGGG) obtained at 500 MHz at 25 °C. (a) COSY, (b) HSQC, and (c) HMBC (EPS 3585 kb)