Abstract
Siraitia grosvenorii (Swingle) is one kind of medical and edible plants with various health-promoting properties. Recently, its hypoglycemic and antidiabetic activities have been reported, but the underlying mechanism remains to be explored. The current study was aimed to investigate the antioxidant and antiglycation activities of mogroside extract (MGE) from Siraitia grosvenorii (Swingle). The results showed that compared to glycated BSA, MGE at middle (125 μg/mL) and high dose (500 μg/mL) significantly inhibited BSA glycation evidenced by decreased fluorescent AGEs formation, protein carbonyls and Nε-(carboxymethyl) lysine (CML) level at 500 μg/mL by 58.5, 26.7 and 71.2%, respectively. Additionally, the antiglycative activity of MGE (500 μg/mL) was comparable to aminoguanidine (AG) at the equal concentration. However, the inhibitory effect of MGE on glycation-induced increase of fructosamine level and decrease of thiol level was not remarkable. MGE was a potent peroxide radicals scavenger (851.8 μmol TE/g), moderate DPPH and ABTS radicals scavenger with IC50 1118.1 and 1473.2 μg/mL, respectively, corresponding to positive controls ascorbic acid of IC50 9.6 μg/mL, and trolox of IC50 47.9 μg/mL, respectively, and mild reducing power. These findings suggest that MGE may serve as a new promising antiglycative agent against diabetic complications by inhibiting protein glycation and glycoxidation.
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3105-2) contains supplementary material, which is available to authorized users.
Keywords: Siraitia grosvenorii (Swingle), Cucurbitane triterpene glycosides, Antiglycation, Antioxidant
Introduction
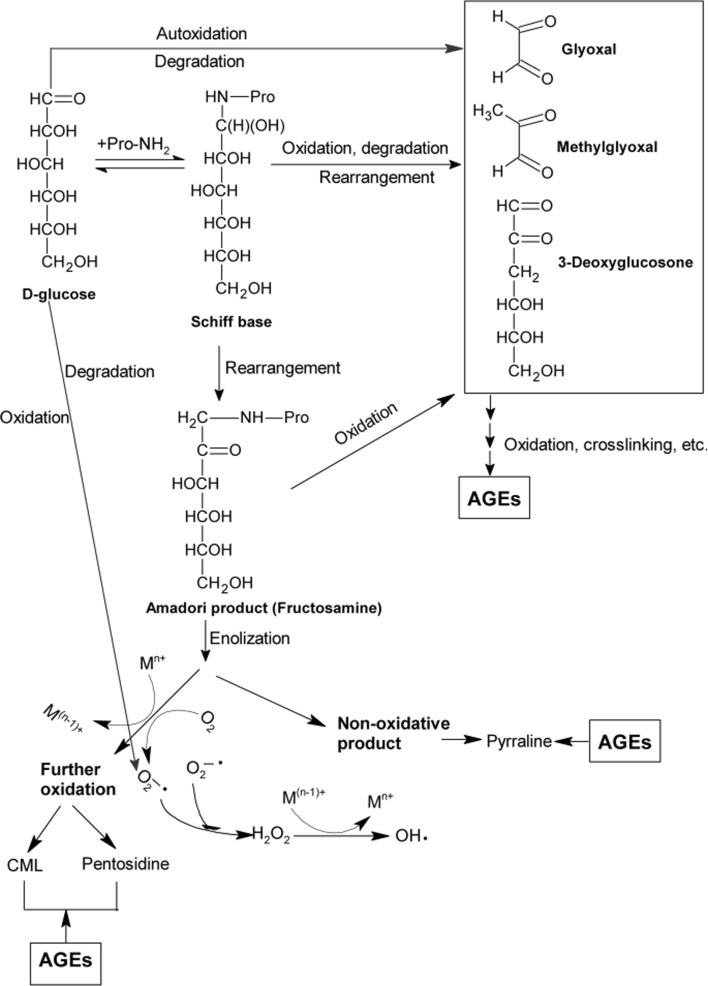
Nonenzymatic glycation reaction, also known as Maillard reaction, occurs between carbonyl groups of monosaccharide and nucleophilic compounds mainly from amino acids, proteins, lipids and nucleic acids (Singh et al. 2001). A complex group of end products, termed as advanced glycation end products (AGEs) are formed through condensation, enolization, dehydration, rearrangement, cyclization, fragmentation, and oxidation reactions during glycation in vitro and in vivo (Fig. 1). Aging and diabetes related hyperglycemia induces an accelerated progression on AGEs formation (Levine and Stadtman 2001). A growing body of evidence indicates that AGEs formation and accumulation is greatly related to microvascular and macrovascular diseases, such as diabetes complications, atherosclerosis and Alzheimer’s disease (Ahmed 2005; Takeda et al. 1996). Thus, AGEs inhibitors are promising therapeutic agents to prevent or alleviate these diseases.
Fig. 1.
General scheme of advanced glycation end products (AGEs) formation pathways in vivo. CML, Nε-(carboxymethyl)lysine; M, transition metal; n, valence of transition metal
Apart from glycation, a large number of studies revealed that reactive oxygen species (ROS) is not only generated by glycoxidation, but also induces and enhances glycation (Hunt et al. 1993; Nagai et al. 1997). Although ROS is not the exclusive mechanism which mediates glycation and AGEs formation, many compounds exhibit powerful antiglycative activity which is mainly ascribed to their ROS-scavenging or antioxidant activities (Aljohi et al. 2016; Jariyapamornkoon et al. 2013). Consequently, compound with potent antioxidant activity may be a promising antiglycative agent. Additionally, the side effects of synthetic AGEs inhibitors, such as aminoguanidine (AG), pyridoxamine, tenilsetam and metformin have been documented. For instance, the side effects and safety concerns of AG in clinical trials have been reported, including gastrointestinal disturbance, liver disorder, flu-like symptoms, rare vasculitis and kidney tumours (Freedman et al. 1999; Thornalley 2003). In light of the fact, much more attention has been drawn to natural origin or food-derived AGEs inhibitors due to minor or no adverse effects. Triterpenoid glycosides characterized as 30-carbon skeletal structure with various glycosyl residues are widely distributed in plant kingdom. Although antioxidant and antiglycative properties of triterpenoid glycosides have not been reported frequently, some specific glycosides or extracts exhibited considerable antioxidant and/or antiglycative activities (Bi et al. 2012; Xi et al. 2010). Mogrosides, principal active components present in Siraitia grosvenorii (Swingle) C. Jeffery, are cucurbitane-type triterpene glycosides and have been granted as GRAS (generally recognized as safe) substances by U.S. Food and Drug Administration with low calorie and extreme sweetness. Recently, antiallergic, anticarcinogenic, and antibacterial activities of mogrosides have been reported (Jin and Lee 2012). Our previous studies showed that mogrosides were effective oxygen radicals scavengers in vitro (Chen et al. 2007) and restored decreased level of antioxidant enzymes in alloxan-induced type 1 diabetic mice model. (Qi et al. 2006, 2008). To our best knowledge, however there are no report on antiglycative activity of mogrosides, and the correlation between antiglycation with its antioxidant properties. Therefore, the present study aimed to explore the antioxidant and antiglycative activities of mogrosides and attempted to clarify the relationship between antiglycation and antioxidation.
Materials and methods
Chemicals and materials
Mogroside extract (MGE) was provided by Guilin Layn Natural Ingredients Corp. Gallic acid, Folin–Ciocalteu’s reagent, bovine serum albumin (BSA), d-(+)-glucose, thioflavin T, l-cysteine and aminoguanidine hydrochloride (AG) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nε-(carboxymethyl) lysine (CML) ELISA kit was purchased from Uscn Life Science, Inc. (Wuhan, China). Fructosamine kit was obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Solvents used for liquid chromatography were of HPLC grade. Water was produced from a Milli-Q purification system (Bedford, MA, USA). Mogroside standards were purchased from Shanghai Tauto Biotech Co., Ltd. (Shanghai, China) or Chengdu Must Biotechnology Co., Ltd. (Chengdu, China). All other reagents were of analytical grade.
In vitro BSA-glucose glycation model
In vitro glycation was performed according to a previous method with minor modifications (Meeprom et al. 2013). Briefly, 0.1 M phosphate buffered saline (PBS), pH 7.4 containing 10 mg/mL BSA, 0.5 M glucose and 0.02% sodium azide without or with 31, 125 and 500 μg/mL MGE was incubated in dark at 37 °C for 4 weeks. Equal amount of BSA, without glucose and MGE, dissolved in the same buffer was prepared as a blank. AG instead of MGE was used as a positive control. Aliquots of these reaction mixtures were taken for determination of AGEs formation, fructosamine level, protein carbonyl and thiol group contents, and amyloid cross-β structure every week. At the last week, CML level was assayed.
AGEs formation
AGEs formation was assayed by measuring fluorescence intensity (FI) of the mixture using an Infinite 200 Pro microplate reader (Tecan, Männedorf, Switzerland) at excitation and emission wavelengths of 355 and 460 nm, respectively. The percent inhibition of AGEs formation was calculated by FI of MGE- or AG-treated group divided by that of BSA-glucose group.
Fructosamine Content
The fructosamine level was analyzed by a commercial kit according to the manufacturer’s protocol. Briefly, 100 μL glycated BSA was incubated with 2.0 mL of 0.5 mM 4-nitro blue tetrazolium (NBT) in 0.1 M, pH 10.4 carbonate buffer at 37 °C for 15 min. The absorbance was measured at 530 nm. The fructosamine level was calculated by calibration curve with 1-deoxy-1-morpholino-fructose (1-DMF) as standard.
Protein carbonyl content
Protein carbonyl group was determined according to the reported method with minor modification (Meeprom et al. 2013). Briefly, 0.8 mL glycated aliquots was added to 3.2 mL 10 mM 2,4-dinitrophenylhydrazine (DNPH) solution (dissolved in 2.0 M hydrochloric acid solution). After incubation in the dark for 1 h, 4.0 mL of 20% (w/v) precooled trichloroacetic acid (TCA) was added to the mixture to precipitate protein and was placed on ice for 5 min. Pellet was collected by centrifugation at 4 °C, 10,000×g for 10 min and washed three times with 2.0 mL of ethanol/ethyl acetate (1:1, v/v) mixture. Finally, it was resuspended in 2.0 mL of 6 M guanidine hydrochloride, and spectrophotometrically measured at 370 nm. The extinction coefficient of DNPH (ε = 2.2 × 104 M−1cm−1) was employed to calculate the carbonyl content (nmol/mg protein) of glycated BSA.
Protein thiol group
Free thiol group of native and modified BSA was measured using earlier method with minor modifications (Meeprom et al. 2013). Briefly, 350 μL of glycated samples were mixed with 650 μL 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) (5 mM) in 0.1 M PBS, pH 7.4 and was left at ambient temperature for 15 min. The absorbance was read at 410 nm and the free thiol concentration of samples was calculated from the l-cysteine standard curve and expressed as nmol thiol group/mg protein.
Amyloid cross-β structure
Amyloid cross-β structure in glycated BSA was measured according to earlier method with slight modifications (Meeprom et al. 2013). Briefly, 2.0 mL of 64 μM thioflavin T in 0.1 M PBS, pH 7.4 was added to 200 μL of the glycated samples and incubated at 25 °C for 60 min. FI was measured at an excitation wavelength and emission wavelength of 435 and 485 nm, respectively. Background FI was subtracted from the same buffer without thioflavin T.
CML level
An enzyme-linked immunosorbant assay (ELISA) kit was applied to determine CML level according to the manufacturer’s protocol.
Oxygen radical absorbance capacity (ORAC) assay
The ORAC assay was performed in a black-walled microplate according to a literature method (Zheng et al. 2012).
DPPH radical scavenging assay
Briefly, 1.0 mL serially diluted MGE aqueous solutions (50 –5000 μg/mL) were added to 3.0 mL DPPH methanolic solution (0.1 mM). The mixture was shaken to mix thoroughly and allowed to stand at room temperature in the dark for 30 min. The absorbance was measured at 517 nm against methanol blank. Distilled water instead of sample was used as control and methanol instead of DPPH solution as sample control for background elimination. Ascorbic acid was used as a positive control. The percent scavenging activity was calculated according to the following equation:
where AC is the absorbance of control (distilled water instead of sample), AS is the absorbance in the presence of the sample, and ASC is the absorbance of sample control (methanol instead of DPPH solution).
ABTS radical scavenging assay
The method reported by Re et al. (1999) was employed with minor modification. Briefly, ABTS radical cation (ABTS·+) stock solution was prepared by mixing 7 mM ABTS aqueous solution with 2.45 mM potassium persulphate, and incubation in the dark at room temperature for 16 h. Prior to use, the ABTS·+ stock solution was diluted to an absorbance of 0.70 ± 0.02 at 734 nm with pH 7.4, 0.1 M sodium phosphate buffer. 0.2 mL sample diluted solutions (50−5000 μg/mL) was mixed well with 3.8 mL diluted ABTS·+ solution and kept in the dark for 6 min before measurement. The absorbance was recorded at 734 nm. Distilled water instead of diluted ABTS·+ solution was used as a blank and trolox as a positive control.
Reducing power assay
The reducing power was determined by the published method with slight modifications (Zheng et al. 2012). 2.0 mL sample aqueous solution (1.0 mg/mL) was mixed with 2.0 mL 0.2 M phosphate buffer (pH 6.6) and 2.0 mL of 0.1% potassium ferricyanide. After incubation at 50 °C for 20 min, 2.0 mL of TCA solution (10%) was added to the mixture. Supernatant was collected by centrifugation at 3000 rpm for 10 min. The supernatant (2.0 mL) was mixed well with equal volume distilled water and 0.4 mL ferric chloride solution (0.3%), and then the absorbance was measured at 700 nm. Ascorbic acid was used as a positive control.
Statistical analysis
All data is obtained from experiments independently performed in triplicate, and is presented as mean ± standard deviation (SD) from triplicate experiments. Difference between groups is analyzed by one-way analysis of variance (ANOVA), followed by Duncan’s post hoc multiple comparisons. P < 0.05 is defined as statistical significance.
Results and discussion
Antioxidant activities of MGE
The chemical composition of MGE was as follows: moisture: 8.69 ± 0.21 g/100 g, total phenolics: 1.43 ± 0.18 g/100 g, total flavonoids: 0.38 ± 0.03 g/100 g, total mogrosides (colorimetric assay): 80.14 ± 0.50 g/100 g. Individual mogrosides were assayed by a HPLC method, and the content of 11-O-mogroside V (11-O-MGV), mogroside V(MG V), mogroside VI (MG VI), mogroside IV (MG IV), mogroside III (MG III) and mogroside IIA2 (MG IIA2) was 7.34 ± 0.16, 44.52 ± 1.33, 4.58 ± 0.45, 0.97 ± 0.05, 0.58 ± 0.03 and 0.32 ± 0.14 g/100 g, respectively (see Electronic Supplementary Material). The results showed that MGE was predominantly composed of mogrosides, particularly MG V, with minor phenolic compounds.
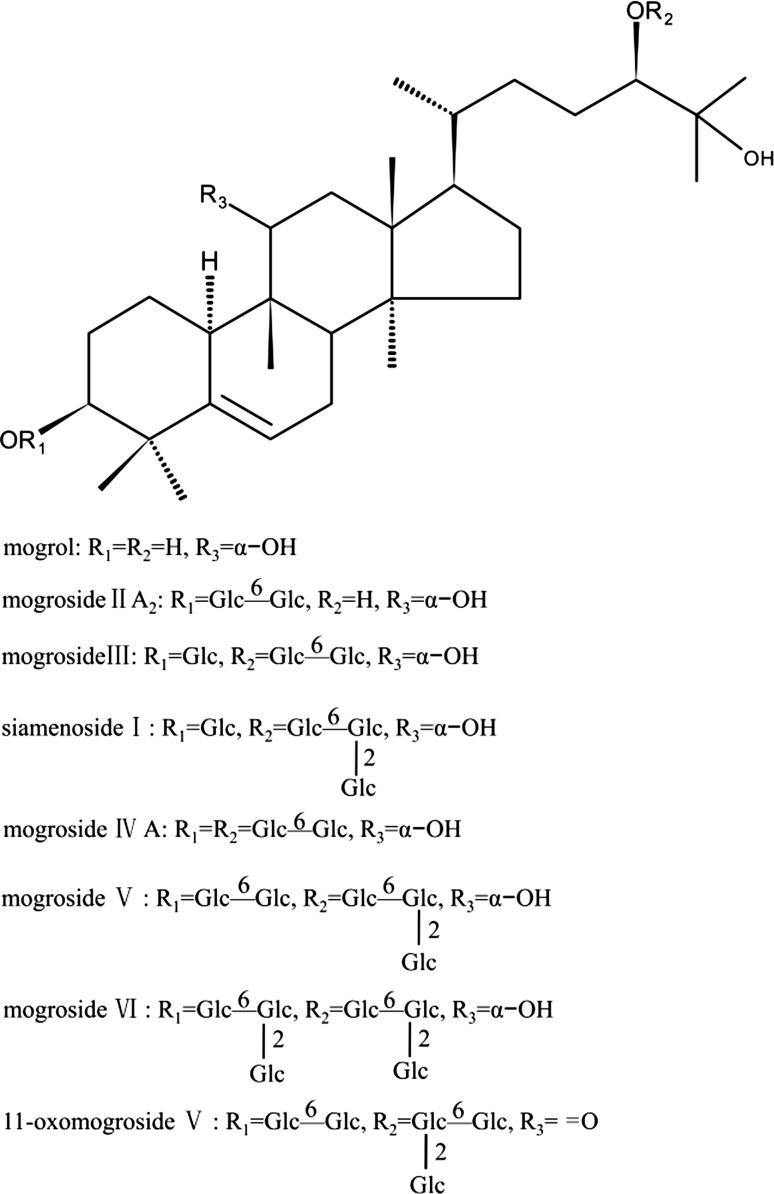
The antioxidant activities of MGE is shown in Table 1. The IC50 of MGE on DPPH and ABTS radicals scavenging assays were 1118.1 and 1473.2 μg/mL, respectively, whereas IC50 of ascorbic acid in DPPH and trolox in ABTS was 9.6 and 47.9 μg/ml, respectively. However, MGE exhibited strong inhibition on peroxyl radicals generated by thermal decomposition of AAPH in ORAC assay. In addition, the data showed that MGE possessed weaker reducing power compared to ascorbic acid. These results indicated that MGE possessed considerable peroxyl radicals-scavenging activities, with moderate DPPH and ABTS radicals-scavenging capacities. Furthermore, the content of total mogrosides was more than 80%, as well as a few amount of phenolic compounds (less than 2%) in MGE. In light of these results, it could be speculated that mogrosides possibly account for the antioxidant activities of MGE, in spite of no direct evidence provided in the study. In fact, a variety of triterpene glycosides with antioxidant or radical-scavenging activities have been recently reported (Allouche et al. 2010; Bi et al. 2012; Xi et al. 2008, 2010). It is noteworthy that these antioxidant terpenoids are mostly pentacyclic triterpene glycosides, with either multiple hydroxyl groups or one hydroxyl group and at least one carboxyl group (Allouche et al. 2010), which may act as an electron or hydrogen donor. However, most types of reported mogrosides, including MG V and 11-O-MG V, are cucurbitane-type tetracyclic triterpene glycosides, and they are not easily prone to provide active electron and hydrogen to quench radicals considering their chemical structures (Fig. 2), which corresponded with weaker DPPH and ABTS radicals-scavenging abilities, compared to ascorbic acid or trolox. On the contrary, ORAC assay showed that MGE was a powerful peroxyl radicals scavenger. This discrepancies may be explained by the synergistic effect of mogrosides with minor phenolics present in MGE or synergistic effect among individual mogrosides with different chemical structures. Actually, Xi et al. (2010) reported that individual triterpene glycosides from Aralia taibaiensis interacted synergistically to enhance free radicals scavenging activity. Another explanation may be that minor polyhydroxy tetracyclic triterpenoids and pentacyclic triterpenoids in MGE, such as 5α, 6α-epoxymogroside I E1, 20-hydroxy-11-oxomogroside I A1, isomultiflorenol and β-amyrin (Jin and Lee 2012) contribute to peroxyl radicals-scavenging activity. These explanations should be studied further.
Table 1.
Antioxidant activities of MGE
| DPPH (IC50, μg/mL) | ABTS (IC50, μg/mL) | ORAC (μmol TE/g MGE) | Reducing power (A700nm)a | |
|---|---|---|---|---|
| MGE | 1118.1 ± 38.6 | 1473.2 ± 85.2 | 851.8 ± 58.7 | 0.387 ± 0.006 |
| Trolox | – | 47.9 ± 2.3 | – | |
| Ascorbic acid | 9.6 ± 1.9 | – | – | 2.850 ± 0.003 |
aAll of the concentrations of MGE, trolox and ascorbic acid are 1.0 mg/mL
Fig. 2.
Chemical structures of major mogrosides from Siraitia grosvenorii
Inhibitory effect of MGE on fluorescent AGEs formation
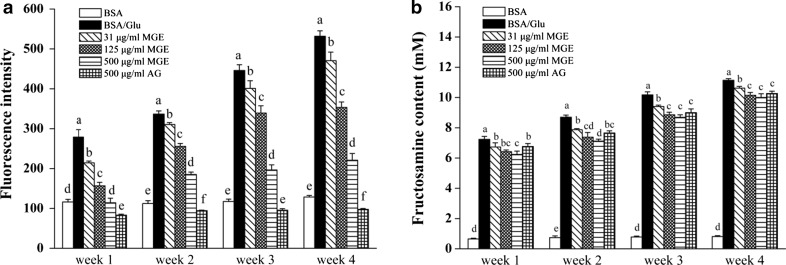
AGEs are a group of complex and heterogeneous compounds with or without fluorescence. Although not all fluorescent compounds formed during protein glycation have been isolated and fully characterized, a variety of fluorescent AGEs such as crossline, pentosidine and pyrrolopyridinium, was proven as fluorophores (Matiacevich et al. 2005; Obayashi et al. 1996). Therefore, specific fluorescence of AGEs has been recommended as an indicator for the formation of AGE-modified proteins (Matiacevich et al. 2005). As observed in Fig. 3a, compared to BSA group, FI of glycated BSA increased greatly with increased incubation time, indicating more fluorescent AGEs formation, while co-incubation with MGE inhibited fluorescent AGEs formation in a dose-dependent manner throughout the experimental period. At the end of experimental period, the percent inhibition of MGE on AGEs formation was 11.6, 33.6 and 58.5% at corresponding concentrations to 31, 125, and 500 μg/mL, respectively, while inhibitory rate of AG was found to 81.6% at 500 μg/mL.
Fig. 3.
Inhibitory effect of MGE on the formation of fluorescent AGEs (a) and fructosamine level (b) in BSA-glucose model. Results are expressed as mean ± SD from triplicate experiments. Different lowercase letters indicated statistical difference between groups (P < 0.05), the same as follow
In view of the structural diversity of reported AGEs inhibitors, various antiglycation mechanisms have been proposed, including scavenging radicals, blocking reactive carbonyls, chelating transition metal ions, inhibiting the formation of Amadori products and breaking cross-link protein structures. With regard to AGEs inhibitor of natural origin, the first three mechanisms are predominant (Wu et al. 2011). Naturally occurring terpenoids, especially triterpene glycosides, are structurally difficult to donate electron or hydrogen to neutralize radicals or oxidants as phenolic compounds act (Chae et al. 2010). Consequently, they are not prone to contribute to antiglycation activity through their radicals-scavenging capacities. Actually, most triterpene glycosides which exhibited strong antiglycation properties perform as either reactive carbonyls blockers or metal ions chelators or both. The study of Chompoo et al. (2011) indicated that aldehyde groups of labdadiene, a diterpenoid from Alpinia zerumbet, blocked active carbonyls and subsequently inhibited AGEs formation, while maslinic acid, a pentacyclic triterpenic acids with vicinal hydroxyl groups at C-2 and C-3 positions, was reported to possess strong copper-chelating activity (Allouche et al. 2010). Although major mogrosides (Fig. 2) are structurally not in agreement with these characteristics described above, MGE exhibited potent antiglycative activity in the present study. The plausible explanation for this is unknown, but it seems that some mogrosides with specific chemical structures, such as carbonyl-containing 11-O-MG V and siraitic acid C, as well as polyhydroxy-containing 5α, 6α-epoxymogroside I E1, present in MGE contribute to antiglycation of MGE. Further studies are needed to confirm it.
Inhibitory effect of MGE on fructosamine
Fructosamine is an oxidative product from Amadori adducts during the early stage of nonenzymatic glycation, and used as a marker for protein glycation (Singh et al. 2001). The inhibition of MGE and AG on fructosamine formation is shown in Fig. 3b. The fructosamine level in BSA-glucose group increased dramatically, while the level in BSA group was very low and almost unchanged throughout the incubation period. In contrast, MGE dose-dependently suppressed the formation of fructosamine, and at week 4, the inhibitory rate of MGE (31−500 μg/mL) on fructosamine formation was 4.7−10.2%, while AG was 7.8% at the concentration of 500 μg/mL, which indicated that MGE was comparable to AG in preventing early protein glycation.
Inhibitory effect of MGE on protein oxidation
Protein oxidation, generated carbonyl groups and deplet free thiols (Singh et al. 2001; Zheng et al. 2016), can not only be mediated by free radicals and/or ROS from monosaccharide autoxidation and glycoxidation (Wolff and Dean 1987), but also by reactive α-oxoaldehydes, such as glyoxal (GO), methylglyoxal (MGO) and 3-deoxyglucosone (3-DG) derived from oxidative degradation of Amadori adducts or from lipid peroxidation.
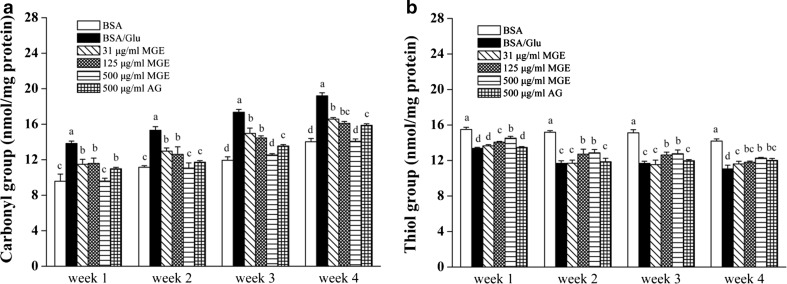
Compared to BSA-glucose group, MGE effectively inhibited carbonyl increase and the effect of MGE at low and middle dose (31 − 125 μg/mL) was comparable to that of AG at 500 μg/mL after 4 week incubation. High dose of MGE was more effective than AG and reversed carbonyl level near to BSA control (Fig. 4a). Likewise, the level of free thiol remarkably decreased in BSA-glucose group compared to BSA control, whereas co-incubation with MGE slightly increased free thiols (Fig. 4b). Increasing carbonylation has been considered as one of the hallmarks of protein oxidation, which is induced by interaction with active carbonyls, mainly from degradation of Amadori products during in vitro glycation. Another hallmark of protein oxidation is depletion of free thiols which are susceptible to oxidative attack by ROS and form adducts with active dicarbonyls (Ahmed 2005; Quiney et al. 2011). It should be noted that AG co-incubation with glucose-BSA mixture for 4 weeks only retained a little more free thiols than glycated BSA (8.6%) (Fig. 4b), although it moderately reduced protein carbonylation (17.3%) (Fig. 4a). It may be explained by the fact that AG exerts anti-glycation effect mainly by neutralizing reactive carbonyls arising from glycoxidation (Thornalley 2003). However, free thiols are depleted by ROS generated from oxidative cleavage of Amadori adducts, which is independent on its carbonyls-trapping property (Glomb and Monnier 1995; Hunt et al. 1993).
Fig. 4.
Inhibitory effect of MGE on protein oxidation in BSA-glucose model. Protein carbonyl level (a) and free thiols level (b)
Inhibitory effect of MGE on CML and amyloid cross-β structure formation
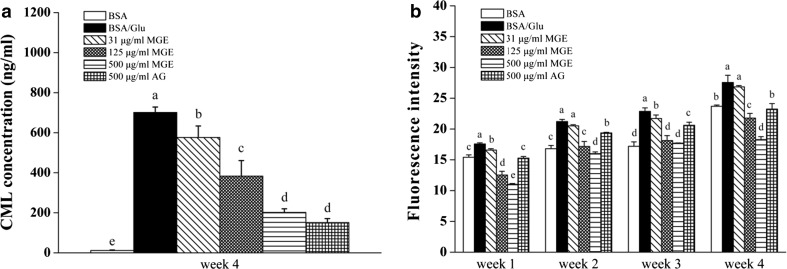
To determine the inhibitory effect of MGE on BSA glycoxidation, non-fluorescent and non-crosslinking CML level was tested by ELISA. As shown in Fig. 5a, CML level of non-glycated BSA was negligible (only 11.70 ng/mL), whereas it rose sharply in glycated BSA (P < 0.05) at the fourth week. AG at 500 μg/mL strongly inhibited CML formation, while MGE treatment dose-dependently reduced CML level and the inhibitory activity of MGE (500 μg/mL) was comparable to AG at 500 μg/mL, suggesting potent inhibition of MGE on CML formation.
Fig. 5.
Inhibitory effect of MGE on CML formation (a) and amyloid cross-β structure (b) in BSA-glucose model
The oxidative cleavage of Amadori products (Odjakova et al. 2012) and Schiff base (Glomb and Monnier 1995), as well as glucose autoxidation-mediated pre-Amadori adducts pathway (Wells-Knecht et al. 1995a, b), are involved in CML formation. Regardless of the three pathways, ROS are involved in the formation of CML (Odjakova et al. 2012). Nagai et al. (1997) found that hydroxyl radical directly accelerated CML formation from Amadori products in vitro. Therefore, it is credible that any compound which possesses strong radicals-, especially hydroxyl radical-scavenging activities, can inhibit CML formation. In the current study, although the scavenging capacity of MGE on hydroxyl radical was not tested, it showed considerable peroxyl radicals and moderate DPPH and ABTS radicals scavenging activities, which may contribute to inhibition of CML (Fig. 5a). Actually, our previous study showed that both MG V and 11-O-MG V were highly reactive to scavenge radicals in chemiluminescence systems, and MG V was more effective than 11-O-MG V on hydroxyl radical, while 11-O-MG V was more potent on hydrogen peroxide and superoxide anion radicals (Chen et al. 2007). Therefore, it can be concluded that the radical-scavenging properties of MGE, at least in part, contribute to its inhibition on CML formation.
Amyloid cross-β structure, as a result of irreversible protein–protein crosslinking and abnormal aggregation, is predominantly drived by nonenzymatic reactions between reactive carbonyls and amino acid residues of protein (Liu et al. 2016). Two effective approaches have been proposed to reduce this crosslinking. One method acts as a carbonyl-quenching agent by inhibiting the formation of amyloid structure, such as AG, and other functions as a dicarbonyl-breaking agent, for example, 4,5-dimethyl-3-phenacylthiazolium chloride (DPTC) by cleaving crosslinked structure (Ulrich and Cerami 2001). As observed in Fig. 5b, compared to non-glycated BSA, glycated BSA exhibited elevated amyloid cross-β structure by 16.3% at the end of this study, while co-incubation with MGE (31−500 μg/mL) reduced the level of amyloid cross-β structure ranging from 2.6−33.6%. Furthermore, treatment with MGE at 125 and 500 μg/mL reversed amyloid cross-β structure near to BSA group, and were more effective than AG at 500 μg/mL. Although the precise antiglycation mechanism needs to be studied further, some carbonyl-containing mogrosides, such as 11-O-MG V (Fig. 2), may be responsible for the antiglycative properties of MGE. It is possible that carbonyl groups present in 11-O-MG V compete with glucose to react with amino groups of protein, which inhibits formation of Schiff base, and/or restricts amino groups to attach glucose (Odjakova et al. 2012). Similarly, carbonyl-containing labdadiene, a diterpenoid compound, showed more antiglycative activity than other diterpenoids without free carbanyl groups (Chompoo et al. 2011).
Conclusion
MGE exhibited considerable antioxidant capacity by scavenging peroxyl radicals and potent antiglycative activities by alleviating glucose-mediated protein glycoxidation and crosslinking in vitro. Although there is no direct evidence that antioxidant properties of MGE contribute to its antiglycation activity, our findings indicate that MGE may be used as a new potential antiglycation agent for treatment of diabetes and its complications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Guilin Layn Natural Ingredients Corp. for generously providing MGE. This work was funded by National Natural Science Foundation of China (No. 31171780), Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Technology and Business University (No. 20171033) and general program from Department of Education of Zhejiang Province, China (Y201738544).
Abbreviations
- 1-DMF
1-Deoxy-1-morpholino-fructose
- 3-DG
3-Deoxyglucosone
- AAPH
2,2′-Azobis (2-amidinopropane) dihydrochloride
- ABTS
2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)
- AG
Aminoguanidine
- AGEs
Advanced glycation end products
- ANOVA
Analysis of variance
- AUC
Area under curve
- BSA
Bovine serum albumin
- CML
Nε-(carboxymethyl)lysine
- DNPH
2,4-Dinitrophenylhydrazine
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- DPTC
4,5-Dimethyl-3-phenacylthiazolium chloride
- DTNB
5,5′-Dithiobis-(2-nitrobenzoic acid)
- ELISA
Enzyme-linked immunosorbent assay
- FI
Fluorescence intensity
- GO
Glyoxal
- GRAS
Generally recognized as safe
- MGE
Mogroside extract
- MGO
Methylglyoxal
- NBT
4-Nitro blue tetrazolium
- ORAC
Oxygen radical absorbance capacity
- PBS
Phosphate buffered saline
- ROS
Reactive oxygen species
- SD
Standard deviation
- TCA
Trichloroacetic acid
- Trolox
6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid
Compliance with ethical standards
Conflict of interest
The authors declare that there are no competing interests.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13197-018-3105-2) contains supplementary material, which is available to authorized users.
References
- Ahmed N. Advanced glycation endproducts—role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Aljohi A, Matou-Nasri S, Ahmed N. Antiglycation and antioxidant properties of Momordica charantia. PLoS ONE. 2016;11:e0159985. doi: 10.1371/journal.pone.0159985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allouche Y, Beltrán G, Gaforio JJ, Uceda M, Mesa MD. Antioxidant and antiatherogenic activities of pentacyclic triterpenic diols and acids. Food Chem Toxicol. 2010;48:2885–2890. doi: 10.1016/j.fct.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Bi L, Tian X, Dou F, Hong L, Tang H, Wang S. New antioxidant and antiglycation active triterpenoid saponins from the root bark of Aralia taibaiensis. Fitoterapia. 2012;83:234–240. doi: 10.1016/j.fitote.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Chae S, Kang KA, Youn U, Park JS, Hyun JW. A comparative study of the potential antioxidant activities of ginsenosides. J Food Biochem. 2010;34:31–43. doi: 10.1111/j.1745-4514.2009.00287.x. [DOI] [Google Scholar]
- Chen WJ, Wang J, Qi XY, Xie BJ. The antioxidant activities of natural sweeteners, mogrosides, from fruits of Siraitia grosvenori. Int J Food Sci Nutr. 2007;58:548–556. doi: 10.1080/09637480701336360. [DOI] [PubMed] [Google Scholar]
- Chompoo J, Upadhyay A, Kishimoto W, Makise T, Tawata S. Advanced glycation end products inhibitors from Alpinia zerumbet rhizomes. Food Chem. 2011;129:709–715. doi: 10.1016/j.foodchem.2011.04.034. [DOI] [PubMed] [Google Scholar]
- Freedman BI, et al. Design and baseline characteristics for the aminoguanidine clinical trial in overt type 2 diabetic nephropathy (ACTION II) Control Clin Trials. 1999;20:493–510. doi: 10.1016/S0197-2456(99)00024-0. [DOI] [PubMed] [Google Scholar]
- Glomb MA, Monnier VM. Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the Maillard Reaction. J Biol Chem. 1995;270:10017–10026. doi: 10.1074/jbc.270.17.10017. [DOI] [PubMed] [Google Scholar]
- Hunt JV, Bottoms MA, Mitchinson MJ. Oxidative alterations in the experimental glycation model of diabetes mellitus are due to protein-glucose adduct oxidation. Some fundamental differences in proposed mechanisms of glucose oxidation and oxidant production. Biochem J. 1993;291:529–535. doi: 10.1042/bj2910529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jariyapamornkoon N, Yibchok-anun S, Adisakwattana S. Inhibition of advanced glycation end products by red grape skin extract and its antioxidant activity. BMC Complement Altern Med. 2013;13:171–179. doi: 10.1186/1472-6882-13-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JS, Lee JH. Phytochemical and pharmacological aspects of Siraitia grosvenorii, luo han kuo. Orient Pharm Exp Med. 2012;12:233–239. doi: 10.1007/s13596-012-0079-x. [DOI] [Google Scholar]
- Levine RL, Stadtman ER. Oxidative modification of proteins during aging. Exp Gerontol. 2001;36:1495–1502. doi: 10.1016/S0531-5565(01)00135-8. [DOI] [PubMed] [Google Scholar]
- Liu F, Teodorowicz M, Wichers HJ, van Boekel MAJS, Hettinga KA. Generation of soluble advanced glycation end products receptor (sRAGE)-binding ligands during extensive heat treatment of whey protein/lactose mixtures is dependent on glycation and aggregation. J Agric Food Chem. 2016;64:6477–6486. doi: 10.1021/acs.jafc.6b02674. [DOI] [PubMed] [Google Scholar]
- Matiacevich SB, Santagapita PR, Buera MP. Fluorescence from the Maillard Reaction and its potential applications in food science. Crit Rev Food Sci Nutr. 2005;45:483–495. doi: 10.1080/10408390591034472. [DOI] [PubMed] [Google Scholar]
- Meeprom A, Sompong W, Chan C, Adisakwattana S. Isoferulic acid, a new anti-glycation agent, inhibits fructose- and glucose-mediated protein glycation in vitro. Molecules. 2013;18:6439–6454. doi: 10.3390/molecules18066439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai R, Ikeda K, Higashi T, Sano H, Jinnouchi Y, Araki T, Horiuchi S. Hydroxyl radical mediates Nϵ-(carboxymethyl)lysine formation from Amadori product. Biochem Biophys Res Commun. 1997;234:167–172. doi: 10.1006/bbrc.1997.6608. [DOI] [PubMed] [Google Scholar]
- Obayashi H, et al. Formation of crossline as a fluorescent advanced glycation end product in vitro and in vivo. Biochem Biophys Res Commun. 1996;226:37–41. doi: 10.1006/bbrc.1996.1308. [DOI] [PubMed] [Google Scholar]
- Odjakova M, Popova E, Sharif MA, Mironova R (2012) Plant-derived agents with anti-glycation activity. Glycosylation. Web. 10.5772/48186
- Qi X, Chen W, Liu L, Yao P, Xie B. Effect of a Siraitia grosvenori extract containing mogrosides on the cellular immune system of type 1 diabetes mellitus mice. Mol Nutr Food Res. 2006;50:732–738. doi: 10.1002/mnfr.200500252. [DOI] [PubMed] [Google Scholar]
- Qi X, Chen W, Zhang L, Xie B. Mogrosides extract from Siraitia grosvenori scavenges free radicals in vitro and lowers oxidative stress, serum glucose, and lipid levels in alloxan-induced diabetic mice. Nutr Res. 2008;28:278–284. doi: 10.1016/j.nutres.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Quiney C, Finnegan S, Groeger G, Cotter TG. Protein oxidation. In: Vidal CJ, editor. Post-translational modifications in health and disease. New York: Springer; 2011. pp. 57–78. [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- Takeda A, et al. Immunohistochemical study of advanced glycation end products in aging and Alzheimer’s disease brain. Neurosci Lett. 1996;221:17–20. doi: 10.1016/S0304-3940(96)13275-4. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys. 2003;419:31–40. doi: 10.1016/j.abb.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW. Identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry. 1995;34:3702–3709. doi: 10.1021/bi00011a027. [DOI] [PubMed] [Google Scholar]
- Wells-Knecht MC, Thorpe SR, Baynes JW. Pathways of formation of glycoxidation products during glycation of collagen. Biochemistry. 1995;34:15134–15141. doi: 10.1021/bi00046a020. [DOI] [PubMed] [Google Scholar]
- Wolff SP, Dean RT. Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem J. 1987;245:243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Huang SM, Lin JA, Yen GC. Inhibition of advanced glycation endproduct formation by foodstuffs. Food Funct. 2011;2:224–234. doi: 10.1039/c1fo10026b. [DOI] [PubMed] [Google Scholar]
- Xi M, Hai C, Tang H, Chen M, Fang K, Liang X. Antioxidant and antiglycation properties of total saponins extracted from traditional Chinese medicine used to treat diabetes mellitus. Phytother Res. 2008;22:228–237. doi: 10.1002/ptr.2297. [DOI] [PubMed] [Google Scholar]
- Xi M, et al. Antioxidant and antiglycation properties of triterpenoid saponins from Aralia taibaiensis traditionally used for treating diabetes mellitus. Redox Rep. 2010;15:20–28. doi: 10.1179/174329210X12650506623041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Su G, Ren J, Gu L, You L, Zhao M. Isolation and characterization of an oxygen radical absorbance activity peptide from defatted peanut meal hydrolysate and its antioxidant properties. J Agric Food Chem. 2012;60:5431–5437. doi: 10.1021/jf3017173. [DOI] [PubMed] [Google Scholar]
- Zheng H, Wu J, Jin Z, Yan LJ. Protein modifications as manifestations of hyperglycemic glucotoxicity in diabetes and its complications. Biochem Insights. 2016;9:1–9. doi: 10.4137/BCI.S36141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.