Abstract
Changes in added polyphosphates throughout the processing of dried salted cod in industrial environment were evaluated. As consumers purchase both dried salted cod and desalted cod, domestic and industrial desalting processes were performed. After brining, total phosphates increased to 11.6 and 16.6 g P2O5/kg in cod processed with 3 and 6% of polyphosphates, respectively. During dry-salting, total phosphates decreased in both cases to ca. 5 g P2O5/kg, suggesting that most polyphosphates were drained with the water released. Cod with polyphosphates needed 85 h extra drying time to achieve regulatory moisture levels. After desalting, total phosphates values in dry weight indicate that phosphates were not removed during this processing step. Free phosphates, and in particular triphosphate contents, were higher in cods desalted following domestic procedures, in comparison with industrial desalting. This study demonstrates that the use of polyphosphates is not recommended for the production of Portuguese dried salted cod. These additives prevent water from being removed during the drying process, increasing the costs. Moreover, in contrast to what was assumed, part of polyphosphates is retained in the final product, even after the desalting process.
Keywords: Phosphates, Triphosphate, Additives, Seafood, bacalhau
Introduction
The use of polyphosphates in wet salted fish was discussed and approved in the European Union (Regulation 1068/2013/EU) at the Standing Committee on the Food Chain and Animal Health meeting, being these authorised since January 1, 2014. The application of phosphates in wet salted fish was requested by producers countries, especially Nordic, allegedly to obtain better quality (avoid oxidation reactions) and appearance (lighter colour), and reduce flavours intensity (Nguyen et al. 2012; SEAFISH 2012). It is allowed the addition of the additives E450 (diphosphates), E451 (triphosphates), and E452 (polyphosphates) to salted fish of the Gadidae family that have been pre-salted by injecting and/or brine salting with an at least 18 g/100 g salt solution and often followed by dry salting (Regulation 1068/2013/EU). The maximum limit level of 5 g P2O5/kg applies to the sum of these additives used individually or in combination (Regulation 1068/2013/EU).
To control the presence/absence of polyphosphates in cod, and also for their quantification, different methodologies can be used. Total phosphorus, which in turn is converted to phosphates, can be determined by the classic spectrophotometric method. Also, ionic chromatography quantifies free water soluble phosphates and is able to distinguish the types of phosphates. In both cases, it is necessary to know the amount of natural phosphates (free orthophosphates and phosphates connected to proteins) to estimate added phosphates.
Nordic countries are the main suppliers of cod, in the form of fresh, frozen or wet salted cod, to the Portuguese industries of dried salted cod, being also competitor producers of cod (wet salted cod). Due to recent legislation changes, cod can be supplied with polyphosphates and thus the availability of polyphosphates-free cod is not assured. In this context, there are concerns from the Portuguese industry of dried salted cod related with the use of polyphosphates in imported cod. These additives improve the water holding capacity, and thus might be a threat to this industry because it may require more drying time and energy costs to achieve the required moisture levels, and hamper the development of the typical colour, texture and taste of cod. Moreover, polyphosphates added by each cod supplier are unknown, as still are undetermined their exact effects on the conditions required to dry the cod with the Portuguese traditional cure process. It is also assumed that polyphosphates are removed during the desalting process.
The dietary phosphate intake and its assimilation are important health issues for the general public. It is known that high phosphates intakes may cause health problems, such as cardiovascular, renal, and a decrease in iron and copper absorption, among others (Bour et al. 1984; Ritz et al. 2012). Phosphates present in food are however not all equal in terms of effect on human health. Organic phosphate esters are the main form of naturally occurring phosphates, being associated with proteins, are slowly hydrolysed in the gastrointestinal tract, and have low absorption (40–60%) (González-Parra et al. 2012; Ritz et al. 2012). In contrast, absorption of inorganic phosphates from additives is very high (> 90%) (González-Parra et al. 2012), and may affect in a higher extension the human health. Controlling the content of added phosphates in food and adequate labelling of its quantity is of great importance for consumers’ health.
A few studies investigated the use of polyphosphates in wet salted cod, in which fillets were treated with these additives in brine or by injection before the salting process and phosphates monitored along the processing chain (Nguyen et al. 2012; Schröder 2010; Thorarinsdottir et al. 2001). In the study of Schröder (2010), Pacific cod was processed in a pilot plant, while the study of Thorarinsdottir et al. (2001) was carried at the laboratory level with Atlantic cod. However, neither study evaluated the different phosphate species. Nguyen et al. (2012) research was also carried out at the laboratory level and the different phosphates species determined in Atlantic cod. Still, these studies do not consider the specific processing of the Portuguese dried salted cod (bacalhau).
Till date, polyphosphates changes have not been evaluated in depth for dried salted cod. Furthermore, is alto taken into consideration that industrywise Atlantic cod is the most important species for the production of dried salted cod in Portugal, being presented as a butterfly cut with spines and skin. The main objective of this study was to evaluate the changes in total and free phosphates in cod at different stages of the industrial processing chain of the Portuguese dried salted cod added with polyphosphates. The aim was to evaluate the impact of added phosphates in the drying process and to evaluate the effect of industrial versus domestic desalting processes.
Materials and methods
Biological material and experimental design
Twenty Atlantic cod (Gadus morhua) were used in the trials developed in an industrial cod processing facility (Riberalves, S.A., Turcifal, Portugal). Gutted and beheaded cod weight ranged between 2 and 3.5 kg. Figure 1 shows the schematic representation of the experimental design. To reduce variability between individuals each cod was divided in two parts, one was used in the control group and the other in one of the polyphosphates treatments. The phosphate blend (BRIFISOL® 512 and 750, ICS Food Specialities, Ladenburg, BK Giulini GmbH) used was a mixture of several sodium and potassium polyphosphates. Salt (NaCl) of food grade (SALDOMAR, Salexpor, Brancanes-Olhão, Portugal) was used to prepare the brine solutions, and also for the dry-salting process. In the control group, cod was immersed in a brine solution of 18 g/100 mL of NaCl. Two concentrations of polyphosphates were tested: 3 and 6% (w/v), each one prepared in a brine solution of 18 g/100 mL of NaCl. Cod was immersed in the brine solutions at a ratio of cod to brine of 1:2 (w:v) during 24 h in refrigerated conditions (7 ± 1 °C).
Fig. 1.
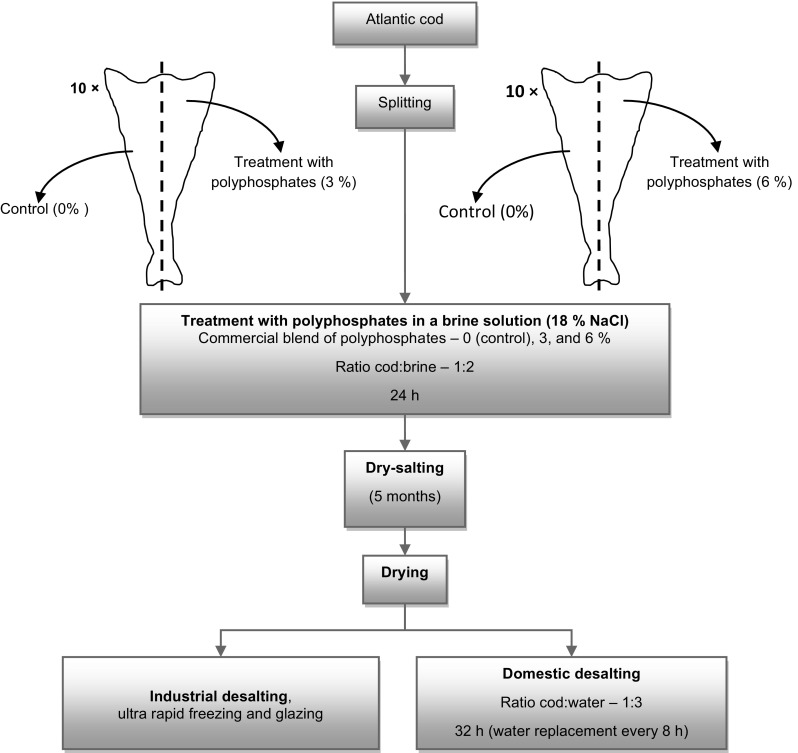
Schematic representation of the experimental design for the study of the effect of polyphosphates through the industrial production of dried salted cod
Dried salted cod with added polyphosphates was produced according to traditional Portuguese processing methods. Briefly, cod was dry salted for 5 months with skin side down and alternated salt layers in a ratio of cod to salt of 2:1 (w:w). Afterwards, the drying process took place in wire trays at 18–24 °C, and a relative humidity of 70% in the beginning, and 40% in the end, during a total of 67 h. Due to the high moisture content of the cod treated with polyphosphates after this period, an extra 85 h drying period was applied. The industrial desalting/rehydration process was performed with continuous cold (5 ± 1 °C) water replacement, until attained a conductivity reference value. Then the cod was frozen by ultra rapid freezing and glazed. The trial in industrial environment took approximately 6 months.
Dried salted cod was also desalted following the domestic procedure during 32 h, with a ratio of cod to water of 1:3 and replacement of water every 8 h.
Sampling
Raw material was sampled before the brining process and in the end of each processing step. Each sample was homogenized and stored at − 20 °C until analysis and measurements were done at least in duplicate.
Cod weight and yield
Cod was weighted throughout the processing steps. Results of changes in weights were presented assuming a standardized initial weight of 1 kg. The yield was calculated as the ratio between the cod weight after and before each processing step, and results presented as percentage.
Chemicals
All chemicals used were analytical grade and were obtained from Sigma-Aldrich (Chemie GmbH, Steinheim, Germany). Deionised water with 18.2 mΩ cm (Millipore Milli-Q water purification system, Millipore S.A.S, 67120 Molsheim, France) was used. Phosphates standards (Sigma-Aldrich) were of the highest available purity as follows: PO4 as sodium phosphate dibasic, P2O7 as sodium pyrophosphate tetrabasic decahydrate, P3O9 as trisodium trimetaphosphate, and P3O10 as sodium triphosphate pentabasic.
Phosphates: spectrophotometric method
Phosphorus was determined according to ISO standard 13730 method (ISO 1996), using UNICAM UV 5 220 spectrophotometer (ATI UNICAM, Cambridge, UK), as described by Lourenço et al. (2009).
Phosphates: chromatographic method
Phosphates were determined according to the method of Nguyen et al. (2012), using high performance ionic chromatography (HPIC). During extract preparation, samples were kept in an ice bath to prevent phosphates degradation. Minced fish muscle (5 g) was weighted and 5 mL of water added. To denature phosphatases, samples were submitted to a microwave treatment (20–25 s, 63% 300 W; Mars 6, CEM corporation, Matthews, NC, USA) according to Dafflon et al. (2003). Then, samples were homogenized using a Polytron homogenizer (10,000 rpm, 1 min; Kinematica, A.G., Luzern, Switzerland), 20 mL of water were added, the mixture was vortexed and centrifuged (5525 × g, 20 min, 4 °C). The supernatant was collected and the pH of an aliquot (4–10 mL) adjusted to 8.5 using potassium hydroxide 0.8 mol/L to prevent phosphates degradation, and then the volume made up to 50 mL. The extract was filtered (0.2 µm pore size), treated with an OnGuard RP-II (Dionex, Sunnyvale, CA 94085-4015, USA), kept in the autosampler of the HPIC (3 ± 1 °C), and injected within 24 h.
Phosphates separation was performed by HPIC in a Dionex ICS-5000+ (Dionex, Sunnyvale, CA 94085-4015, USA) system equipped with a Dionex IonPac AS16 (2 mm × 250 mm) column, a guard column AG16 (2 mm × 50 mm), an anion Dionex Electrolytically Regenerated Suppressor ERS 500 (2 mm), and a Dionex conductivity detector. Chromatographic conditions followed Dionex application notes 71 and 172 (Dionex 2002, 2010). Briefly, separation was done with a 25–100 mmol/L KOH gradient program at 0.25 mL/min for 90 min and column and detector temperature set to 30 °C. Data acquisition was performed with Dionex Chromeleon 7 software.
Phosphates identification and quantification were assessed by comparison with retention times and peak areas of standards within the range 0.5–80 mg/L using cod extract as matrix medium. PO4, P2O7, P3O9, and P3O10 were prepared with H2NaO4P·H2O, Na4O7P2·10H2O, Na3O9P3, and Na5O10P3, respectively. Phosphates contents were expressed as phosphorus pentoxide in g P2O5/kg of product.
Protein
The protein content was determined according to the Dumas method (Saint-Denis and Goupy 2004) in a FP-528 LECO nitrogen analyser (LECO, St. Joseph, USA) calibrated with EDTA (nitrogen—9.57 ± 0.03 g/100 g). A conversion factor of 6.25 was used.
Moisture
Moisture content was obtained by drying the sample (10 g) overnight at 105 °C, as described in the AOAC (AOAC 1990).
pH
The pH was measured directly on minced cod using a calibrated pH insertion electrode (WTW Sentix sp; Weilhein, Germany) connected to a pH meter (WTW 7110 pH meter; Weilhein, Germany).
Statistical analysis
The effect of polyphosphate processing (0, 3, and 6%) and processing steps on total and free phosphates, protein, and moisture contents, and pH were tested with a two-way analysis of variance followed by a multiple comparisons test (Tukey HSD) to identify the differences. The Dunnett test was used to identify differences between the different treatments and the raw material, after one-way ANOVA. In the case of yield and weight changes, one-way analysis of variance followed by Tukey HSD was applied to identify the differences between polyphosphates treatments, and a t test for independent samples was used to study the effect of desalting (industrial versus domestic desalting). All statistical analyses were tested at a 0.05 significance level with the software STATISTICA© from StatSoft Inc. (Tulsa, OK, USA, version 7.0, 2004).
Results and discussion
Raw material
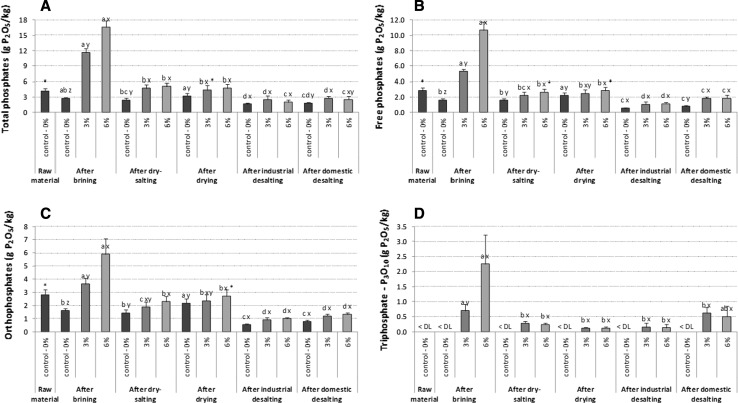
The total phosphate content in the raw material, measured using the spectrophotometric method, was 4.2 ± 0.5 g P2O5/kg, while the free phosphate content analysed by HPIC as PO4 was 2.8 ± 0.4 g P2O5/kg (Fig. 2). Comparable phosphate contents were obtained by Nguyen et al. (2012) in raw Atlantic cod (3.0 mg PO4/g = 2.3 g P2O5/kg; chromatographic method) and by Schröder (2010) in raw Pacific cod (3.6–4.4 g P2O5/kg; spectrophotometric method). Di-, tri- and other polyphosphates more complexes were not detected in the raw material, being all free phosphates in the orthophosphates form (Fig. 2).
Fig. 2.
Total phosphate (A), free phosphate (B), free orthophosphate (C), and free triphosphate (D) contents (g P2O5/kg) in cod treated with polyphosphates through the industrial production of dried salted cod. Data were obtained by the spectrophotometric (A) and chromatographic (B–D) methods. Processing with polyphosphates (3 and 6%) was performed in brining solution with 18 g/100 mL of NaCl. Different letters denote significant differences between processing steps (a–d), polyphosphate batches (x–z), and the absence of * denotes differences with the raw material. < DL—values below detection limit. The number of cods was 20 in control—0%, 10 in 3% of polyphosphates, and 10 in 6% of polyphosphates
The moisture and protein contents and the pH of the cod raw material (Table 1) were within several published results of cod (Oliveira et al. 2012; Schröder 2010; Thorarinsdottir et al. 2001).
Table 1.
Moisture and protein contents and pH in cod treated with polyphosphates through the industrial production of dried salted cod
| Processing steps | Polyphosphates batches | Moisture (%) | Protein (%) | pH |
|---|---|---|---|---|
| Raw material | Control—0% | 82.2 ± 0.6* | 17.6 ± 0.8* | 6.9 ± 0.2* |
| After brining | Control—0% | 79.0 ± 2.6abxy | 13.2 ± 0.8dx | 6.5 ± 0.2ay |
| 3% | 77.4 ± 0.9aby | 13.4 ± 0.6dx | 6.6 ± 0.2ay | |
| 6% | 80.0 ± 0.8ax | 12.7 ± 0.9dx | 7.0 ± 0.1ax | |
| After dry-salting | Control—0% | 59.3 ± 0.4cx | 18.9 ± 0.7bx | 6.0 ± 0.1cx |
| 3% | 59.1 ± 0.6cx | 18.8 ± 1.2cx | 6.0 ± 0.0dxy | |
| 6% | 59.1 ± 0.9cx | 18.6 ± 1.0bcx | 5.9 ± 0.1dy | |
| After drying | Control—0% | 53.5 ± 2.0dy | 24.3 ± 1.4ax | 6.0 ± 0.1cx |
| 3% | 54.9 ± 2.1dxy | 23.0 ± 1.8ay | 5.9 ± 0.0dx | |
| 6% | 55.6 ± 1.0dx | 22.0 ± 1.0ay | 5.9 ± 0.0dx | |
| After industrial desalting | Control—0% | 80.3 ± 1.6ax | 17.2 ± 0.6cx* | 6.4 ± 0.0ax |
| 3% | 79.4 ± 2.1ay | 18.7 ± 0.9cx | 6.4 ± 0.0bx | |
| 6% | 80.3 ± 0.6axy | 18.0 ± 0.6cx* | 6.4 ± 0.0bx | |
| After domestic desalting | Control—0% | 78.3 ± 1.2bx | 18.8 ± 1.3bx | 6.2 ± 0.1bx |
| 3% | 75.8 ± 0.7bx | 20.4 ± 0.9bx | 6.1 ± 0.0cx | |
| 6% | 77.1 ± 0.9bx | 20.0 ± 1.5bx | 6.1 ± 0.1cx |
Processing with polyphosphates (3 and 6%) was performed in brining solution with 18 g/100 mL of NaCl. Different letters denote significant differences between processing steps (a–d), polyphosphate batches (x–y), and the absence of * denotes differences with the raw material. The number of cods was 20 in control—0%, 10 in 3% of polyphosphates, and 10 in 6% of polyphosphates
Polyphosphates blend
The commercial blend was analysed by HPIC and contained a total amount of 57.6 g P2O5/100 g, being 3.8 g P2O5/100 g in the form of orthophosphates, 6.6 g P2O5/100 g as diphosphates, 0.8 g P2O5/100 g as trimetaphosphates, 38.4 g P2O5/100 g as triphosphates (P3O10), and 8.0 g P2O5/100 g as polyphosphates with at least four phosphate groups.
Changes during the brining step
In the control group, the brining process caused a reduction in total phosphates in cod to 2.8 g P2O5/kg (spectrophotometric method) and in free phosphates to 1.6 g P2O5/kg (chromatographic method) (Fig. 2). This decrease is explained by the diffusion of natural phosphates, especially free phosphates, from the cod muscle to the surrounding water.
In the groups treated with polyphosphates, the total phosphate content of cod increased, being higher in the samples processed with 6% of polyphosphates. Results obtained with the spectrophotometric method showed a higher increase, compared with the chromatographic method, although added phosphates were in the free form. In detail, total phosphates increased to levels of 11.6 and 16.6 g P2O5/kg, and free phosphates increased to 5.3 and 10.7 g P2O5/kg in cod with 3 and 6% of polyphosphates, respectively. Added phosphates quantified with the spectrophotometric method reached the legal limit (5 g P2O5/kg) in both groups, while when the chromatographic method is used, the limit level was only reached in the treatment with 6% of polyphosphates.
A previous study reported that a brine with 2% polyphosphates (42 h) led to a phosphate content of 6.0 g P2O5/kg (spectrophotometric method) in Atlantic cod fillets (Thorarinsdottir et al. 2001). Nguyen et al. (2012) obtained ca. of 7 g P2O5/kg (chromatographic method) in cod processed with 3% of polyphosphates in brine for 2 days. Schröder (2010) performed a 4% polyphosphates injection in Pacific cod, but obtained lower phosphate contents (8.1 g P2O5/kg; spectrophotometric method). The divergences observed among studies might be due to differences in the polyphosphates blend, size of cod, species, and/or due to the method (injection versus brine; time of brining) of polyphosphates addition.
Considering the different phosphate species, after brining with 3 and 6% of polyphosphates, the cod muscle presented a higher proportion of orthophosphates (more than 50%) followed by triphosphates (more than 20%) (Fig. 2). In particular, in the 3% treatment the content was 3.6 ± 0.5 g P2O5/kg of orthophosphate, 0.2 ± 0.0 g P2O5/kg of diphosphate, 0.4 ± 0.1 g P2O5/kg of trimetaphosphate, 0.7 ± 0.2 g P2O5/kg of triphosphate, and 0.4 ± 0.2 g P2O5/kg of phosphate with at least four phosphates groups. In the 6% treatment higher amounts were found: 5.9 ± 1.2 g P2O5/kg of orthophosphate; 0.4 ± 0.0 g P2O5/kg of diphosphate, 1.3 ± 0.3 g P2O5/kg of trimetaphosphate, 2.3 ± 1.0 g P2O5/kg of triphosphate, and 0.8 ± 0.3 g P2O5/kg of phosphates with at least four groups of phosphates. The concentration of added phosphates doubled with the increase from 3 to 6% in the brine solution in the case of orthophosphates, diphosphates, and phosphates with at least four groups of phosphates, while triphosphates concentration increased about 3 times. In a previous study, Nguyen et al. (2012) obtained higher diffusion rates in the phosphates with lower molecular weights. The diffusion of phosphates between brine and cod muscle seems to depend not only in the concentrations of the different compounds, but also in their ionic strength, while the high concentration of sodium chloride may have an antagonistic effect in phosphates diffusion (Nguyen et al. 2012). Although these authors suggested that polyphosphates might degrade during the brining process, in the current study there is no clear evidence of such degradation.
The weight and yield of cods changed with the brining step, and the results obtained throughout the industrial processing of dried salted cod are shown in Table 2 and Fig. 3. After brining, the weight of cods increased in all groups, being the 6% treatment significantly different from the control group. Comparable yields were obtained in cod fillets after brining with 2.0–3.0% of polyphosphates in previous studies (Thorarinsdottir et al. 2001, 2004b). The increase in weight might be explained by the incorporation of salt and polyphosphates in the muscle of cod. Thorarinsdottir et al. (2001) determined a salt content of ca. 7.2–8.1 g/100 g in cod fillets after brining (≈ 17.5 g/100 mL NaCl, 42 h) with and without polyphosphates.
Table 2.
Average weight (kg) of cod treated with polyphosphates through the industrial production of dried salted cod
| Treatments | Brining | Dry-salting | 1st drying | 2nd drying | Industrial desalting | Freezing | Glazing | Domestic desalting |
|---|---|---|---|---|---|---|---|---|
| Control—0% (n = 20) |
1.052y | 0.762y | 0.634y | 0.634x | 0.774ax | 0.773x | 0.819x | 0.729bx |
| 3% (n = 10) |
1.061xy | 0.806x | 0.724x | 0.610x | 0.735ax | 0.723x | 0.777x | 0.690ax |
| 6% (n = 10) |
1.087x | 0.813x | 0.733x | 0.623x | 0.760ax | 0.753x | 0.809x | 0.706ax |
Processing with polyphosphates (3 and 6%) was performed in brining solution with 18 g/100 mL of NaCl. Results are presented considering a standardized cod with an initial weight of 1 kg. Standard deviations were lower than 50 g. Different letters denote significant differences between polyphosphate batches (x–y) and between industrial and domestic desalting (a–b)
Fig. 3.
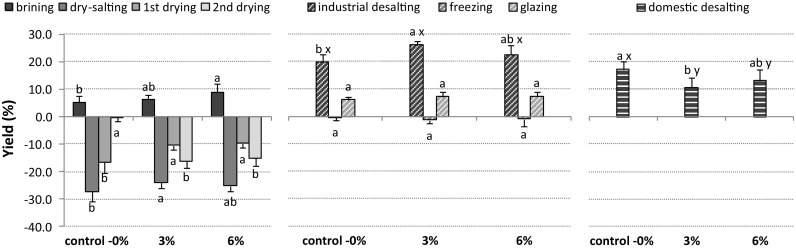
Yield (%) of cod treated with polyphosphates through the industrial production of dried salted cod. Processing with polyphosphates (3 and 6%) was performed in brining solution with 18 g/100 mL of NaCl. Different letters denote significant differences between the polyphosphate batches (a–c) and between industrial and domestic desalting (x–y). The number of cods was 20 in control—0%, 10 in 3% of polyphosphates, and 10 in 6% of polyphosphates
After brining, the moisture content decreased in the control group and in samples treated with polyphosphates (Table 1), which might be explained by the diffusion of water from the cod muscle to the brine due to the different ionic strength between the cod muscle and the brine solution. Comparing the two levels of polyphosphates, it can be noticed that the moisture content was higher in the group with 6% of polyphosphates, which might be due to a higher water retention as a result of the higher amount of polyphosphates.
In a previous study, a 2% polyphosphates brine did not improve the water holding capacity of cod fillets (Thorarinsdottir et al. 2001). Moreover, Schröder (2010) observed that in Pacific cod injected with 4% polyphosphates (with 24 g/100 mL of salt) the moisture content reduced slightly after the treatment, while in control fillets (without polyphosphates) it remained constant. However, the brining step performed after that led to a considerable moisture loss in both treatments (water content reduced from 80% to ca. 75–76%) (Schröder 2010).
During brining, the protein content decreased to values of ca. 13% with no statistical differences between the three groups (Table 1). This can be due to the leaching of soluble proteins in the surrounding water, but also to a dilution effect caused by the incorporation of salt and polyphosphates in cod muscle. Although a similar decrease was observed in the work of Thorarinsdottir et al. (2001) with cod fillets, it was not so marked.
The pH decreased significantly after brining in the control group and in the group processed with 3% of polyphosphates (Table 1). In a previous study, similar pH values were obtained in cod fillets treated with 2% of polyphosphates (Thorarinsdottir et al. 2001). In the group with 6% of polyphosphates, the pH was significantly higher. The presence and the different amount of polyphosphates in the brining solutions can result in different pH values. The changes in the pH values might be due to protein conformational changes caused by the presence of salts (both NaCl and polyphosphates) (Thorarinsdottir et al. 2001).
Changes during the dry-salting step
In the control group, the total and free phosphate contents after dry-salting was similar to that found after brining (Fig. 2). However, considering data in dry weight (not shown), values decreased to half after dry-salting in the control group. In the groups processed with polyphosphates, phosphate content also decreased to 4.7–5.1 g P2O5/kg (spectrophotometric method) and to 2.2–2.6 g P2O5/kg (chromatographic method) (Fig. 2), suggesting that these were drained out. In the dry-salting process, a part of added salt was solubilizes in water that leached and drained out from the fish tissues (Oliveira et al. 2012). Similarly, soluble polyphosphates can also be drained together.
In a previous study, more phosphates were retained (6.1 g P2O5/kg; spectrophotometric method) after dry-salting in Pacific cod fillets injected with 4% of polyphosphates (Schröder 2010). The duration of the dry-salting process might also affect the amount of polyphosphates retained in the muscle of cod. Thorarinsdottir et al. (2001) showed that after dry-salting for only 12–14 days (and storage for 3 weeks), cod fillets presented 3.4 g P2O5/kg (spectrophotometric method) in control samples (1 g P2O5/kg less than in the raw material) and 4.2 g P2O5/kg in the fillets treated with 2% of polyphosphates.
Considering the different phosphate species, orthophosphate contents were similar in the control group before and after dry-salting. In the samples processed with polyphosphates, orthophosphate contents decreased to ca. 1.9–2.3 g P2O5/kg, showing the 6% processed samples significantly higher levels than the control group (Fig. 2). In the case of diphosphate, the contents decreased to undetectable levels, while trimetaphosphate and phosphates with at least 4 phosphate groups decrease to levels below the quantification limits. Triphosphate (P3O10) content decrease to levels of 0.2–0.3 g P2O5/kg in the groups processed with polyphosphates (Fig. 2). In a previous study the content of the different phosphates species did not decrease to such lower levels after dry-salting (Nguyen et al. 2012). However, in this study this processing step was performed in 2 weeks, and not during the 5 months period that is necessary to achieve the maturation required in the traditional Portuguese dried salted cod. Furthermore, Nguyen et al. (2012) also showed that the levels of orthophosphates increased in cod fillets stored up to 6 months as a result of the degradation of long chain phosphates. In the current study, it is not clear that polyphosphates degradation occurred, but that hypothesis is not left aside. In the dry-salting process the drain out of polyphosphates seems to be more relevant in the elimination of the different phosphate species present in cod muscle. Moreover, natural phosphates that are connected with proteins might also degrade and contribute for differences in the free phosphates composition.
As expected, the moisture decreased abruptly after the dry-salting process, and no significant differences were found among groups (Table 1). Similar results were obtained in Pacific cod fillets (Schröder 2010) and Atlantic cod fillets (Thorarinsdottir et al. 2001). The decrease in the water holding capacity of dried salted cod was attributed by Thorarinsdottir et al. (2001) to the protein denaturation at high salt concentration.
Protein content increased to 18.6–18.9% due to draining of water during the dry-salting process, and no differences were found among the different groups (Table 1). This increase was similar to that described earlier by Thorarinsdottir et al. (2001), although different values were observed.
After dry-salting, the pH decreased to values near 5.9–6.0 in all treatments (Table 1). Schröder (2010) also observed a continuous decrease in the pH of Pacific cod throughout the processing chain (from 6.8 in the raw material to 6.2–6.3 in wet salted cod). Thorarinsdottir et al. (2001) also registered a similar pH evolution in Atlantic cod. The increase of salt content in cod muscle, during the dry-salting step, added to a decrease in moisture content seems to be responsible for such variations. In a previous study, the pH of cod also decreased associated with an increase in salt content and decreasing water content (Thorarinsdottir et al. 2004a).
The control group showed the highest weight loss (− 27.3 ± 3.5%) during dry-salting process, and in contrast cod processed with 3% of polyphosphates had the lowest weight loss (− 23.9 ± 2.0%) (Fig. 3). On average, a cod with an initial weight of 1 kg presented higher weight after the dry-salting step in both groups with polyphosphates (Table 2). Higher yields were also reported in cod fillets treated with polyphosphates, when compared with treatments without additives (Thorarinsdottir et al. 2001).
Changes during the drying step
Published works dealing with the addition of polyphosphates to cod did not studied changes induced by the drying process. In Portugal, this processing step gives unique texture characteristics to cod which are extremely appreciated, in such a way that even desalted cod (sold frozen) is dried before the industrial desalting process.
After drying, the total and free phosphate contents increased in the control samples, but not in the samples processed with polyphosphates (Fig. 2). The higher values observed after drying can be due to the lower moisture content of dried cod. Among batches, total phosphate content was significantly higher in the samples processed with polyphosphates (4.4 ± 0.9 and 4.7 ± 0.8 g P2O5/kg in the 3 and 6% treatments, respectively). Regarding free phosphates, their content was only significantly higher in the group processed with 6% of polyphosphates (2.8 ± 0.5 g P2O5/kg), when compared with control samples (2.2 ± 0.4 g P2O5/kg). In particular, the orthophosphate content was higher in control and 3% treatment after drying, in comparison with those observed after dry-salting (Fig. 2). The increase in the orthophosphate content might also be due to the degradation of phosphates, as triphosphates decreased in both treatments with polyphosphates. However, diphosphate contents were bellow the detection limit.
The drying procedure caused a decrease in the moisture content in the control group (Table 1). It was denoted visually that cod treated with polyphosphates were not so dried, because they were very flexible, while those of the control group were rigid. Thus, as expected, the moisture content of cod treated with polyphosphates was higher, being only significantly different in the samples processed with 6% of polyphosphates. Similarly, in the study of Nguyen et al. (2010), performed with a heat pump drying in laboratory, polyphosphates had an effect on decreasing the water mobility in cod samples and a lower drying rate during the first 2 days of drying. The protein content increased during the drying process, and the cod from the groups processed with polyphosphates presented significantly lower protein content than control samples (Table 1). This difference may be explained by the water retention caused by the presence of polyphosphates. The drying step did not change significantly the pH of the cod, independently of the type of processing (Table 1).
The application of polyphosphates showed to have implications in the drying time, which increased from 67 to 152 h. This represents an increase in the energetic costs for the production of cod previously treated with polyphosphates. Additionally, it has also repercussions in the production of dried cods, as the drier during this extra period is not available for other products in the processing line. It also needs to be taken into consideration that in our experiment only a few cod were in these conditions in the industrial drying chamber, together with 10 tons of other cod (without polyphosphates). If a higher proportion of cod was treated with polyphosphates, the moisture content in the products and the humidity levels in the chamber would be considerable higher, and higher drying times would be expected before reaching the characteristic moisture content of dried salted cod. This effect can also be magnified when cods with higher sizes are processed. Thus, the use of polyphosphates in fresh/frozen cod imported for the production of dried salted cod can effectively have detrimental consequences for the Portuguese industry.
As expected, the weight loss after the first drying step (67 h) was higher in the control group, but after the second drying step (extra 85 h), the weight of the cod from the added polyphosphate groups was similar (Table 2).
Changes during the desalting/rehydration
Before cooking, dried salted cod needs to be desalted/rehydrated and this processing step can be done by the industry or at home. The desalting process caused a decrease in total and free phosphate content of the cod in all groups (Fig. 2). In the control group, the values of total and free phosphates were 1.6 ± 0.2 and 0.5 ± 0.1 g P2O5/kg, respectively, after industrial desalting, and 1.8 ± 0.2 and 0.8 ± 0.1 g P2O5/kg, respectively, after domestic desalting.
After industrial desalting, in the samples processed with polyphosphates the total and free phosphate contents were slightly higher, but not significantly different from those observed in the control group. Considering total phosphates, the domestic desalting was as efficient as the industrial one to remove phosphates, but in terms of free phosphates differences were found between the two desalting processes, being the domestic desalting not so efficient. Results indicate that even in the case of an abusive addition of polyphosphates in the brine, the final product (desalted) presents a much lower amount of polyphosphates than that added initially.
Previous studies determined the phosphate content in desalted cod fillets (spectrophotometric method): Thorarinsdottir et al. (2001) obtained ca. 1 g P2O5/kg in desalted cod, in both control and polyphosphates (2.0–2.5%) added samples; Schröder (2010) obtained 2.9 g P2O5/kg in desalted Pacific cod initially injected with 4% of polyphosphates, while control samples had 0.7 g P2O5/kg. The difference in the phosphates contents between these treatments was due, as suggested by the last author, to the injection method that enhanced strong interaction of phosphates to protein of fish muscle.
Considering total phosphates in dry weight, there were no statistical differences between processing steps (before and after desalting) neither among groups. Taking into account the free phosphate content in dry weight, values increased with the domestic desalting, but not with the industrial one. Differences observed in wet weight can be due to the rehydration of cod muscle.
Average orthophosphate content decreased to less than half with the desalting process, but there were no statistical differences in the orthophosphate contents among groups, neither between industrial and domestic desalting processes (Fig. 2). Triphosphates contents were higher in the 3 and 6% processed samples after domestic desalting (ca. 0.5–0.6 g P2O5/kg), when compared with the industrial desalting (ca. 0.1–0.2 g P2O5/kg) (Fig. 2). These triphosphates can be the result of degradation of trimetaphosphate and phosphates with higher number of phosphate groups, which were bellow detection limits. Diphosphates were also bellow detection limit. These results indicate that added polyphosphates were not completely eliminated during the domestic desalting process.
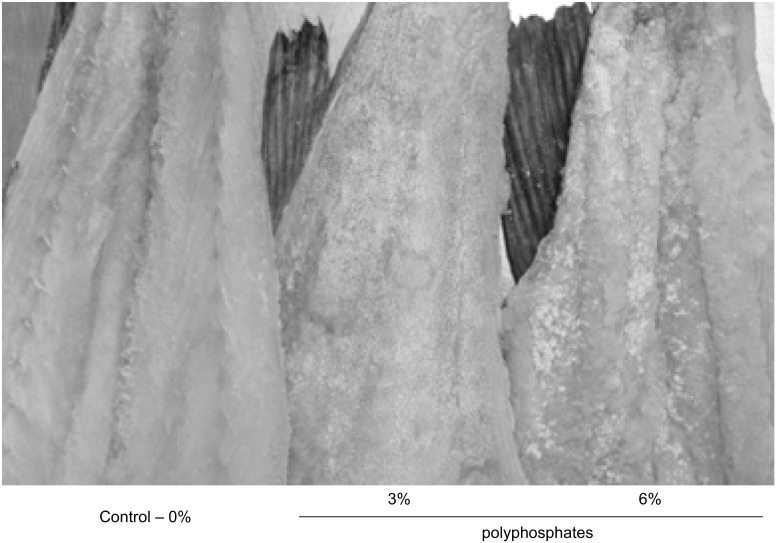
The visual observation of cod showed after domestic desalting, small white dots spread all over the muscle surface in both groups with polyphosphates. In the cod with 3% polyphosphates the dots were regularly dispersed, while in the treatment with 6% polyphosphates small agglomerates of white dots appeared which resulted from the increase in size of the precipitates and their coalescence (Fig. 4). Thorarinsdottir et al. (2001) observed a similar appearance of cod fillets after dry-salting. For evaluation of the precipitates a thin layer (2 mm) of the surface of cod muscle was analyzed. Results revealed the presence of polyphosphates, mainly triphosphates (ca. 85%). The inner muscle of cod presented a phosphate concentration 8 times lower. These results evidence the existence of a gradient accumulation of polyphosphates, but it also confirms that polyphosphates are not completely eliminated with the desalting process and affects negatively the appearance of the products.
Fig. 4.
Aspect of cod processed with polyphosphates (0, 3, and 6%) after the domestic desalting process
The rehydration process increased the moisture contents in cod from all processing methods, but values did not attain those in the raw material (Table 1). Lower water holding capacity after rehydration may have been due to some irreversible degradation of proteins in the salting process (Thorarinsdottir et al. 2001). Moisture content was significantly lower after domestic desalting than after industrial desalting, reflecting once again the lower efficiency of the domestic procedure.
The desalting process resulted in a decrease in the protein content, especially in samples from the industrial desalting, to values closer to those observed in the raw material (Table 1). The differences observed might be explained by the lower efficiency of the domestic desalting process. In a previous study, the protein content in desalted cod fillets not submitted to the drying process decrease to values lower (15–16% of protein) than the initial ones (17.5% of protein), with no significant differences between control and polyphosphates treated samples (Thorarinsdottir et al. 2001).
The desalting process caused an increase in the pH values of cod, being more evident after industrial desalting, when compared with the domestic desalting (Table 1). Among processing methods no significant differences were found. Still, the initial values observed in the raw material were not achieved. Higher values (pH = 6.8) were reported by Schröder (2010), after desalting Pacific cod (not dried).
The cod from the control group presented similar weight changes between industrial and domestic desalting processes (Fig. 3). However, the weight percentage showed higher increase in cod treated with polyphosphates when the desalting process was performed in the industrial environment, in comparison with the domestic desalting. In the industry, the water is continuously renewed in the rehydration tanks either for a fixed period of time or until a conductivity reference value is achieved. This maximizes the salt and water transfer and guarantees a similar salt content among the rehydrated cod. However, in domestic environment, the rehydration of cods is a process somehow intuitive, with differences in the number of water exchanges, time, and temperature, which mainly depends on the familiarity with the desalting process from who performs it and on the height of the cod steak. This assay showed that, for a domestic desalting of 32 h with water replacement every 8 h, the weight increase (in percentage) was lower in the cod treated with polyphosphates, which might indicate that salts were not removed efficiently, and the water uptake was lower. In this sense, the domestic desalting of cod with polyphosphates can lead to different final products.
Regarding the standardized weight of cod through the different processing steps, no major significant differences were found after rehydration between the different groups and the desalting process (domestic versus industrial) (Table 2). In a previous work, Thorarinsdottir et al. (2001) observed a lower yield in cod fillets treated with 2.0–2.5% of polyphosphates after the rehydration process, compared with control samples. Still, those yield values (98–100%) were higher than those observed in the current study. The drying process performed in the current study might have contributed for this difference.
Conclusion
The polyphosphates uptake by cod muscle was influenced by the phosphates concentration in the brine. In the dry-salting processing step, most of the polyphosphates were drained away with water. During drying and desalting, there were no significant variations in the phosphate content in cod muscle. Inclusively, triphosphates were not completely eliminated during the desalting process, being the lower levels obtained a result of the incorporation of water. The industrial desalting process was more efficient than the domestic one.
Although EU-regulation has accepted polyphosphates addition in cod products, their use presents detrimental consequences for the Portuguese dried salted cod industry as a result of the water retention properties of these additives. The costs involved for a proper drying of cods might make vulnerable the sustainability of this industry.
In fact, desalted cod had lower phosphate levels than raw material. However, the presence of triphosphates in the final product indicates that these inorganic phosphates, which are efficiently absorbed by the gastrointestinal tract, might represent a risk to consumers health (Ritz et al. 2012).
Acknowledgements
This study was supported by the National Project POLIFOSFATOS “Use of polyphosphates in the fish processing industry: control of levels and effects of processing” (PROMAR 31.03.01.FEP.0167). Authors also thank to Riberalves, S.A. (Turcifal, Portugal), a Portuguese industry of dried salted cod, by their support in the development of the trials with cod in their facilities. B. Teixeira acknowledges the Portuguese Foundation for Science and Technology (FCT), the European Social Fund (FSE) and the Ministry of Education and Science for supporting a grant (Ref. SFRH/BPD/92929/2013).
References
- AOAC . Offical methods of analysis. 15. Arlington: Association of Official Analytical Chemistry; 1990. [Google Scholar]
- Bour NJS, Soullier BA, Zemel MB. Effect of level and form of phosphorus and level of calcium intake on zinc, iron and copper bioavailability in man. Nutr Res. 1984;4(3):371–379. doi: 10.1016/S0271-5317(84)80098-6. [DOI] [Google Scholar]
- Dafflon O, Scheurer L, Gobet H, Bosset JO. Polyphosphate determination in seafood and processed cheese using high-performance anion exchange chromatography after phosphatase inhibition using microwave heat shock. Mitt Lebensmittelunters Hys. 2003;94(2):127–135. [Google Scholar]
- Dionex (2002) Determination of polyphosphates using ion chromatography with suppressed conductivity detection. Thermo Scientific (Application Note 71):4
- Dionex (2010) Determination of polyphosphates using ion chromatography. Thermo Scientific (Application Update 172):8
- González-Parra E, Gracia-Iguacel C, Egido J, Ortiz A. Phosphorus and nutrition in chronic kidney disease. Int J Nephrol. 2012;2012:597605. doi: 10.1155/2012/597605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO (1996) ISO 13730: meat and meat products—determination of total phosphorus content—spectrometric method. ISO Technical Committee TC 34/SC 6, Geneva
- Lourenço HM, Anacleto P, Afonso C, Martins MF, Carvalho ML, Lino AR, Nunes ML. Chemical characterisation of Nephrops norvegicus from Portuguese coast. J Sci Food Agr. 2009;89(15):2572–2580. doi: 10.1002/jsfa.3754. [DOI] [Google Scholar]
- Nguyen MV, Jonsson A, Gudjonsdottir M, Arason S. Drying kinetics of salted cod in a heat pump dryer as influenced by different salting procedures. As J Food Ag Ind. 2010;4(1):22–30. [Google Scholar]
- Nguyen MV, Jonsson JO, Thorkelsson G, Arason S, Gudmundsdottir A, Thorarinsdottir KA. Quantitative and qualitative changes in added phosphates in cod (Gadus morhua) during salting, storage and rehydration. LWT Food Sci Technol. 2012;47(1):126–132. doi: 10.1016/j.lwt.2011.12.024. [DOI] [Google Scholar]
- Oliveira H, Pedro S, Nunes ML, Costa R, Vaz-Pires P. Processing of salted cod (Gadus spp.): a review. Compr Rev Food Sci F. 2012;11(6):546–564. doi: 10.1111/j.1541-4337.2012.00202.x. [DOI] [Google Scholar]
- Ritz E, Hahn K, Ketteler M, Kuhlmann MK, Mann J. Phosphate additives in food—a health risk. Dtsch Arztebl Int. 2012;109(4):49–55. doi: 10.3238/arztebl.2012.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Denis T, Goupy J. Optimization of a nitrogen analyser based on the Dumas method. Anal Chim Acta. 2004;515(1):191–198. doi: 10.1016/j.aca.2003.10.090. [DOI] [Google Scholar]
- Schröder U. Changes in phosphate and water content during processing of salted pacific cod (Gadus macrocephalus) J Aquat Food Prod T. 2010;19(1):16–25. doi: 10.1080/10498850903445597. [DOI] [Google Scholar]
- SEAFISH (2012) Review of polyphosphates as additives and testing methods for them in scallops and prawns. Campden BRI report: BC-REP-125846-01
- Thorarinsdottir KA, Arason S, Bogason SG, Kristbergsson K. Effects of phosphate on yield, quality, and water-holding capacity in the processing of salted cod (Gadus morhua) J Food Sci. 2001;66(6):821–826. doi: 10.1111/j.1365-2621.2001.tb15180.x. [DOI] [Google Scholar]
- Thorarinsdottir KA, Arason S, Bogason SG, Kristbergsson K. The effects of various salt concentrations during brine curing of cod (Gadus morhua) Int J Food Sci Tech. 2004;39(1):79–89. doi: 10.1046/j.0950-5423.2003.00757.x. [DOI] [Google Scholar]
- Thorarinsdottir KA, Gudmundsdottir G, Arason S, Thorkelsson G, Kristbergsson K. Effects of added salt, phosphates, and proteins on the chemical and physicochemical characteristics of frozen cod (Gadus morhua) fillets. J Food Sci. 2004;69(4):SNQ144–SNQ152. [Google Scholar]