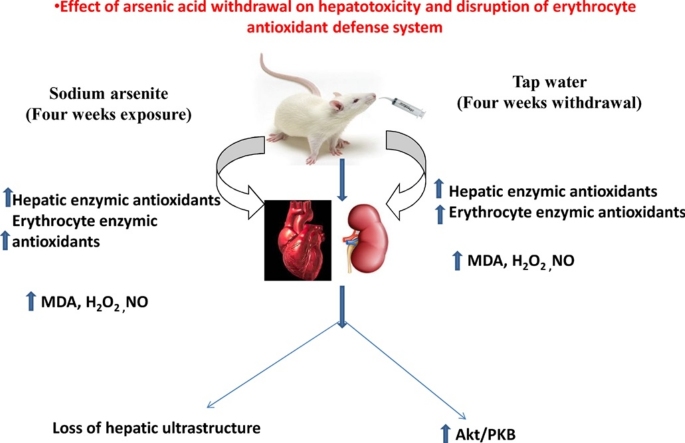
Graphical abstract
Keywords: Liver, Erythrocyte, Arsenic acid, Hepatotoxicity, Oxidative stress, Akt/PKB signaling
Highlights
-
•
Arsenic acid increased both hepatic and erythrocyte enzymic and non-enzymic antioxidant defense system following exposure.
-
•
Arsenic acid induced hepatotoxicity via free radical generation and oxidative stress.
-
•
Hepatic antioxidant defense system partially recovered after withdrawal of the exposed arsenic acid.
-
•
Arsenic acid toxicity enhanced protein kinase B (Akt/PKB) signaling pathway.
Abstract
We investigated the effects of withdrawal from Sodium arsenite (NaAsO2) on the hepatic and antioxidant defense system in male Wistar rats using a before and after toxicant design. Rats were orally gavaged daily with varying doses of NaAsO2 for a period of 4 weeks. One half of the population was sacrificed and the remaining half had the toxicant withdrawn for another further 4 weeks. Biochemical and immunohistochemical techniques were used to assess the impact of withdrawal on the erythrocyte and hepatic systems. Exposure of Wistar rats to NaASO2 led to a significant (p < 0.05) increase in hepatic and erythrocyte markers of oxidative stress (malondialdehyde, thiol contents and hydrogen peroxide generation). Concurrently, there was a significant (p < 0.05) increase in hepatic and erythrocyte antioxidant enzymes (glutathione-S-transferase, glutathione peroxidase and superoxide dismutase) following exposure. Withdrawal from NaAsO2 exposure led to a decline in both erythrocyte and hepatic markers of oxidative stress and together with a significant improvement in antioxidant defense system. Histopathology and immunohistochemistry revealed varying degrees of recovery in hepatocyte ultrastructure alongside increased expression of the pro-survival protein Kinase B (Akt/PKB) after 4 weeks of NaAsO2 withdrawal. Conclusively, withdrawal from exposure led to a partial recovery from oxidative stress-mediated hepatotoxicity and derangements in erythrocyte antioxidant system through Akt/PKB pathway.
1. Introduction
Of the many naturally occurring elements found abundantly distributed in the earth’s crust, arsenic has found its way into prominence as a toxicant of significant public health risk [1]. A heavy metal, specifically classed as a metalloid [2], it is naturally produced during processes such as volcanic eruptions and the biodegradation of other organic minerals and rocks [3]. Increased dependency on arsenic among other heavy metals for anthropogenic causes has resulted in widespread release of arsenic by-products into the environment [4]. Human exposure is then unavoidable, not just from occupational causes, but also from atmospheric pollution, ingestion of contaminated food and water sources and from contact with certain finished industrial products [5].
Of the routes to exposure, the most important source, by far appears to be dietary. Tchounwou et al. [1] report that each individual has an average intake of 50 μg per day. Recent studies show a rise in the levels of inorganic arsenic in food items, especially rice, a staple of third world and underdeveloped countries [6], [7], [8]. Arsenic was found to accumulate more in the liver than other tissues after one month of exposure, when administered orally and subcutaneously. However, after 3 months of exposure, was found to accumulate more in the kidney than in the liver or other tissues. [9]. With a high correlation established between exposure and increased health risks, and an uptick in the incidence of health-related conditions among affected populations, it is no wonder arsenic is listed as one of the high malignancy causing elements today [10], [11].
Certain heavy metals as cobalt and copper have been classified as essentials due to their absolute requirements in trace quantities for normal biochemical and physiological functions in the human body [12]. Arsenic, on the other hand, has been found to have no beneficial physiologic role in the human body and is classified as a non-essential metal [13]. Even at low exposure levels, it has been shown to cause multiple organ damage by generating reactive oxygen species (ROS) and promoting oxidative stress: a redox deficiency or imbalance that can cause bimolecular damage [2], [14], disrupting cellular structures and components [15], affecting detoxification and repair enzymes [16], causing DNA damage, disrupting normal cell cycle regulation and in extreme cases, cancer and cell death [17].
Free radicals are produced in living organisms during normal metabolism (e.g., the reactions of mitochondrial respiratory chain and cytochrome P450), inflammation, phagocytosis and other physiological processes. The most important category of free radicals is constituted by ROS, such as superoxide radical anion (O2•−), hydroxyl radical (OH•), peroxyl radical (RO•2) and hydrogen peroxide (H2O2) [18]. The mechanisms of liver toxicity and damage in chronic arsenic toxicity are primarily a consequence of increased free radical activity and oxidative stress [19].
From this point, two possible routes to hepatic damage have been reported: The activation of kinase signaling molecules such as C-Jun N-terminal kinases (JNK), p38 Mitogen activated protein kinase (p38 MAPK) and cytochrome P450, leading to cell lysis, apoptosis and accumulation of bile acids [20], [21], or increased peroxidation of lipids which also cause hepatocyte damage [22]. It has been reported that in erythrocytes, NaAsO2 can absorb into blood circulation and bind to hemoglobin. This might lead to the oxidation of sulfhydryl groups, which are critical for heme synthesis, with a concomitant reduction in oxygen uptake capabilities [19]. Also, significant decreases are triggered in erythrocyte reduced glutathione levels, coupled with increases in malondialdehyde and protein carbonyl levels, all indicative of oxidative damage to erythrocyte membranes. Grossly, this is evident as distorted, misshapen erythrocytes [23].
In poor resource regions of the world where government regulatory mechanisms are weakened or virtually non-existent, and where indiscriminate dumping of heavy metals and their metabolites occur on a daily basis, the dangers to health from arsenic exposure persist. We explore to see the effects of withdrawal from exposure on the known disease states arising from the multi-organ damage associated with this toxicant.
2. Materials and methods
2.1. Chemicals
Epinephrine, NaAsO2, Hydrogen peroxide (H2O2), hydrochloric acid, sulphuric acid, xylenol orange, sodium hydroxide, potassium iodide, reduced glutathione (GSH), potassium dichromate, O-dianisidine, sodium potassium tartarate, copper sulphate, glacial acetic acid, ethanol, sodium azide, 2-dichloro-4-nitrobenzene (CDNB), thiobarbituric acid (TBA), Trichloroacetic acid, Ellman’s reagent (DTNB), ammonium ferrous sulphate, sorbitol was purchased from Sigma (St Louis, MO, USA). Normal goat serum, Biotinylated antibody and Horse Radish Peroxidase (HRP) System was purchased from (KPL, Inc., Gaithersburg, Maryland, USA). Akt/PKB antibody was purchased from (Bioss Inc. Woburn, Massachusetts, USA) while 3, 3′-Diaminobenzidine (DAB) tablets were purchased from (AMRESCO LLC. Ohio, USA). All other chemicals used were of analytical grade and obtained from British Drug Houses (Poole, Dorset, UK).
2.2. Experiment and design
80 healthy adult male Wistar rats (200–250 g) obtained from the Experimental animal unit of the Faculty of Veterinary Medicine, University of Ibadan were used for this study. The animals were handled humanely according to the criteria outlined in the ‘Guide for the Care and Use of Laboratory Animals’ prepared by the National Academy of Science and published by the National Institute of Health. Ethical regulations were followed in accordance with national and institutional guidelines for the protection of animal welfare during experiments [24].
All animals were kept in wire mesh cages under controlled light cycle (12 h light/12 h dark) and temperature of 32 ± 3 °C. The rats were fed with commercial rat chow (Ladokun Feeds Nigeria Limited) ad libitum and liberally supplied with clean tap water. Randomly, they were assigned into four groups comprising twenty animals per group. Group A was provided tap water with no test substance added. Group B was treated with tap water into which NaAsO2 was dissolved at 10 mg/kg/day; Group C was treated with tap water into which NaAsO2 was dissolved at 20 mg/kg/day; Group D was treated with tap water into which NaAsO2 was dissolved at 40 mg/kg/day.
One half of the population was sacrificed at the end of 4 weeks of NaAsO2 exposure. NaAsO2 was withdrawn from the remaining rats and they were administered clean tap water only for another 4 weeks. The rats were monitored weekly for clinical signs of toxicity. After four weeks, the remaining animals were sacrificed. For each phase of sacrifice, the rats were starved overnight and the following procedure was observed. Blood was drawn from the retro-orbital venous plexus of the animals into vials containing heparin as an anticoagulant. The animals were then sacrificed by cervical dislocation. The liver was removed, rinsed in 1.15% KCl and homogenized in aqueous potassium phosphate buffer (0.1 M, pH 7.4). The homogenates were centrifuged at 10,000g for 20 min to obtain the supernatant fraction stored at 4 °C till use.
2.3. Preparation of erythrocytes for biochemical assays
The preparation of erythrocytes was described according to the method of Steck and Kant [25]. The erythrocytes were washed three times with ice-cold phosphate buffer saline at pH 7.4, and centrifuged. The erythrocyte membrane was lysed and the pellets were resuspended in PBS at 1:109 dilutions until the time of use. The pellets thus obtained were washed repeatedly in the same buffer to obtain haemoglobin-free white membranes.
2.4. Biochemical assays and hematological parameters
Protein concentration was carried out as described by Gornall et al. [26]. Hydrogen peroxide generation was evaluated as described by Wolff [27]. The malondialdehyde concentration was determined according to Farombi et al. [28]. Nitric oxide was quantified as described by Olaleye et al. [29], while the thiol contents were estimated by the method of Ellman [30].
The erythrocyte and hepatic reduced glutathione (GSH) concentration was determined using the method of Jollow et al. [31]. The superoxide dismutase (SOD) activity was evaluated by the method of Misra and Fridovich [32] with slight modification [33]. Glutathione peroxidase (GPx) and Glutathione-S-transferase (GST) activities were measured by the method of Rotruck et al. [34] and Habig et al. [35], respectively. The packed cell volume (PCV) was determined by microhaematocrit method. The haemoglobin (Hb) concentration was determined by Cyanmethaemoglobin method while red blood cell (RBC) and white blood cell (WBC) counts were determined using an haemocytometer.
2.5. Histopathology
Small pieces of liver tissues were collected in 10% buffered formalin for proper fixation. These tissues were processed and embedded in paraffin wax. Sections of 5–6 μm in thickness were made and stained with haematoxylin and eosin for histopathological examination [36].
2.6. Immunohistochemistry of protein kinase B
Immunohistochemistry of paraffin embedded tissue of the liver was performed after the tissues were obtained from buffered formalin perfused rats. The paraffin sections were melted at 60 °C in the oven. Dewaxing of the samples in xylene was followed by passage through ethanol of decreasing concentration (100-80%). Peroxidase quenching in 3% H2O2/methanol was carried out with subsequent antigen retrieval performed by microwave heating in 0.01 M citrate buffer (pH 6.0) to boil. All the sections were blocked in normal goat serum (10%, HistoMarkR, KPL, Gaithersburg MD, USA) and probed with PKB antibody (AbclonalR, Baltimore, MD, USA), 1:200 overnight at 4 °C in a refrigerator. Detection of bound antibody was carried out using biotinylated (goat anti-rabbit, 2.0 μg/ml) secondary antibody and subsequently, streptavidin peroxidase (Horse Radish Peroxidase-streptavidin system) according to manufacturer’s protocol (HistoMarkR, KPL, Gaithersburg MD, USA).
Reaction product was enhanced with diaminobenzidine (DAB, AmrescoR, USA) for 6–10 min and counterstained with high definition hematoxylin (EnzoR, NY − USA), with subsequent dehydration in ethanol. The slides were covered with coverslips and sealed with DPx mountant. The immunoreactive positive expression of intensive regions were viewed starting from low magnification on each slice then with 100 × magnifications using a photo microscope (Olympus) and a digital camera (ToupcamR, Touptek Photonics, Zhejiang, China).
2.7. Statistical analysis
All values are expressed in mean ± S.D. Parameters obtained from the experimental groups were compared with the control. The data obtained were analyzed using repeated measures one-way analysis of variance (ANOVA) with the Tukey post-hoc analysis for the analysis of scientific data using GraphPad Prism® 5.01 Values were considered statistically significant at p < 0.05.
3. Results
3.1. Hepatic antioxidant status and markers of oxidative stress following NaAsO2 exposure and withdrawal
Rats exposed to NaAsO2 showed significant (p < 0.05) reduction of hepatic total protein (Table 1). Concurrently, there were significant (p < 0.05) increases in thiol, malondialdehyde (MDA), hydrogen peroxide (H2O2) generated and nitric oxide levels following exposure at 10 mg/kg, 20 mg/kg and 40 mg/kg of NaAsO2 (Table 1). These markers of oxidative levels were normalized after the withdrawal of NaAsO2 (Table 1). On the other hand, the activities of anti-oxidant enzymes including glutathione peroxidase (GPx), glutathione-S-transferase (GST) and superoxide dismutase (SOD) were observed to increase significantly (p < 0.05) following exposure to NaAsO2 at 10 mg/kg, 20 mg/kg and 40 mg/kg, respectively (Table 2). Furthermore, upon NaAsO2 withdrawal, there was a significant increase in the activity of hepatic GPx might be due to adaptive response (Table 2).
Table 1.
Liver total protein and markers of oxidative stress.
| Experimental groups | Group A | Group B | Group C | Group D | |
|---|---|---|---|---|---|
| Total Protein | Exposure | 2.64 ± 0.51 | 2.23 ± 0.24 | 2.09 ± 0.19* | 2.09 ± 0.16* |
| Withdrawal | 2.56 ± 0.24 | 2.53 ± 0.31 | 2.47 ± 0.33 | 2.43 ± 0.26 | |
| Total Thiol | Exposure | 73.76 ± 4.81 | 95.83 ± 4.88* | 86.12 ± 5.48* | 85.51 ± 4.92* |
| Withdrawal | 64.62 ± 8.13 | 45.21 ± 5.32* | 63.90 ± 4.12 | 61.62 ± 8.02 | |
| Nitric Oxide | Exposure | 3.41 ± 0.23 | 4.21 ± 0.24* | 4.29 ± 0.30* | 4.65 ± 0.25* |
| Withdrawal | 2.88 ± 0.51 | 1.82 ± 0.26* | 2.92 ± 0.25 | 2.45 ± 0.23 | |
| MDA | Exposure | 0.17 ± 0.02 | 0.28 ± 0.03* | 0.44 ± 0.04* | 0.45 ± 0.02* |
| Withdrawal | 0.09 ± 0.02 | 0.11 ± 0.01 | 0.10 ± 0.02 | 0.10 ± 0.02 | |
| H2O2 generated | Exposure | 21.54 ± 0.38 | 23.53 ± 0.86* | 24.89 ± 1.46* | 25.29 ± 1.43* |
| Withdrawal | 26.38 ± 3.44 | 27.03 ± 3.74 | 26.23 ± 2.13 | 27.08 ± 2.75 | |
Values are presented as mean ± standard deviation.
Group A (Control), Group B (Sodium arsenic (10 mg/kg; p.o.), Group C (Sodium arsenic (20 mg/kg; p.o.) Group D (Sodium arsenic (40 mg/kg; p.o.).
Asterisk (*) Indicates significant difference from control at p < 0.05 in each row. Total protein (Mg/dl), H2O2 (hydrogen peroxide generation; μmol/mg protein), MDA (malondialdehyde; μmol of MDA formed/mg protein), Nitric oxide (μmol/mg protein) Total thiol (μmol/L).
Table 2.
Liver antioxidant enzyme profile following exposure and withdrawal of Sodium Arsenite.
| Experimental groups | Group A | Group B | Group C | Group D | |
|---|---|---|---|---|---|
| GSH | Exposure | 61.71 ± 3.75 | 60.60 ± 2.75 | 61.79 ± 2.27 | 59.96 ± 1.31 |
| Withdrawal | 52.30 ± 2.00 | 50.91 ± 1.64 | 53.33 ± 3.36 | 52.04 ± 1.32 | |
| SOD | Exposure | 16.10 ± 2.27 | 18.54 ± 1.90 | 19.75 ± 1.82* | 19.79 ± 1.49* |
| Withdrawal | 19.80 ± 0.18 | 20.60 ± 0.15 | 20.70 ± 0.27 | 20.80 ± 0.20 | |
| GST | Exposure | 1.74 ± 0.28 | 2.02 ± 0.21 | 2.14 ± 0.20* | 2.12 ± 0.16* |
| Withdrawal | 1.76 ± 0.16 | 1.83 ± 0.13 | 1.85 ± 0.27 | 1.85 ± 0.17 | |
| GPx | Exposure | 89.08 ± 13.88 | 103.54 ± 10.57 | 110.14 ± 10.20* | 109.42 ± 7.52* |
| Withdrawal | 83.32 ± 7.73 | 86.68 ± 6.15* | 80.05 ± 5.48 | 90.31 ± 5.97* | |
Values are presented as mean ± standard deviation.
Group A (Control), Group B (Sodium arsenic (10 mg/kg; p.o.), Group C (Sodium arsenic (20 mg/kg; p.o.) Group D (Sodium arsenic (40 mg/kg; p.o.).
Asterisk (*) Indicates significant difference from control at p < 0.05 in each row. GSH (reduced glutathione; μmol/mg protein), SOD (Superoxide dismutase; Units/mg protein), GST (Glutathione S-Transferase; μmol/min/mg protein), GPx (Glutathione peroxidase; μmol/mg protein).
3.2. Erythrocyte antioxidant profile and indicators of oxidative stress following NaAsO2 exposure and withdrawal
As observed in the liver, there was significant (p < 0.05) declines in erythrocyte total protein levels following 4 weeks’ exposure to NaAsO2 (Table 3). The markers of oxidative stress followed the similar pattern as observed in the liver. A significant (p < 0.05) increase in MDA, H2O2 and nitric oxide levels was observed following exposure to NaAsO2 (Table 3).
Table 3.
Erythrocyte total protein and markers of oxidative stress.
| Experimental groups | Group A | Group B | Group C | Group D | |
|---|---|---|---|---|---|
| Total Protein | Exposure | 1.15 ± 0.19 | 0.99 ± 0.02* | 0.98 ± 0.06* | 1.26 ± 0.27 |
| Withdrawal | 1.14 ± 0.04 | 1.08 ± 0.05* | 1.09 ± 0.05 | 0.99 ± 0.04* | |
| Total Thiol | Exposure | 54.33 ± 8.74 | 55.06 ± 10.41 | 52.39 ± 10.44 | 47.61 ± 10.39 |
| Withdrawal | 99.21 ± 13.56 | 82.30 ± 15.82 | 71.31 ± 15.66* | 40.13 ± 8.85* | |
| Nitric Oxide | Exposure | 0.47 ± 0.05 | 0.49 ± 0.04 | 0.44 ± 0.07 | 0.97 ± 0.03* |
| Withdrawal | 0.83 ± 0.09 | 0.59 ± 0.05* | 0.51 ± 0.11* | 0.37 ± 0.10* | |
| MDA | Exposure | 0.28 ± 0.01 | 0.31 ± 0.01* | 0.35 ± 0.02* | 0.24 ± 0.01* |
| Withdrawal | 0.16 ± 0.02 | 0.16 ± 0.03 | 0.17 ± 0.02 | 0.17 ± 0.02 | |
| H2O2 generated | Exposure | 13.98 ± 0.25 | 14.31 ± 0.58 | 14.23 ± 1.39 | 14.64 ± 0.63 |
| Withdrawal | 15.73 ± 0.74 | 15.73 ± 0.79 | 15.77 ± 1.05 | 14.73 ± 0.84 | |
Values are presented as mean ± standard deviation.
Group A (Control), Group B (Sodium arsenic (10 mg/kg; p.o.), Group C (Sodium arsenic (20 mg/kg; p.o.) Group D (Sodium arsenic (40 mg/kg; p.o.).
Asterisk (*) Indicates significant difference from control at p < 0.05 in each row. Total protein (Mg/dl), H2O2 (hydrogen peroxide generation; μmol/mg protein), MDA (malondialdehyde; μmol of MDA formed/mg protein), Nitric oxide (μmol/mg protein) Total thiol (μmol/L).
Upon withdrawal of the toxicant, values for total protein, and the other markers of oxidative stress appeared to return to pre-exposure levels (Table 3). Further, significant increases were observed in the erythrocyte GSH content and the activities of SOD, GPx and GST activity both at exposure and withdrawal phases. However, there was no significant changes in the values of erythrocyte GSH contents during the withdrawal period of NaAsO2 (Table 4).
Table 4.
Erythrocyte antioxidant enzyme profile following exposure and withdrawal of Sodium arsenite.
| Experimental groups | Group A | Group B | Group C | Group D | |
|---|---|---|---|---|---|
| GSH | Exposure | 51.41 ± 0.70 | 52.26 ± 0.37* | 51.98 ± 0.83 | 53.11 ± 1.31* |
| Withdrawal | 53.33 ± 0.53 | 53.40 ± 0.42 | 54.11 ± 0.72 | 53.61 ± 0.26 | |
| SOD | Exposure | 41.33 ± 7.04 | 47.16 ± 1.31* | 47.33 ± 3.29* | 40.25 ± 5.68 |
| Withdrawal | 42.90 ± 0.16 | 46.10 ± 0.18* | 45.20 ± 0.18* | 49.70 ± 0.19* | |
| GST | Exposure | 3.39 ± 0.49 | 3.89 ± 0.18* | 3.95 ± 0.25* | 3.60 ± 0.33 |
| Withdrawal | 3.31 ± 0.11 | 3.49 ± 0.16* | 3.43 ± 0.14 | 3.74 ± 0.15* | |
| GPx | Exposure | 208.51 ± 33.39 | 238.49 ± 4.79* | 239.59 ± 14.81* | 193.48 ± 37.74 |
| Withdrawal | 188.85 ± 7.52 | 200.72 ± 9.19* | 199.27 ± 7.58* | 217.17 ± 8.12* | |
Values are presented as mean ± standard deviation.
Group A (Control), Group B (Sodium arsenic (10 mg/kg; p.o.), Group C (Sodium arsenic (20 mg/kg; p.o.) Group D (Sodium arsenic (40 mg/kg; p.o.).
Asterisk (*) Indicates significant difference from control at p < 0.05 in each row. GSH (reduced glutathione; μmol/mg protein), SOD (Superoxide dismutase; Units/mg protein), GST (Glutathione S-Transferase; μmol/min/mg protein), GPx (Glutathione peroxidase; μmol/mg protein).
3.3. Hematological parameters following exposure and withdrawal of NaAsO2
Following four weeks of exposure to NaAsO2, anemia was observed with a significant (p < 0.05) reductions in PCV, WBC and RBC indices across all treatment groups compared to the control (Table 5). On the other hand, platelets showed dose dependent increases of 20.7%, 28.8% and 45.2% in the order of 10 mg/kg, 20 mg/kg and 40 mg/kg treatment NaAsO2, respectively (Table 5). Upon withdrawal of NaAsO2 however, a reverse picture of the hematological parameters was observed. Across the treatment groups, upon withdrawal of increases NaAsO2, in PCV, Hb, RBC and WBC values were obtained when compared to the control (Table 5). This demonstrated that the anemia observed upon exposure was responsive, and regenerative.
Table 5.
Hematological parameters following sodium arsenite intoxication.
| Experimental groups | Group A | Group B | Group C | Group D | |
|---|---|---|---|---|---|
| PCV (%) | Exposure | 46.30± 1.50 | 42.30 ± 1.20* | 43.30 ± 1.50* | 42.80 ± 1.64* |
| Withdrawal | 47.00 ± 1.90 | 50.40 ± 1.50* | 50.00 ± 4.20 | 46.70 ± 1.53 | |
| Hb (g/dl) | Exposure | 14.50 ± 0.70 | 14.50 ± 0.90 | 14.30 ± 0.50 | 14.10 ± 0.75 |
| Withdrawal | 14.60 ± 0.50 | 15.40 ± 0.40* | 15.90 ± 1.50 | 14.40 ± 0.44 | |
| PLATELETS (x103/μL) | Exposure | 590.20 ± 190.00 | 712.40 ± 199.10 | 760.70 ± 166.40 | 857.50 ± 75.74* |
| Withdrawal | 675.80 ± 97.90 | 577.00 ± 156.00 | 696.20 ± 98.00 | 544.70 ± 17.47* | |
| RBC(x1012/L) | Exposure | 8.10 ± 0.20 | 7.50 ± 0.50* | 7.80 ± 0.20* | 7.60 ± 0.28* |
| Withdrawal | 8.10 ± 0.20 | 8.60 ± 0.20* | 8.80 ± 0.80* | 8.20 ± 0.20 | |
| WBC (x109/L) | Exposure | 17.8 ± 5.80 | 15.9 ± 4.00 | 15.1 ± 2.50 | 7.3 ± 5.82 |
| Withdrawal | 11.60 ± 3.50 | 15.10 ± 4.90 | 16.90 ± 8.10 | 13.40 ± 2.50 |
Values are presented as mean ± standard deviation.
Group A (Control), Group B (Sodium arsenic (10 mg/kg; p.o.), Group C (Sodium arsenic (20 mg/kg; p.o.) Group D (Sodium arsenic (40 mg/kg; p.o.).
Asterisk (*) Indicates significant difference from control at p < 0.05 in each row.
3.4. Histopathology
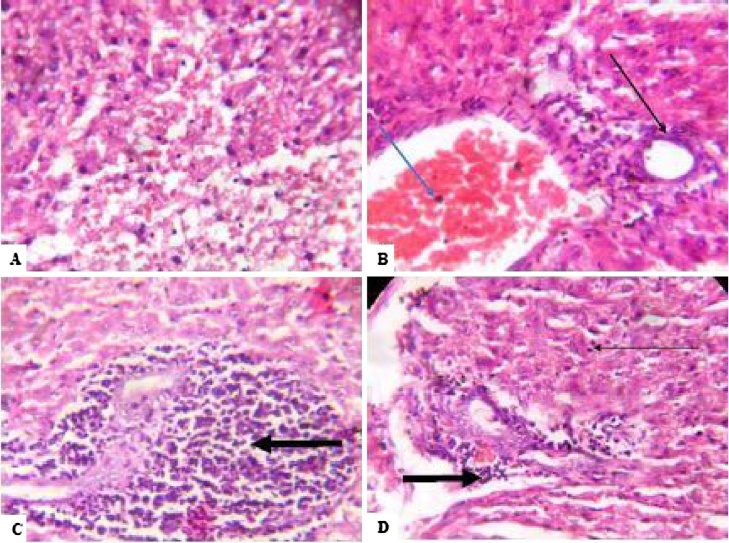
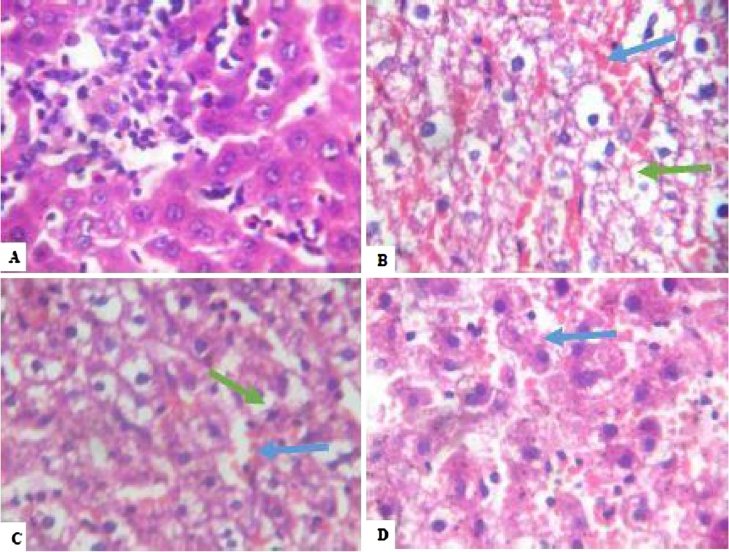
After four weeks’ exposure to NaAsO2, the liver ultrastructure of the control group showed no visible lesions. Conversely, across all treated groups, peri-portal inflammation was observed around the hepatic portal veins alongside congestion of the hepatic blood vessels (Plate 1). Following withdrawal of NaAsO2, the hepatocytes showed varying degrees of macrovesicular steatosis, indicative of long-term fat deposition within the hepatocytes together with different degrees of congestion in the hepatic vessels (Plate 2).
Plate 1.
Photomicrograph showing Liver of rats. (A) − Control: Plate shows no significant lesion (B) −10 mg/Kg: Plate shows congestion of vessels (light blue arrow) and mild periportal inflammation (light black arrow). (C) −20 mg/Kg: Plate show severe disseminated periportal inflammation (black arrows) (D) −40 mg/Kg: Plate shows mild periportal inflammation (black arrow). All plates are after 4 weeks exposure to Sodium Arsenite. Magnification: 100× with Haematoxylin and Eosin stains. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Plate 2.
Photomicrograph showing Liver of rats. (A) – Control: Plate shows no significant lesion. Groups B-D show disseminated macrovesicular steatosis (green arrows) as well as congestion of vessels and sinusoid (blue arrow). All plates are after 4 weeks exposure to Sodium Arsenite. Magnification: 100× with Haematoxylin and Eosin stains. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
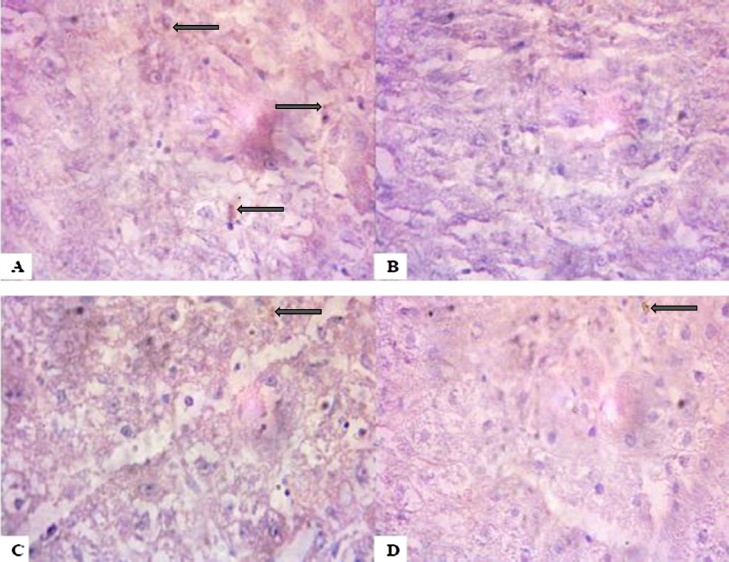
3.5. Immunohistochemistry
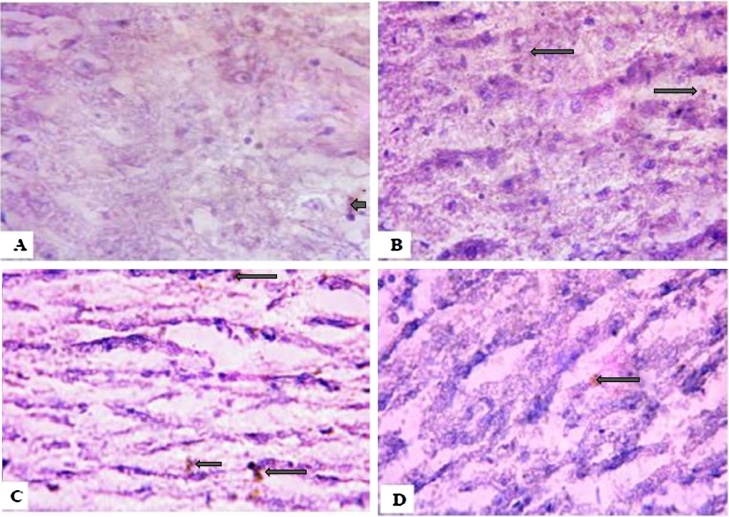
Following four weeks’ exposure to NaAsO2, the 10 mg/kg, 20 mg/kg and 40 mg/kg treated groups showed lower PKB expressions when compared with the control group (Plate 3). Interestingly, upon withdrawal of the NaAsO2, the exposed rats showed even higher expressions of PKB when compared with the control group (Plate 4).
Plate 3.
Control group (A) showed higher expression of the survival protein PKB. Groups B–D treated with Sodium arsenite showed lower PKB expression 4 weeks after exposure. (Immunohistology slides were prepared with 100× magnification using a digital microscope.).
Plate 4.
Control group (A) showed lower PKB expressions. Groups B–D treated with Sodium arsenite showed higher PKB expression 4 weeks after withdrawal. (Immunohistochemistry slides were prepared with 100× magnification using a digital microscope.).
4. Discussion
In this study, we investigated the effects of long term exposure and withdrawal of NaAsO2 on the liver and erythrocyte. It was well established that arsenic causes toxicity by the process of oxidative stress [37], [38], [39], [40]. Also, reactive oxygen and nitrogen species (ROS, RNS) are generated and are due to the presence of unpaired electrons in their outer orbit that ultimately make them extremely reactive [41]. Such free radicals are needed in trace quantities for normal physiologic reactions, including defense mechanisms against infectious agents and in maintaining redox homeostasis [42]. When in excess, these oxidants initiate a chain reaction, culminating in the oxidation of lipids (lipid peroxidation), proteins and DNA, creating imbalances, disrupting redox signaling and causing molecular damage while incapacitating the antioxidant defense systems [43].
Our results showed significant arsenic acid (10 mg/kg, 20 mg/kg and 40 mg/kg) induced increases in MDA content, H2O2 generated and nitric oxide content in the liver and erythrocytes following exposure to arsenic with the non-significant increase in the values of H2O2 generated in the erythrocytes. This is an indication of oxidative stress precipitated by NaAsO2 exposure. However, upon withdrawal of the NaAsO2 from the exposed rats, the markers of oxidative stress were found to return to near normal values suggesting that withdrawal of NaAsO2 following exposure might show some degree of amelioration.
On the one hand, the hepatic GSH, (which provides the reduction of hydroperoxides in membrane phospholipids via glutathione-S-transferases and glutathione peroxidase, and thereby the activation of glutathione antioxidant system to interrupt lipid peroxidation chain reaction) was largely unaffected following exposure to NaAsO2. On the other hand, significant increases in erythrocyte and hepatic GPx, GST and SOD activity was observed. We propose two possible explanations for this development, both involving the up-regulation of antioxidant enzyme activity by Nrf2 [44], [45].
First, is the cysteine-thiol modification of the Keap 1 arm of the Nrf2-Keap1 pathway [44]. In the absence of stressors, Nrf2 is kept at low levels by Keap 1 [45], but in the presence of ROS, the sulfhydryl groups present on the Keap 1 arm oxidizes cysteine residues, especially Cys 151, changing the critical conformation of Keap 1 and inhibiting it from binding to Nrf2 [46], [47].
Subsequently, Nrf2 is released within the cytosol, moves into the nucleus to heterodimerize with small Maf proteins and then, bind to regulatory gene regions known as the antioxidant response elements (ARE) [48]. The substances which elicit a response at the ARE include quercetin, environmental contaminants, metals and certain oxidants like nitric oxide, and hydrogen peroxide [49]. The Nrf2-Maf-ARE complex subsequently activates a whole array of antioxidant and detoxification genes including those of glutathione-S-transferase and many other enzymes involved in antioxidant defense, thus increasing antioxidant activity and detoxifying free radicals [42].
Secondly, arsenic itself has been discovered to activate the Nrf2-Keap1 antioxidant pathway and prolong Nrf2 activation [50], [51], [52]. But, unlike ROS and other free radicals, it works independent of the Keap 1 and Cys 151 mechanism. Studies suggest that it involves the autophagy protein p62 binding to Keap 1, thereby disrupting the Nrf2-Keap1 complex and releasing Nrf2, which then initiates the activation of antioxidant enzymes [53], [54].
Following withdrawal of the animals from exposure, there was a stepwise reduction in the hepatic and erythrocyte markers of oxidative stress. Due to the apparent diminishing of the toxicant in systemic circulation, a negative feedback mechanism could be responsible for this, possibly through Keap 1 signaling to auto-regulate Nrf2 concentrations back to minimum levels as reported by [45]. Interestingly, the hepatic and erythrocyte antioxidant enzyme activities improved significantly both at exposure and withdrawal of NaAsO2. During oxidative stress, the activities of antioxidant enzymes should decline, but the reverse was recorded in the present study. We therefore hypothesize that the observable increase in the acidities of SOD, CAT, GST and GPx might be due to adaptive response to the NaAsO2 toxicity through Nrf2 activation and (Akt/PKB) signaling.
Our results showed sections of mild to severe periportal inflammation, steatosis and congestion of portal vessels, but this was still mild when compared to established histo-pathological manifestations of arsenic toxicity [55], [56]. We speculate that the lesions seen were initial signs of toxicity and that the up-regulation of hepatic and erythrocyte antioxidant enzyme activity, secondary to Nrf2 activation, prevented further fibrotic damage to the liver ultrastructure. This agrees with similar reports where Nrf2 activity was shown to protect against liver damage [57], [58], [59].
Following exposure to NaAsO2, our results showed a progressive, dose-dependent pancytopenia across all treatment groups. This corroborates established literature demonstrating arsenic to have a wide-ranging suppressive effect on the hematopoietic system, spanning the bone marrow, spleen and erythrocytes [60]. Increased cytosolic calcium levels, ceramide formation, and disruption in erythrocyte membrane integrity alongside decreased ATP availability all contribute to cell death. The short life span of arsenic laden RBCs has been shown to impact a direct negative effect on erythrocyte and hemoglobin values, leading to anemia [19], [61].
An exception to the pancytopenia observed in this study was the thrombocytosis seen in response to exposure and the concomitant thrombocytopenia in response to withdrawal. The exact cause is unclear as this differs from established platelet responses in arsenic toxicity [60], [62], but a possible explanation is reported by [63] who linked iron deficiency with secondary thrombocytosis and platelet activation. The oxidation of the sulfhydryl groups on hemoglobin by arsenic and the subsequent reduction in heme synthesis could have led to depleted iron stores, possibly triggering a secondary thrombocytosis. Withdrawal of the inciting toxicant should have the reverse effect, freeing up the iron stores and reducing platelet levels as was seen in this study. Overall, withdrawal from NaAsO2 appeared to alleviate and reverse arsenic induced anemia, and improve blood count across all groups.
Protein kinase B, synonym Akt is a serine/threonine kinase that is expressed to varying degrees in response to stimuli which impact cell homeostasis or destruction Nicholson and Anderson [64]. A marker of cell survival, it is central to homeostasis, replication and existence [65]. There is thus a positive association to be expected between its expression and cell survival. In this study, we observed a decrease in the expression of Akt/PKB in the exposed groups. This could be interpreted that exposure to NaAsO2 had deleterious effects on cell survivability. On withdrawal from the toxicant however, higher expressions of Akt/PKB were observed in NaAsO2 treated rats. This suggests that Akt/PKB could be involved in promoting cell survival after withdrawal from NaAsO2. We surmise that withdrawal from NaAsO2 reduced the impact of toxicant on cell ultrastructure, reversed the progression of cell destruction and improved the survival of hepatic tissue.
5. Conclusion
Taking all into account, NaAsO2 toxicity even at low doses is still of grave public health concern. NaAsO2 induced oxidative stress, causing significant damage to the liver and hematopoietic systems. However, withdrawal of the exposed animals seemed to reverse the aforementioned NaAsO2 toxicity by increasing the antioxidant defense system through Akt/PKB signaling as speculatively demonstrated by immunohistochemistry. It is possible that Akt/PKB signaling might play a protective role in human populations from NaAsO2 contamination following withdrawal. A future research direction is to focus on the molecular analysis of Akt/PKB signaling pathway.
References
- 1.Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012;3:133–164. doi: 10.1007/978-3-7643-8340-4_6. In: Luch A, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jomova K., Jenisova Z., Feszterova M., Baros S., Liska J., Hudecova D., Rhodes C.J., Valko M. Arsenic: toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011;31(2):95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- 3.Mandal B.K., Suzuki K.T. Arsenic round the world: a review. Talanta. 2002;58(1):201–235. [PubMed] [Google Scholar]
- 4.Garelick H., Jones H., Dybowska A., Valsami-Jones E. Arsenic pollution sources. Rev. Environ. Contam. Toxicol. 2008;197:17–60. doi: 10.1007/978-0-387-79284-2_2. [DOI] [PubMed] [Google Scholar]
- 5.ATSDR . CSEM; 2000. Arsenic Toxicity Case Study: Where Is Arsenic Found? | ATSDR – Environmental Medicine & Environmental Health Education. Available from: http://www.atsdr.cdc.gov/csem/csem.asp?csem=1&po=5 (Accessed 10 March 2016). [Google Scholar]
- 6.Brammer H., Ravenscroft P. Arsenic in groundwater: a threat to sustainable agriculture in South and South-east Asia. Environ. Int. 2009;35(3):647–654. doi: 10.1016/j.envint.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Meharg A.A., Williams P.N., Adomako E., Lawgali Y.Y., Deacon C., Villda A., Cambell R.C., Sun G., Zhu Y.G., Feldmann J., Raab A., Zhao F.J., Islam R., Hossain S., Yanai J. Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ. Sci. Technol. 2009;43(5):1612–1617. doi: 10.1021/es802612a. [DOI] [PubMed] [Google Scholar]
- 8.Mondal D., Polya D.A. Rice is a major exposure route for arsenic in Chakdaha block, Nadia district, West Bengal, India: a probabilistic risk assessment. Appl. Geochem. 2008;23(11):2987–2998. [Google Scholar]
- 9.Ezedom T., Asagba S.O. Effect of a controlled food-chain mediated exposure to cadmium and arsenic on oxidative enzymes in the tissues of rats. Toxicol. Rep. 2016;3(2016):708–715. doi: 10.1016/j.toxrep.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tchounwou P.B., Patlolla A.K., Centeno J.A. Carcinogenic and systemic health effects associated with arsenic exposure–a critical review. Toxicol. Pathol. 2003;31(6):575–588. doi: 10.1080/01926230390242007. [DOI] [PubMed] [Google Scholar]
- 11.International Agency for Research on Cancer (IARC) vol. 1–42. 1987. pp. 100–106. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans). Lyons, France. Supplement 7., Overall Evaluation of Carcinogenicity: An updating of Monographs. [PMC free article] [PubMed] [Google Scholar]
- 12.WHO/FAO/IAEA . 1996. World Health Organization Switzerland: Geneva.T Race Elements in Human Nutrition and Health. [Google Scholar]
- 13.Sears M.E., Kerr K.J., Bray R.I. Arsenic, cadmium, lead, and mercury in sweat: a systematic review. J Environ Public Health. 2012;184745 doi: 10.1155/2012/184745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yedjou C.G., Tchounwou P.B. Oxidative stress in human leukemia (HL-60), Human liver carcinoma (HepG2), and human (JURKAT-T) cells exposed to arsenic trioxide. Met. Ions Biol. Med. 2006;9:298–303. [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S., Shi X. Molecular mechanisms of metal toxicity and carcinogenesis. Mol. Cell. Biochem. 2001;222(1–2):3–9. [PubMed] [Google Scholar]
- 16.Hughes M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002;133(1):1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- 17.Stevens J.J., Graham B., Walker A.M., Tchounwou P.B., Rogers C. The effects of arsenic trioxide on DNA synthesis and genotoxicity in human colon cancer cells. Int. J. Environ. Res. Public Health. 2010;7(5):2018–2032. doi: 10.3390/ijerph7052018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goutzourelas N., Stagos D., Housmekeridou A., Karapouliou C., Kerasioti E., Aligiannis N., Skaltsounis A.L., Spandidos D.A., Tsatsakis A.M., Kouretas D. Grape pomace extract exerts antioxidant effects through an increase in GCS levels and GST activity in muscle and endothelial cells. Int. J. Mol. Med. 2015;36(2):433–441. doi: 10.3892/ijmm.2015.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdul K.S.M., Jayasinghe S.S., Chandana E.P.S., Jayasumana C., De Silva P.M. Arsenic and human health effects. A review. Environ. Toxicol. Pharmacol. 2015;40:828–846. doi: 10.1016/j.etap.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Seki E., Brenner D.A., Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143(2):307–320. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh R., Czaja M.J. Regulation of hepatocyte apoptosis by oxidative stress. J. Gastroenterol. Hepatol. 2007;22(1):S45–S48. doi: 10.1111/j.1440-1746.2006.04646.x. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto H., Kanno K., Ikuta T., Arihiro K., Sugiyama A., Kishikawa N., Tazuma S. Enhancing hepatic fibrosis in spontaneously hypertensive rats fed a choline-deficient diet: a follow-up report on long-term effects of oxidative stress in non-alcoholic fatty liver disease. J. Hepatobiliary Pancreat. Sci. 2016;23(5):260–269. doi: 10.1002/jhbp.333. [DOI] [PubMed] [Google Scholar]
- 23.Biswas D., Banerjee M., Sen G., Das J.K., Banerjee A., Sau T.J., Pandit S., Giri A.K., Biswas T. Mechanism of erythrocyte death in human population exposed to arsenic through drinking water. Toxicol. Appl. Pharmacol. 2008;230(1):57–66. doi: 10.1016/j.taap.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Garber J.C., Barbee R.W., Bielitzki J.T., Clayton L.A., Donovan J.C., Kohn D.F., Lipman N.S., Wood G.A., Wurbel H. Vol. 8. The National Academic Press; Washington DC: 2010. p. 220. (Guide for the Care and Use of Laboratory Animals). [Google Scholar]
- 25.Steck T.L., Kant J.A. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- 26.Gornall A.G., Bardawill C.J., David M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 27.Wolff S.P. Ferrous ion oxidation in the presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994;233:182–189. [Google Scholar]
- 28.Farombi E.O., Tahnteng J.G., Agboola A.O., Nwankwo J.O., Emerole G.O. Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron–a Garcinia kola seed extract. Food Chem. Toxicol. 2000;38:535–541. doi: 10.1016/s0278-6915(00)00039-9. [DOI] [PubMed] [Google Scholar]
- 29.Olaleye S.B., Adaramoye O.A., Erigbali P.P., Adeniyi O.S. Lead exposure increases oxidative stress in the gastric mucosa of HCl/ethanol-exposed rats. World J. Gastroenterol. 2007;13:5121–5126. doi: 10.3748/wjg.v13.i38.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 31.Jollow D.J., Mitchell JR, Zampaglione N., Gillete JR Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 32.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 33.Oyagbemi A.A., Omobowale T.O., Akinrinde A.S., Saba A.B., Ogunpolu B.S., Daramola O. Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ. Toxicol. 2015;37:1202–1211. doi: 10.1002/tox.21994. [DOI] [PubMed] [Google Scholar]
- 34.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 35.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;25(249):7130–7139. [PubMed] [Google Scholar]
- 36.Drury R.A.B., Wallington E.A., Cancerson R. 4th ed. Oxford University Press; Oxford/London/New York: 1976. Carleton’s Histopathological Techniques. [Google Scholar]
- 37.Guo Y., Zhao P., Guo G., Hu Z., Tian L., Zhang K., Zhang W., Xing M. The role of oxidative stress in gastrointestinal tract tissues induced by arsenic toxicity in cocks. Biol. Trace Elem. Res. 2015;168(2):490–499. doi: 10.1007/s12011-015-0357-9. [DOI] [PubMed] [Google Scholar]
- 38.Li L., Chen F. Oxidative stress, epigenetics, and cancer stem cells in arsenic carcinogenesis and prevention. Curr. Pharmacol. Rep. 2016;2(2):57–63. doi: 10.1007/s40495-016-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sener U., Uygur R., Aktas C., Uygur E., Erboga M., Balkas G., Caglar V., Kumral B., Gurel A., Erdogan H. Protective effects of thymoquinone against apoptosis and oxidative stress by arsenic in rat kidney. Ren. Fail. 2016;38(1):117–123. doi: 10.3109/0886022X.2015.1103601. [DOI] [PubMed] [Google Scholar]
- 40.Xu Z., Wang Z., Li J., Chen C., Zhang P.C., Dong L., Chen J.H., Chen Q., Zhang X.T., Wang Z.L. Protective effects of selenium on oxidative damage and oxidative stress related gene expression in rat liver under chronic poisoning of arsenic. Food Chem. Toxicol. 2013;58:1–7. doi: 10.1016/j.fct.2013.03.048. [DOI] [PubMed] [Google Scholar]
- 41.Niedzwiecki M.M. Columbia University; 2017. Mechanisms of Arsenic Toxicity in Humans: Interplay of Arsenic, Glutathione, and DNA Methylation in Bangladeshi Adults. Available from: http://academiccommons.columbia.edu/catalog/ac:185812 (Accessed 14 March, 2017) [Google Scholar]
- 42.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P., Cadenas S., Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baird L., Dinkova-Kostova A.T. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011;85(4):241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 45.Zhang D.D., Lo S.-C., Cross J.V., Templeton D.J., Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 2004;24(24):10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fourquet S., Guerois R., Biard D., Toledano M.B. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J. Biol. Chem. 2010;285(11):8463–8471. doi: 10.1074/jbc.M109.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto T., Suzuki T., Kobayashi A., Wakabayashi J., Maher J., Motohashi H., Yamamoto M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol. Cell. Biol. 2008;28(8):2758–2770. doi: 10.1128/MCB.01704-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rushmore T.H., Morton M.R., Pickett C.B. The antioxidant responsive element: activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 1991;266(18):11632–11639. [PubMed] [Google Scholar]
- 49.Ma Q., He X. Molecular basis of electrophilic and oxidative defense: promises and perils of Nrf2. Pharmacol. Rev. 2012;64(4):1055–1081. doi: 10.1124/pr.110.004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen W., Sun Z., Wang X.J., Jiang T., Huang Z., Fang D., Zhang D.D. Direct interaction between Nrf2 and p21 (Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol. Cell. 2009;34(6):663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y., Ji L., Wang H., Wang Z. Intracellular glutathione plays important roles in pyrrolizidine alkaloids-induced growth inhibition on hepatocytes. Environ. Toxicol. Pharmacol. 2009;28(3):357–362. doi: 10.1016/j.etap.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Lau A., Whitman S.A., Jaramillo M.C., Zhang D.D. Arsenic-mediated activation of the Nrf2-Keap1 antioxidant pathway. J. Biochem. Mol. Toxicol. 2013;27(2):99–105. doi: 10.1002/jbt.21463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y., Sou Y.S., Ueno I., Sakamoto A., Tong K.I., Kim M., Nishito Y., Iemura S., Natsume T., Ueno T., Kominami E., Motohashi H., Tanaka K., Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12(3):213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 54.Lau A., Wang X.-J., Zhao F., Villeneuve N.F., Wu T., Jiang T., Sun Z., White E., Zhang D.D. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol. Cell. Biol. 2010;30(13):3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazumder D.N.G. Effect of chronic intake of arsenic-contaminated water on liver. Toxicol. Appl. Pharmacol. 2005;206(2):169–175. doi: 10.1016/j.taap.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 56.Santra A., Maiti A., Das S., Lahiri S., Charkaborty S.K., Mazumder D.N. Hepatic damage caused by chronic arsenic toxicity in experimental animals. J. Toxicol. Clin. Toxicol. 2000;38(4):395–405. doi: 10.1081/clt-100100949. [DOI] [PubMed] [Google Scholar]
- 57.Cai Z., Lou Q., Wang F., Li E., Sun J., Fang H., Xi J., Ju L. N-acetylcysteine protects against liver injure induced by carbon tetrachloride via activation of the Nrf2/HO-1 pathway. Int. J. Clin. Exp. Pathol. 2015;8(7):8655–8662. [PMC free article] [PubMed] [Google Scholar]
- 58.Lu Y.-F., Liu J., Wu K.C., Klaassen C.D. Protection against phalloidin-induced liver injury by oleanolic acid involves Nrf2 activation and suppression of Oatp1b2. Toxicol. Lett. 2015;232(1):326–332. doi: 10.1016/j.toxlet.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pang C., Zheng Z., Shi L., Sheng Y., Wei H., Wang Z., Ji L. Caffeic acid prevents acetaminophen-induced liver injury by activating the Keap1-Nrf2 antioxidative defense system. Free Radic. Biol. Med. 2016;91:236–246. doi: 10.1016/j.freeradbiomed.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 60.Ezeh P.C., Lauer F.T., MacKenzie D., McClain S., Liu K.J., Hudson L.G., Gandolfi A.J., Burchiel S.W. Arsenite selectively inhibits mouse bone marrow lymphoid progenitor cell development in vivo and in vitro and suppresses humoral immunity in vivo. PLoS One. 2014;9(4):e93920. doi: 10.1371/journal.pone.0093920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keshavarzi B., Seradj A., Akbari Z., Moore F., Shahraki A.R., Pourjafar M. Chronic arsenic toxicity in sheep of kurdistan province, western Iran. Arch. Environ. Con. Tox. 2015;69:44–53. doi: 10.1007/s00244-015-0157-4. [DOI] [PubMed] [Google Scholar]
- 62.Sánchez-Virosta P., Espín S., García-Fernández A.J., Eeva T. A review on exposure and effects of arsenic in passerine birds. Sci. Total Environ. 2015;512:506–525. doi: 10.1016/j.scitotenv.2015.01.069. [DOI] [PubMed] [Google Scholar]
- 63.Kulnigg-dabsch S., Schmid W., Howaldt S., Stein J., Mickisch O., Waldhör T., Evstatiev R., Kamali H., Volf I., Gasche C. Iron deficiency generates secondary thrombocytosis and platelet activation in IBD: the randomized, controlled ThromboVIT. Trial. Inflamm. Bowel Dis. 2013;19(8):1609–1616. doi: 10.1097/MIB.0b013e318281f4db. [DOI] [PubMed] [Google Scholar]
- 64.Nicholson K.M., Anderson N.G. The protein kinase B/Akt signalling pathway in human malignancy. Cell. Signal. 2002;14(5):381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 65.Fayard E., Tintignac L.A., Baudry A., Hemmings B.A. Protein kinase B/Akt at a glance. J. Cell Sci. 2005;118(24):6541–6551. doi: 10.1242/jcs.02724. [DOI] [PubMed] [Google Scholar]