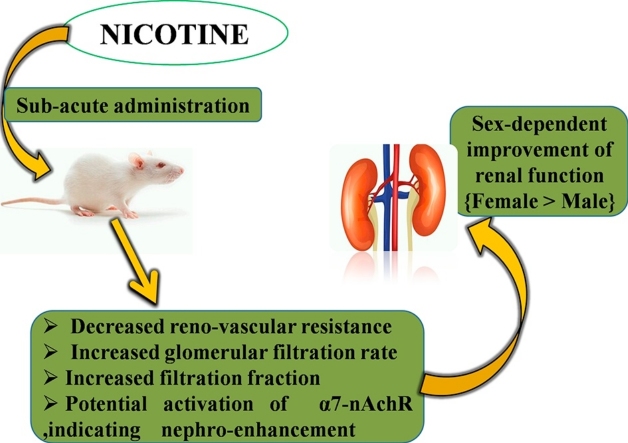
Graphical abstract
Keywords: Nicotine, Wistar rats, Creatinine clearance, Plasma and urine electrolytes, Renal function
Highlights
-
•
Exposure to nicotine is associated with sex-dependent variation in electrolyte disturbances.
-
•
Lower doses of sub-acute nicotine administration enhanced renal function.
-
•
Nicotine-enhanced renal function is more pronounced in female than in male Wistar rats.
-
•
Nicotine-enhanced renal function may be mediated through α7-nAchR.
Abstract
The adverse and beneficial health effects of nicotine (NIC), the major alkaloid found in cigarettes and tobacco, are controversial. Most studies on NIC have focused on its effects on cardiovascular and nervous functions. This study aimed at determining dose- and sex-specific effects of sub-acute (28 days) NIC administration on some indices of kidney function in Wistar rats. Forty rats (20 males and 20 females), 8–9 weeks old (each housed in separate metabolic cage), were used for this study such that graded doses of NIC (1, 2 and 4 mg/kg i.p. for 28 days) were administered to both sexes while each control received distilled water at 0.2 mL/100 g i.p. Blood was collected under ketamine anesthesia (10 mg/kg i.m) for analyses and results obtained were compared at p < 0.05. The result showed beneficial alterations in plasma and urine level of creatinine, urea and uric acid (p < 0.05) as well as plasma and urine electrolyte level (Na+ and K+) in both sexes (p < 0.05). Also, there was significant improvement in creatinine clearance (p < 0.05) with no appreciable difference in their histological examination. Although these beneficial effects were more pronounced in the female than in the male (p < 0.05), administration at the highest dose showed potentially deleterious alterations from normal beneficial trend (p < 0.05) in both sexes. It was concluded that sub-acute administration of lower doses of NIC improves kidney function of Wistar rats; an effect that was more pronounced in the females than their male counterparts.
1. Introduction
Nicotine, a natural alkaloid found in the plant Nicotiana tabacum, is considered an important component of cigarettes [1], [2]. Nicotine chewing gums and dermal patches are other ways in which nicotine is consumed through non-prescription nicotine replacement therapy [3]. Although available in synthetic forms, it also constitutes a portion of human dietary intake as it naturally occurs in small amounts in plants belonging to the Solanaceae family such as eggplants, potatoes and tomatoes [4]. However, in man it is consumed primarily through cigarettes, pipes or cigars [5]. Nicotine can also be found in electronic cigarettes (e-cig), which is a contemporary and wide spread way of smoking [6], [7]. Invented and patented by a Chinese Pharmacist named Hon Lik in 2003 [8], e-cig was introduced to the market, a year later, as an alternative nicotine delivery device [9]. Simulating the operation of conventional cigarette, e-cig is a battery –operated device containing e-liquid which is composed of concentrated flavours, propylene glycol (humectants), glycerol and various amount of nicotine [10]. To date, the adverse and beneficial effects of nicotine are stranded in controversy.
Reputed to be the tobacco alkaloid that is responsible for addiction, nicotine affects the body by causing modulation in dependence and behavior resulting in repeated exposure and consumption [5], [11], [12]. With an elimination half-life of about two hours when absorbed in the body [12], nicotine is readily transported in the blood to various body organs where it exert its effects. More than 80% of absorbed nicotine is metabolized by the liver [13] while several of its metabolites reach the central nervous system, crossing the blood – brain barrier within 10–20 s after inhalation [12], [14]. A vast body of literatures exist on its effects on the nervous system [15], [16], [17], [18], [19], [20]; showing that it is both a stimulant and relaxant, as well as on the cardiovascular system [21], [22], [23], [24], [25], [26]. However, there is dearth of literature on the sub-acute effects of its graded doses on the renal system.
The kidney, an important organ of the renal system, plays a principal role in homeostasis by excreting urine [27], [28]. It achieves this by filtering waste products from the blood stream and converting the ultra filtrate to urine. Therefore, any compromise of kidney function can cause deleterious health effects due to build up of unwanted substances in the body [29]. Measurements of some biological (biochemical) markers can demonstrate beneficial or harmful changes in kidney function, thereby serving as indices of renal function. Variations in these biochemical markers play an important role in accurate diagnosis and also in assessing risk and adopting therapy that improves clinical outcomes [30]. Important biochemical markers that are often used for routine analysis of kidney function include creatinine, urea, uric acid as well as some important electrolytes such as sodium and potassium [30], [31]. These biochemical markers were assayed for in the plasma to assess kidney function after a sub-acute duration of nicotine study.
Sub-acute, a condition between acute and chronic, study implies a study between 2 and 29 days while acute study is less than 2 days to within few minutes or hours [32]. Between 30–90 days comprises sub-chronic study while a study beyond 90 days is considered as chronic [33], [34], [35].
A comprehensive understanding of nicotine’s effects on renal function can point towards novel approach to its use or disuse in both social and clinical settings with regards to renal implications. This study aimed at contributing to the body of existing knowledge by providing information on the renal implication of dose- and sex-specific effects of sub-acute nicotine administration using Wistar rat model.
2. Materials and methods
2.1. Metabolic cages
Metabolic cages used for this study were fabricated by Central Technological Laboratory and Workshops (CTLW), OAU, Ile-Ife, Osun State, Nigeria.
2.2. Chemicals and assay kits
Nicotine hydrogen tartarate (04441416) ≥98% was purchased from Sigma-Aldrich Company while the standard laboratory kits for the assay of urea and uric acid were purchased from Randox Laboratories Limited, United Kingdom.
Creatinine was assayed using laboratory protocol as described Jaffe [36], Na+ and K− by Flame Photometry while HCO3− was determined by titrimetry.
2.3. Preparation of stock solution for different concentration of nicotine
Creatinine clearance was determined using conventional formula as follows;
| Clearance = UcV/Pc (mL/min) |
Where Uc = concentration of creatinine in urine; V = urine flow rate = amount of urine/time (secs); Pc = concentration of creatinine in plasma.
2.4. Animal management and experimental design
Both distilled water and the different nicotine concentration were administered at 0.2 mL/100 g of rat. Different concentration of nicotine at 1 mg/kg, 2 mg/kg and 4 mg/kg were prepared by dissolving 10 mg, 20 mg and 40 mg of nicotine salt in 20 mL, each, of distilled water respectively. Eventually, the entire groups received 0.2 mL/100 g of both distilled water and nicotine.
2.5. Measurement of body and organ weight
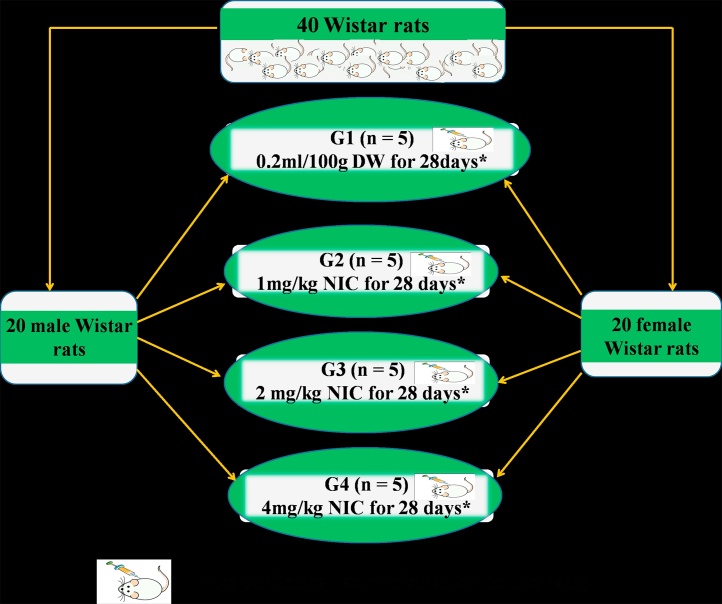
All experimental protocols were in strict compliance with the guidelines for animal research, as detailed in the NIH Guidelines for the Care and Use of Laboratory Animals (Guide for the care and use of laboratory animals, 2011) [37] and approved by local Institutional Research Committee. Forty Wistar rats comprising 20 males and 20 females (8–9 weeks old), between 120 and 150 g, were used for this study. They were purchased from the Animal Holdings of the College of Health Sciences, OAU, Ile – Ife, Osun State, Nigeria where the study was carried out. Each rat was housed in separate metabolic cage under natural light and dark cycle and allowed to have access to food and water ad libitum. Each sex was divided into 4 groups of 5 rats each as follows; Group 1 (control) received 0.2 mL/100 g of distilled water (i.p. for 28 days) after which they were sacrificed, while groups 2, 3 and 4 received nicotine (NIC) at 1, 2 and 4 mg/kg i.p. respectively for the same period after which they were sacrificed (Fig. 1). Twenty four hours urine samples were collected before the rats were sacrificed. Rats were sacrificed under ketamine anesthesia (10 mg/kg i.m.) and blood samples were collected by cardiac puncture into separate lithium heparinized bottles. Blood samples were thereafter centrifuged at −4 °C using a cold centrifuge (Centurium Scientific, Model 8881) that was set at 4000 revolutions per minute for 15 min. The plasma obtained was decanted with sterile syringes into separate plain bottles for biochemical assays.
Fig. 1.
Experimental Protocol and Dose Regimen.
G1 = Group 1; G2 = Group 2; G3 = Group 3; G4 = Group 4; n = number of rats in the group; DW = Distilled water; NIC Nicotine; * = Point at which rats were sacrificed.
The right kidney of each Rat was fixed in 10% formal-saline solution and thereafter processed for histopathological examination using Hematoxylin – Eosin (H & E) staining technique.
2.6. Determination of creatinine clearance
Weekly body weight was measured using Hanson digital weighing balance (Hanson, China) to assess weekly weight change while organ weights were determined using Camry sensitive weighing balance (Camry, China). Both percentage weight change and relative kidney weight were calculated using the formula below;
[38].
[39].
2.7. Statistical analysis
Using one-way analysis of variance (ANOVA), data obtained were expressed as mean ± S.E.M (standard error of mean) and subjected to Tukey’s post-hoc test for multiple comparisons. For paired observations, data were subjected to Student’s t-test and values at p < 0.05 were considered statistically significant. Data were analyzed using Graph Pad Prism 5.03 (Graph Pad Software Inc., CA, USA).
3. Results
3.1. Plasma and urine level of creatinine, urea and uric acid of Wistar rats following sub-acute nicotine administration
While plasma creatinine level (μmol/L) was not significantly higher in group 1 of the male sex when compared with their female counterpart, urine creatinine level was significantly higher in the female sex when compared with their male counterparts (p < 0.05). In the male sex, following nicotine administration the plasma creatinine level was significantly higher in group 2 and not significantly lower in group 4 when both were compared with group 1 (p < 0.05). The female sex, however, showed a significantly lower plasma creatinine level in groups 3 and 4 when compared with group 1. Plasma creatinine level was significantly lower in the female sex as shown in groups 3 and 4 when compared with their male counterparts in groups 3 and 4 respectively (p < 0.05). Both sexes recorded a non-significantly lower level of urine creatinine in groups 2 and 3 when compared with their respective group 1 (p < 0.05). However, while group 4 showed insignificantly higher level of urine creatinine in the male sex, it was shown to be significantly lower in the female sex when both were compared with their respective group 1 (p < 0.05). It was shown that the urine plasma levels were significantly higher in the female sex in groups 1–3 while this level was significantly lower in group 4 when compared with the respective groups of their male counterparts (p < 0.05) (Table 1).
Table 1.
Effects of Sub-acute Nicotine Administration on Plasma and Urine Level of Creatinine (μmol/L), Urea (mmol/L) and Uric Acid (mg/dL) of Wistar Rats.
| Creatinine (μmol/L) | Plasma Level |
Urine Level |
||
|---|---|---|---|---|
| GROUPS (n = 5) | Male | Female | Male | Female |
| [1] Control | 166.00 ± 2.79 | 158.20 ± 3.98 | 2400.00 ± 149.40 | 3166.00 ± 98.97a |
| [2] 1 mg/kg | 165.60 ± 7.30 | 144.80 ± 6.11 | 2274.00 ± 61.85 | 2698.00 ± 68.95e, b |
| [3] 2 mg/kg | 163.80 ± 2.50 | 95.80 ± 6.14e, f, c | 2210.00 ± 96.18 | 2522.00 ± 91.18e, c |
| [4] 4 mg/kg | 158.60 ± 3.31 | 80.00 ± 8.52e, f, g, d | 2410.00 ± 105.30 | 1418.00 ± 113.00e, f, g, d |
| Urea (mmol/L) | ||||
| [1] Control | 11.16 ± 0.30 | 9.38 ± 0.13a | 337.20 ± 3.72 | 254.80 ± 15.39a |
| [2] 1 mg/kg | 11.94 ± 0.24 | 8.14 ± 0.41b | 299.80 ± 16.73 | 283.20 ± 11.26 |
| [3] 2 mg/kg | 12.86 ± 0.44e | 10.02 ± 0.35f, c | 394.20 ± 4.31e, f | 318.20 ± 15.58e, c |
| [4] 4 mg/kg | 11.22 ± 0.46g | 7.42 ± 0.38e, g, d | 337.40 ± 12.25g | 205.20 ± 5.93f, g, d |
| Uric Acid (mg/dL) | ||||
| [1] Control | 2.88 ± 0.05 | 3.24 ± 0.18 | 30.40 ± 1.40 | 17.00 ± 2.35a |
| [2] 1 mg/kg | 3.74 ± 0.13e | 4.34 ± 0.20e, b | 22.20 ± 4.10 | 18.20 ± 2.54 |
| [3] 2 mg/kg | 4.12 ± 0.26e | 3.68 ± 0.31 | 21.60 ± 4.09 | 27.20 ± 4.55 |
| [4] 4 mg/kg | 2.54 ± 0.17f, g | 4.30 ± 0.22e, d | 19.60 ± 4.68 | 22.20 ± 4.95 |
Each value represents mean ± standard error of mean at p < 0.05.
significantly different from Male control group.
significantly different from Male group 2.
significantly different from Male group 3.
significantly different from Male group 4.
significantly different from control.
significantly different from group 2 of same sex.
significantly different from group 3 of same sex.
Both plasma and urine levels of urea (mmol/L) were shown to be significantly higher in group 1 of the male sex when compared with their female counterparts (p < 0.05). Nicotine administration was associated with a significantly higher level of plasma urea in group 3 of both sexes when compared with their respective group 1 (p < 0.05). However, while group 4 showed a non-significantly higher level when compared with group 1 in the male sex, this was shown to be significantly lower in the respective groups of the female sex (p < 0.05). Urine urea level was significantly higher in group 3 of both sexes when compared with their respective group 1 (p < 0.05). While group 4 of the male sex showed no significant difference in urine urea level when compared with group 1, this was shown to be significantly reduced in the respective groups of their female counterparts (p < 0.05). The urine urea level was significantly lower in groups 3 and 4 of the female sex when compared with their male counterparts (p < 0.05) (Table 1).
Plasma uric acid level (mg/dL) was not significantly lower in group 1 of the male sex, however, the urine uric acid level was significantly higher in group 1 of the male sex when compared with their female counterparts respectively (p < 0.05). While groups 2 and 3 of the male sex showed a significantly higher level of plasma uric acid when compared with group 1, in the female sex groups 1 and 4 showed a significantly higher level of plasma uric acid when compared with group 1 (p < 0.05). At the end of the study, plasma uric acid level was shown to be significantly higher in groups 2 and 4 of the female groups when compared with their male counterparts (p < 0.05). Although there was no significant difference in the urine uric acid level in the nicotine-treated groups of both the male and female sexes, while the male sex recorded a steady decline with increasing doses their female counterparts showed an increase which peaked in group 3 (p < 0.05) (Table 1).
3.2. Creatinine clearance of Wistar rats following sub-acute nicotine administration
Although group 1 of the male sex showed a non-significantly lower creatinine clearance (mL/min) when compared with their female counterparts (p < 0.05), creatinine clearance was significantly increased in groups 2 and 3 of both sexes when compared with their respective group 1 (p < 0.05). This was, however, accompanied by a sharp decline that was significantly lower than groups 2 and 3 of both sexes (p < 0.05) but not significantly lower than their respective group 1 (p < 0.05). At the end of the study, the nicotine-treated groups recorded a significantly higher creatinine clearance in the female groups when compared with their respective male groups (p < 0.05) (Table 2).
Table 2.
Effects of Sub-acute Nicotine Administration on Creatinine Clearance (mL/min) of Wistar Rats.
| Creatinine Clearance × 10−3 (mL/min) | ||
|---|---|---|
| GROUPS (n = 5) | Male | Female |
| [1] Control | 4.67 ± 0.66 | 6.28 ± 0.37 |
| [2] 1 mg/kg | 7.38 ± 0.53a, e | 9.45 ± 0.49e, b |
| [3] 2 mg/kg | 7.66 ± 0.40a, e | 14.29 ± 0.62e, f, c |
| [4] 4 mg/kg | 3.41 ± 0.38f, g | 4.61 ± 0.34f, g, d |
Each value represents mean ± standard error of mean at p < 0.05.
significantly different from Male control group.
significantly different from Male group 2.
significantly different from Male group 3.
significantly different from Male group 4.
significantly different from control.
significantly different from group 2 of same sex.
significantly different from group 3 of same sex.
3.3. Plasma and urine level of Na+, K+ and HCO3− of Wistar rats following sub- acute nicotine administration
In the male sex, plasma Na+ level (mmol/L) was not significantly higher in group 1 but was shown to be significantly higher in urine level when compared with their respective group 1 of the female sex (p < 0.05). There was no significant difference in plasma Na+ in the nicotine-treated groups of both the male and female sexes (p > 0.05), however, group 4 of the female sex showed a significantly lower level of plasma Na+ when compared with their male counterparts (p < 0.05). While urine Na+ level was significantly higher in groups 2 and 3 of the male sex when compared with group 1 (p < 0.05) only group 2 recorded a significantly higher level of urine Na+ when compared with group 1 (p < 0.05). At the end of the study, the nicotine-treated groups of the female sex showed a significantly lower level of urine Na+ when compared with their male counterparts (p < 0.05) (Table 3).
Table 3.
Effects of Sub-acute Nicotine Administration on some Electrolyte Concentrations in the Urine and Plasma of Wistar Rats.
| Na+ Concentration (mmol/L) | Plasma Level |
Urine Level |
||
|---|---|---|---|---|
| GROUPS (n = 5) | Male | Female | Male | Female |
| [1] Control | 135.80 ± 2.08 | 130.00 ± 1.67 | 100.60 ± 4.43 | 70.40 ± 2.36a |
| [2] 1 mg/kg | 129.60 ± 1.36 | 128.20 ± 2.33 | 126.40 ± 4.60e | 72.80 ± 2.08b |
| [3] 2 mg/kg | 133.80 ± 2.40 | 132.60 ± 1.40 | 174.40 ± 3.67e, f | 92.80 ± 5.16e, f, c |
| [4] 4 mg/kg | 133.80 ± 1.39 | 128.20 ± 1.28d | 120.80 ± 7.52g | 76.60 ± 6.17d |
| K+ Concentration (mmol/L) | ||||
| [1] Control | 4.20 ± 0.06 | 3.68 ± 0.12a | 129.80 ± 7.43 | 160.80 ± 4.32a |
| [2] 1 mg/kg | 3.54 ± 0.10e | 3.26 ± 0.07e, b | 132.60 ± 4.16 | 227.60 ± 7.33e, b |
| [3] 2 mg/kg | 3.72 ± 0.05e | 3.16 ± 0.06e, c | 228.60 ± 7.31e, f | 185.20 ± 7.79f |
| [4] 4 mg/kg | 3.32 ± 0.08e, g | 3.22 ± 0.10e | 157.20 ± 9.71g | 176.00 ± 6.33f |
| HCO3− Concentration (mmol/L) | ||||
| [1] Control | 20.80 ± 0.49 | 21.20 ± 0.49 | ||
| [2] 1 mg/kg | 19.60 ± 0.75 | 22.40 ± 0.75b | ||
| [3] 2 mg/kg | 21.60 ± 0.75 | 20.80 ± 0.49 | ||
| [4] 4 mg/kg | 22.00 ± 0.63 | 21.20 ± 0.80 | ||
Each value represents mean ± standard error of mean at p < 0.05.
significantly different from Male control group.
significantly different from Male group 2.
significantly different from Male group 3.
significantly different from Male group 4.
significantly different from control.
significantly different from group 2 of same sex.
significantly different from group 3 of same sex.
Although the plasma K+ level (mmol/L) was significantly higher in group 1 of the male sex, urine K+ was shown to be significantly reduced in this group when compared with their respective female counterparts (p < 0.05). The nicotine-treated groups showed a significantly reduced level of plasma K+ in both the male and female sexes when compared with their respective group 1 (p < 0.05). At the end of the study, groups 2 and 3 of the female sex showed a significantly lower plasma K+ level when compared with their respective male counterparts (p < 0.05). Apart from group 3 that showed a significantly higher level of urine K+, groups 2 and 4 showed a non-significantly higher level of urine K+ when compared with group 1 (p > 0.05). With the exception of group 2 of the female sex which showed a significantly higher level of urine K+, groups 3 and 4 showed a non-significant increase in the urine K+ when compared with group 1 (p < 0.05). At the end of the study, only group 2 of the female sex showed a significant increase in urine K+ level when compared with group 1 of their male counterparts (p < 0.05) (Table 3).
The study showed no significant difference in the level of plasma HCO3− (mmol/L) between group 1 and the nicotine-treated groups of both sexes (p > 0.05). However, group 2 of the female sex showed a significant increase in plasma HCO3− when compared with their respective group 2 of the male sex (p < 0.05) (Table 3).
3.4. Sub-acute effects of nicotine on percentage weight change (PWC) and relative kidney weight (RKW) of Wistar rats
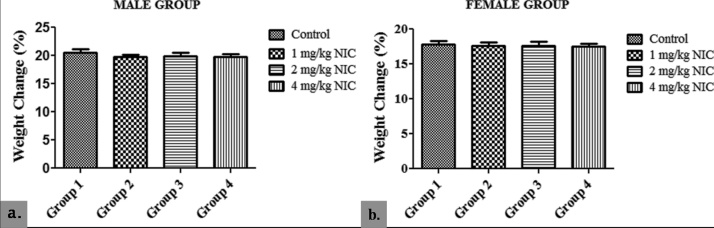
In the male group, there was no significant difference in PWC of the nicotine-treated groups 2, 3 and 4 (19.80 ± 0.40; 19.90 ± 0.60 and 19.75 ± 0.50 respectively) when compared with their control (20.50 ± 0.60) (p > 0.05). The same is true for the female groups 2, 3 and 4 (17.60 ± 0.50; 17.65 ± 0.60 and 17.50 ± 0.40 respectively) when compared with their control (17.85 ± 0.50) (p > 0.05), at the end of the study. However, the male groups showed a significantly higher PWC aht the end of the study when compared with their respective female counterparts (p < 0.05) (Fig. 2).
Fig. 2.
Effects of Sub-acute Nicotine Administration on Percentage Weight Change (%) of Wistar Rats.
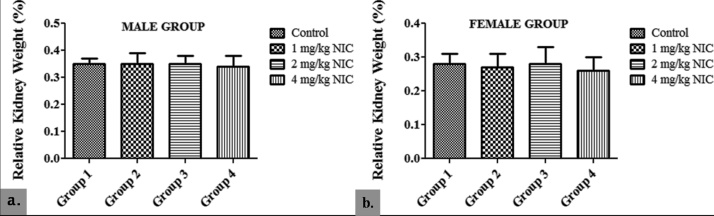
The study showed no significant difference in RKW in the male groups 2, 3 and 4 (0.35 ± 0.04; 0.35 ± 0.03 and 0.34 ± 0.04 respectively) when compared with their control (0.35 ± 0.02) (p > 0.05). Similarly, no significant difference in RKW was recorded in the female groups 2, 3 and 4 (0.27 ± 0.04; 0.28 ± 0.05 and 0.26 ± 0.04 respectively) when compared with their control (0.28 ± 0.03) (p > 0.05). The female groups, however, showed a significantly lower RKW at the end of the study when compared with their respective male counterparts (p < 0.05) (Fig. 3).
Fig. 3.
Effects of Sub-acute Nicotine Administration on Relative Kidney Weight (%) of Wistar Rats.
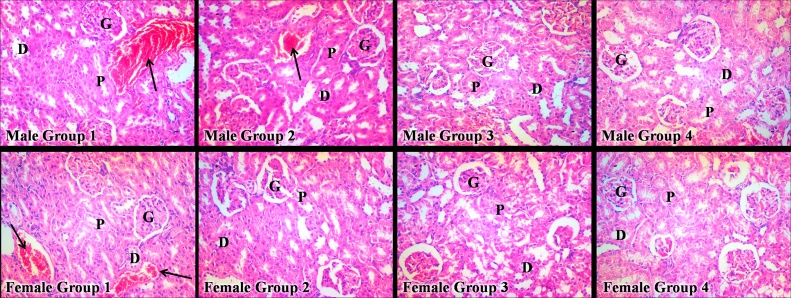
3.5. Histological examination
The representative photomicrograph of the nicotine-treated groups of both sexes did not reveal any appreciable difference in kidney histoarchitecture when compared with that of their respective control group (Fig. 4).
Fig. 4.
Histological Effects of Sub-acute Nicotine Administration in Wistar Rats.
P = Proximal convoluted tubule; D = Distal convoluted tubule; G = Glomerulus; Black arrow = Blood vessel.
4. Discussion
The study appraised both dose- and sex-dependent effects of sub-acute nicotine administration on the renal function of Wistar rats. This was aimed at investigating its use or disuse in both social and clinical settings with regards to dose- and sex-dependent renal implications.
In this study, the apparently normal rats which served as control groups for both sexes showed that the assayed markers of renal function were sexually dimorphic. This was expressed as a relative decrease in plasma creatinine and urea with an increase in plasma uric acid in the female rats when compared with their male counterparts. Following exposure to nicotine administration, perturbations in the plasma and urine levels of the assayed markers were relatively more in the female groups than their respective male counterparts. Consequently, these culminated in a higher creatinine clearance (an index of renal function) in the female than their male counterparts, although creatinine clearance increased with lower doses of nicotine in both sexes. This study therefore suggests that sub-acute administration of lower doses of nicotine potentiates sex-dependent perturbation in markers of renal function resulting in a higher index of renal function in female than in male Wistar rats. The possible mechanism(s) by which this is achieved is nicotine-enhanced renal vasodilation, causing a decrease in renovascular resistance as well as increased filtration fraction. The expression of a better renal function in females than their male counterparts, as shown in this study, is consistent with existing literatures of both clinical and animal models of various renal conditions [39], [40], [41], [42], [43], [44], [45], [46]. Worthy of note is the fact that cotinine levels (stable metabolite of nicotine) in Wistar rats appear to be similar with that of smokers after cigarette consumption [47]. This study by Mohammed and Ian provides further validation of animal model of nicotine dependence. According to Hiroshi et al. [48], human blood concentrations of cotinine (a bio-monitoring marker for tobacco smoke) can be directly extrapolated from nicotine metabolism in rats using physiologically based pharmacokinetic modeling. These facts buttress the suitability of Wistar rat models in the study of clinical conditions of nicotine exposure.
Renal vasoconstriction is enhanced by nicotine in smokers, possibly through alterations of cyclic-GMP-dependent vasoactive mechanism [49]. A study by Ritz et al. showed that during smoking, glomerular filtration rate decreased in all but one volunteer, accompanied by an increased renovascular resistance with a consequent significant decrease in filtration fraction [50]. The implication of these studies is that nicotine potentiates hemodynamic disturbances/damage, with further alterations in the renal handling of fluid and electrolytes as well as renal clearance. It is possible that lower doses of sub-acute nicotine administration increased creatinine clearance in both sexes by reversing the aforementioned mechanism; that is, enhanced renal vasodilation possibly through modulations of cyclic-GMP-dependent vasodilative mechanism. This could have affected the renal clearance by causing a decrease in renovascular resistance as well as an increase in glomerular filtration and filtration fraction.
The insignificant alteration in the plasma level of some electrolytes like Na+ and HCO3− in the nicotine-treated rats of both sexes is consistent with the findings of Waheeb who reported that no significant variations in plasma Na+ level was observed in a group of subjects smoking 3–5 cigarettes per day [51]. This, like similar findings [39], further buttresses the suitability of Wistar rat model for the study of clinical models of renal conditions. The implication of this result is that the adopted doses fall below the threshold of nicotine amount that can potentiate deleterious alterations in body electrolyte levels. However, significant increase in urine Na+ level was more expressed in the male than their female counterparts while significant decrease in urine K+ level was more in the female groups. The perturbation in urine Na+ level associated with lower doses of sub-acute nicotine administration can be attributed to nicotine’s influence on the hypothalamus/osmoreceptor system which can be of clinical relevance if a more comprehensive study on its diuretic effects is undertaken. Nicotine has been reported to easily cross the blood-brain barrier and exert its effects on the brain within 10–20 s of ingestion [13]. A note of caution is emphasized in that sub-acute administration of low doses of nicotine potentiated reduction in urine K+ excretion; as low urinary K+ excretion has been found to be associated with an increased risk of developing hypertension [52].
The study showed that the highest dose of nicotine administration in both sexes caused significant alterations in most of the measured parameters. This suggests that, as opposed to lower doses, sub-acute exposure to higher doses of nicotine potentiates a high risk profile of renal dysfunction. This has been well demonstrated in some existing literatures on the effects of nicotine on renal function [53], [54], [55], [56], [57]. In a study reported by Anshu and co-workers, a single low dose of nicotine (0.25 mg/kg) administered for a period of 5 months (consecutively) resulted in nephrotoxicity as indicated by deleterious alterations in kidney markers of oxidative stress as well as apoptotic markers [58]. The disparity in results, even though both experiments involved low doses of nicotine, can be attributed to the duration of nicotine exposure. While Anshu et al. studied renal effects of nicotine after 5 months exposure (chronic study), this study was performed to ascertain renal effects after 28 days exposure (sub-acute study). The low dose that was administered for the chronic period may have cumulated biological mechanisms that simulate the effects of a high dose when administered for a shorter period. This study showed that, contrary to popular opinion which tends to associate nicotine ingestion with toxicity in biological systems, sub-acute administration of lower doses caused sex-dependent improvement of renal function in Wistar rats.
This study shows that Wistar rats express sexual dimorphism in percentage weight change and relative kidney weight following sub-acute administration of nicotine. These features are, however, higher in the male than their female counterparts. Both percentage weight change and relative kidney weights in the nicotine-treated groups were insignificantly lowered when compared with their respective control groups and in some cases they happened to be of similar values; suggesting that administration of the adopted nicotine doses are not sufficient to elicit significant changes in body and kidney weights over a sub-acute period. The apparent non-significant difference in percentage weight change can be attributed to the sustained appetite/food consumption that was observed during the course of the study since weight gain or loss is a function of a balance between food consumption and the rate of energy expenditure [59]. A sensitive indicator of an effect of an experimental compound may be significant differences in organ weights between treated and untreated animals in the absence of morphological changes [60]. Therefore, in order to avoid any complication that may arise as a result of differences in body weight between groups, the ratio of body to organ weight (generally described as relative organ weight) is commonly used for analysis [60]. The non-significant difference in relative kidney weights across the groups of both sexes provides additional support for the histological examination of the kidneys; suggesting that lower doses of nicotine have the potential to improve renal homeostasis over a sub-acute period.
In this study, histological examination of the nicotine-treated groups showed no appreciable difference in kidney histoarchitecture when compared with the control. This supports the findings of some existing literatures which suggest that nicotine administration is associated with anti-inflammatory effects [61], [62], [63], [64], [65]. There are receptors for nicotine on monocytes and endothelial cells. Inflammation is sustained if these cells are activated [66]. However, the anti-inflammatory effects of nicotine are usually mediated through α7-nAchR; binding of nicotine to α7-nAchR which is present on macrophages and endothelial cells consequently results in down-regulation of inflammation by deactivation of these cells. By this action, in many observational studies of diseases of inflammatory origin, nicotine has been found to be associated with a more favourable prognosis [11], [66]. Studies have shown that nicotine attenuates ischemia-reperfusion-induced kidney injury in animal models [67], [68], [69]. Thus, whereas there is an apparent world-wide suggestion of the nephrotoxic potential of nicotine as a component of cigarette smoke by inducing ischemia and intra-renal vasoconstriction [11], [54], [55], nicotine administration could also have reno-protective potentials by ameliorating intra-renal inflammation (as reported by the aforementioned studies) as well as potentially inhibiting the expression of intra-renal inflammation with a consequent improvement in the indices of renal function (as reported in this study) particularly at relatively lower doses. According to Tsuyoshi et al. [70], vagus nerve stimulation mediates protection from ischemia-reperfusion injury through α7-nAchR+ splenocytes. This implies that α7-nAchR potentially shows activation that may indicate nephro-protection or enhancement of kidney function.
5. Conclusion
It was concluded that sub-acute administration of lower doses of nicotine alters the plasma and urine electrolyte balance and increases creatinine clearance of Wistar rats, possibly by causing a decrease in reno-vascular resistance as well as increase in both glomerular filtration rate and filtration fraction; an effect that was more pronounced in the females than their male counterparts.
Acknowledgements
The authors wish to appreciate Mr. Afolayan and other members of staff of the Animal Holding Unit of the College of Health Sciences, Obafemi Awolowo University as well as the Faculty of Pharmacy, Obafemi Awolowo University for their kind support and technical assistance.
References
- 1.Henningfield J.E., Zeller M. Nicotine psychopharmacology research contributions to United States and global tobacco regulation: a look back and a look forward. Psychopharmacology (Berl.) 2006;184:286–291. doi: 10.1007/s00213-006-0308-4. [DOI] [PubMed] [Google Scholar]
- 2.Tariq M., Khan H.A., Al Deeb S., Al Moutaery K. Neuroprotec-tive effect of nicotine against 3-nitropropionic acid (3-NP)-inducedexperimental Huntington’s disease in rats. Brain Res. Bull. 2005;67:161–168. doi: 10.1016/j.brainresbull.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Mahar R.C., Bagot M.A., Davoli S., Miksys R.F., Tyndale C., Walker M., Maheu S., Huang T.P., Mechawar Wong N. Developmental hip-pocampal neuroplasticity in a model of nicotine replacement therapyduring pregnancy and breastfeeding. PLoS One. 2012;7:37219. doi: 10.1371/journal.pone.0037219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Science Daily, Nicotine 2017, (Retrieved from https://www.sciencedaily.com/terms/nicotine.htm [Accessed January 2017].
- 5.Ross C.G. Effect of tobacco smoking on renal function. Indian J. Med. Res. 2006;124:261–268. [PubMed] [Google Scholar]
- 6.Flouris A.D., Chorti M.S., Poulianiti K.P., Jamurtas A.Z., Kostikas K., Tzatzarakis M.N., Wallace H.A., Tsatsakis A.M., Koutedakis Y. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal. Toxicol. 2013;25:91–101. doi: 10.3109/08958378.2012.758197. [DOI] [PubMed] [Google Scholar]
- 7.Matthaios P.K., Polychronis D.S., Manolis N.T., Dimitrios K., Jyrki L., Athanasios K.A., Dionysios V., Aristidis M.T. Multicomponent analysis of replacement liquids of electronic cigarettes using chromatographic techniques. Anal. Toxicol. 2015;39:262–269. doi: 10.1093/jat/bkv002. [DOI] [PubMed] [Google Scholar]
- 8.Pauly J.L., Barry M.B. Tobacco-free electronic cigarettes and cigars deliver nicotine and generate concern. Tob. Control. 2007;16:357. doi: 10.1136/tc.2006.019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.P.S. Gardiner, The vapour this time? In: university of California Office of the President (ed.), Tobacco Related Disease Research Program (TRDRP). UCSF Health Sciences West 301, San Francisco, CA (2013) 1–19.
- 10.Schaller K., Rupper L., Kahnert S., Bethke C., Nair U., Pothschke-Langer M. German Cancer Research Centre (DFKZ); Heidelberg, Germany: 2013. Electronic Cigarettes –An Overview, Res Series Tobacco Prevention and Tobacco Control; pp. 1–59. [Google Scholar]
- 11.Agarwal P.K. Dissertation University of Groningen; 2012. Smoking, Nicotine and the Kidney. (ISBN: 978-94-6203-069-5) [Google Scholar]
- 12.U.S. Department of Health and Human Services . U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta (GA): 2010. How Tobacco Smoke Causes Disease—The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. [Google Scholar]
- 13.Benowitz N.L., Hukkanen J., Jacob P., Jr. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crooks P.A., Dwoskin L.P. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem. Pharmacol. 1997;54:743–753. doi: 10.1016/s0006-2952(97)00117-2. [DOI] [PubMed] [Google Scholar]
- 15.Risso F., et al. Chronic nicotine causes functional upregulation of ionotropic glutamate receptors mediating hippocampal noradrenaline and striatal dopamine release. Neurochem. Int. 2004;44:293–301. doi: 10.1016/s0197-0186(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 16.Osterhout C.A., et al. Induction of tyrosine hydroxylase in the locus coeruleus of transgenic mice in response to stress or nicotine treatment: lack of activation of tyrosine hydroxylase promoter activity. J. Neurochem. 2005;94:731–741. doi: 10.1111/j.1471-4159.2005.03222.x. [DOI] [PubMed] [Google Scholar]
- 17.Dickinson J.A., Kew J.N., Wonnacott S. Presynaptic alpha 7- and beta 2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol. Pharmacol. 2008;74:348–359. doi: 10.1124/mol.108.046623. [DOI] [PubMed] [Google Scholar]
- 18.Parikh K., Man M.W., Sarter Decker M. Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex. J. Neurosci. 2008;28:3769–3780. doi: 10.1523/JNEUROSCI.5251-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarter M., Parikh V., Howe W.M. nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem. Pharmacol. 2009;78:658–667. doi: 10.1016/j.bcp.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dos Santos C.R., Granon S. Prefrontal neuromodulation by nicotinic receptors for cognitive processes. Psychopharmacology (Berl.) 2012;221:1–18. doi: 10.1007/s00213-011-2596-6. [DOI] [PubMed] [Google Scholar]
- 21.Astrup P. Carbon monoxide, smoking, and cardiovascular disease. Circulation. 1973;48:1167–1168. doi: 10.1161/01.cir.48.6.1167. [DOI] [PubMed] [Google Scholar]
- 22.Hayano J., Yamada M., Sakakibara Y., Fujinami T., Yokoyama K., Watanabe Y. Short and long-term effects of cigarette smoking on heart rate variability. Am. J. Cardiol. 1990;65:84–88. doi: 10.1016/0002-9149(90)90030-5. [DOI] [PubMed] [Google Scholar]
- 23.Cope G.F., Battersby N. Smoking verification and the risk of myocardial infarction. J. Epidemiol. Commun. Health. 2004;58:156–157. doi: 10.1136/jech.58.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnoya J., Glantz S.A. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 25.Tonstad S., Johnston A.J. Cardiovascular risks associated with smoking: a review for clinicians. Eur. J. Cardiovasc. Prev. Rehab. 2006;13:507–515. doi: 10.1097/01.hjr.0000214609.06738.62. [DOI] [PubMed] [Google Scholar]
- 26.D’Alessandro A., Boeckelmann I., Hammwhoner M., Goette A. Nicotine, cigarette smoking and cardiac arrhythmia: an overview. Eur. J. Prev. Cardiol. 2012;19:297–305. doi: 10.1177/1741826711411738. [DOI] [PubMed] [Google Scholar]
- 27.Walter F.B. 1st ed. Elsevier; Saunders: 2004. Medical Physiology: A Cellular and Molecular Approach. [Google Scholar]
- 28.Stuart F. 12th ed. McGraw-Hill; 2011. Human Physiology. [Google Scholar]
- 29.Valerie C., Scanlon T.S. 5th ed. F.A. Davis Company; Philadelphia: 2007. Essentials of Anatomy and Physiology. [Google Scholar]
- 30.Shivaraj G., Prakash B.D., Shruthi S.K., Vinayak V.H., Avinash A.K.M., Sonal N.V. Markers of renal function tests. North Am. J. Med. Sci. 2010;2:170–173. [PMC free article] [PubMed] [Google Scholar]
- 31.Edmund L., David J. In: Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 4th ed. Carl A.B., Edward R., David E., editors. Elsevier Inc.; New Delhi: 2006. Kidney function tests; pp. 797–808. [Google Scholar]
- 32.Medical dictionary . 2017. Meaning of Sub-acute. (Retrieved from www.medical-dictionary.thefreedictionary.com/subacute [Accessed 07.07.17]) [Google Scholar]
- 33.Determination of acute . 2017. Sub-acute Chronic and Sub-chronic Doses. (Retrieved from http://www.researchgate.net/post/How_does_one_determine_doses_for_acute_sub-acute_chronic_and_sub-chronic_toxicity [Accessed 07.07.17]) [Google Scholar]
- 34.Muralidhara S., Ramanathan R., Metha S.M., Lash L.H., Acosta D., Bruckner J.V. Acute, sunacute and subchronic oral toxicity studies of 1, 1-dichloroethane in rats: application to risk evaluation. Toxicol. Sci. 2001;64:135–145. doi: 10.1093/toxsci/64.1.135. [DOI] [PubMed] [Google Scholar]
- 35.Pilar M.O., Lozano M.C., Botero L., Rincon J., Guerrero M.F. Evaluation of the acute and subchronic oral toxicity of ethanol extract from Valeriana pavonii species in Wistar rats. Colomb. Med. 2010;41:256–266. [Google Scholar]
- 36.Jaffe M. Ueberden Niederschlag welchen Pikrinsaure in normalen Harn erzeugt und uber eine neue reaction des Kreatinins. Z. Phys. Chem. 1886;10:391–400. [Google Scholar]
- 37.8th ed. 2011. Guide for the Care and Use of Laboratory Animals. (Retrieved from http://www.google.com.ng/?gfe_rd=cr&ei=KWkSVvy7MtPH8gehuLvQDg#q=nih+guidelines+animal+care [Accessed February 2017]) [Google Scholar]
- 38.Imafidon C.E., Olatoye T.R., Bamidele F.S., Ojo O.E., Ademoye K.A. Cadmium-induced testicular toxicity, oxidative stress and histopathology in Wistar rats: sustained effects of polyphenol-rich extract of Vernonia amygdalina (Del.) leaf. J. Interdiscip. Histopathol. Scopemed. 2016;4:54–62. doi: 10.5455/jihp.20160618041629. [DOI] [Google Scholar]
- 39.Imafidon C.E., Akomolafe R.O., Oladele A.A. Sexually dimorphic proteinuria in Wistar rats: relevance to clinical models. Pathophysiology. 2016;23:51–59. doi: 10.1016/j.pathophys.2016.02.001. (Elsevier) [DOI] [PubMed] [Google Scholar]
- 40.Silbiger S.R., Neugarten J. The impact of gender on the progression of chronicrenal disease. Am. J. Kidney Dis. 1995;25:515–533. doi: 10.1016/0272-6386(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 41.Tsjitske J.T., van der Graaf A.M., Folkert W.V., Hendrik B., Gerjan N., Marijke M.F., Lely A.T. Gender differences in response to acute and chronic angiotensin-II infusion: a translational approach. Physiol. Rep. 2015;3:e12434. doi: 10.14814/phy2.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Telma B., Tamy I.R., Lenir C.P., de Souza A.S., Iandara S.S. Renal biomarkers of male and female Wistar rats (Rattus norvegicus) undergoing renal ischemia and perfusion. Acta Cir. Bras. 2015;30:277–289. doi: 10.1590/S0102-865020150040000007. [DOI] [PubMed] [Google Scholar]
- 43.Mehdi N., Shadi E., Mona T., Eshraghi-Jazi F., Ardeshir T., Farzaneh A. Gender difference in cisplatin-induced nephrotoxicity in a rat model: greater intensity of damage in male than female. Nephro Urol. Mon. 2013;5:818–821. doi: 10.5812/numonthly.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin Z., Uzi G., Eberhard R. Renal function and renal disease in males or females –vive la petite difference. Nephrol. Dial. Transplant. 1998;13:2195–2198. doi: 10.1093/oxfordjournals.ndt.a027910. [DOI] [PubMed] [Google Scholar]
- 45.Jose L.R., Estela M., Augusto A., Jaramillo-Juarez F. Influence of sex differences on the renal secretion of organic anions. Endocrinology. 1998;139:1581–1587. doi: 10.1210/endo.139.4.5930. [DOI] [PubMed] [Google Scholar]
- 46.Susan E.M., Craig W., Michele J., Carlo P. Gender difference in renal growth and function after uninephrectomy in adult rats. Kidney Int. 1999;56:944–953. doi: 10.1046/j.1523-1755.1999.00647.x. [DOI] [PubMed] [Google Scholar]
- 47.Mohammed S., Ian P.S. Plasma nicotine and cotinine levels following intravenous nicotine self-administration in rats. Psychopharmacology (Berl.) 1999;143:318–321. doi: 10.1007/s002130050954. [DOI] [PubMed] [Google Scholar]
- 48.Hiroshi Y., Horiuchi K., Takano R., Nagano T., Shimizu M., Kitajima M., Murayama N., Shong F. Human blood concentrations of cotinine, a biomonitoring marker for tobacco smoke, extrapolated from nicotine metabolism in rats and humans and physiologically based pharmacokinetic modeling. Int. J. Environ. Res. Public Health. 2010;7:3406–3421. doi: 10.3390/ijerph7093406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jean-Michel H., Claude P., Albert M. Contrasting renal effects of nicotine in smokersand non-smokers. Nephrol. Dial. Transplant. 1998;13:940–944. doi: 10.1093/ndt/13.4.940. [DOI] [PubMed] [Google Scholar]
- 50.Ritz E., Benck U., Franek E., Keller C., Seyfarth M., Clorius J. Effects of smoking on renal hemodynamics in healthy volunteers and in patients with glomerular disease. J. Am. Soc. Nephrol. 1998;9:1798–1804. doi: 10.1681/ASN.V9101798. [DOI] [PubMed] [Google Scholar]
- 51.Waheeb D.M.A. Electrolyte changes in cigarette smoking male students. Pak. J. Pharmacol. 2012;29:33–38. [Google Scholar]
- 52.Lyanne M.K., Ron T.G., Kenneth J.M., deBoer R.A., Gerjan N., Stephen J.L.B., Michel M.J. Urinary potassium excretion and risk of developing hypertension. Hypertension. 2014;64:769–776. doi: 10.1161/HYPERTENSIONAHA.114.03750. [DOI] [PubMed] [Google Scholar]
- 53.Gauray J., Edgar A.J. Nicotine signalling and progression of chronic kidney disease in smokers. Biochem. Pharmacol. 2014;86:1215–1223. doi: 10.1016/j.bcp.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orth S.R., Hiroaki O., Eberhard R. Smoking and the kidney. Nephrol. Dial. Transplant. 2000;15:1509–1511. doi: 10.1093/ndt/15.10.1509. [DOI] [PubMed] [Google Scholar]
- 55.Orth S.R., Eberhard R., Robert W.S. The renal risk of smoking. Kidney Int. 1997;51:1669–1677. doi: 10.1038/ki.1997.232. [DOI] [PubMed] [Google Scholar]
- 56.Ross G.C. Effect of tobacco smoking on renal function. Indian J. Med. Res. 2006;124:261–268. [PubMed] [Google Scholar]
- 57.Stein H., Orth S.R. Smoking is a risk factor in the progression to kidney failure. Int. Soc. Nephrol. 2011;80:516–523. doi: 10.1038/ki.2011.157. [DOI] [PubMed] [Google Scholar]
- 58.Anshu J., Shruti A., Swaran J.S.F. Arsenic and nicotine co-exposure lead to some synergistic effects on oxidative stress and apoptotic markers in young rat blood, liver, kidneys and brains. Toxicol. Rep. 2015;2:1334–1346. doi: 10.1016/j.toxrep.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katherine A.S., Niamh M.M., Steve R.B. Hypothalamic regulation of apetite. Expert Rev. Endocrinol. Metab. 2008;3:577–592. doi: 10.1586/17446651.3.5.577. [DOI] [PubMed] [Google Scholar]
- 60.Steven A.B., Robert H.Z., Richard W.P. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol. Pathol. 2004;32:448–466. doi: 10.1080/01926230490465874. [DOI] [PubMed] [Google Scholar]
- 61.Roma K., Shashi P.S., Pena-Philippides J.C., Raymond J.L., Razani-Boroujerdi S., Mohan L.S. Immunosuppressive and anti-inflammatory effects of nicotine administered by patch in animal model. Clin. Diagn. Lab. Immunol. 2004;11:563–568. doi: 10.1128/CDLI.11.3.563-568.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shaheen E.L., Annette K. Anti-inflammatory effects of nicotine in obesity and ulcerative colitis. J. Transl. Med. 2011;9:1–10. doi: 10.1186/1479-5876-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Claude S., Gwen T., Guert S., Carole K., Nike C., Fabrice M., Patrizia L., Bilo D., Luc B., Michel G., Sandrine F., Le Moine A. Nicotine protects kidney from renal ischemia/reperfusion injury through the cholinergic anti-inflammatory pathway. PLoS One. 2007;5:e469. doi: 10.1371/journal.pone.0000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adriana M.G., Wallace C.F., Leonardo Tadeu S.R., Raphael G.F., Andre Luiz L.P., Leonardo A.M., Nascimento E.B., Jr., Leandro Francisco S.B., Marcio M.C. Nicotinic acid induces antinociceptive and anti-inflammatory effects in different experimental models. Pharmacol. Biochem. Behav. 2012;101:493–498. doi: 10.1016/j.pbb.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 65.Alex C., Valentin G., Igor B.S., Sergei A.G. Anti-Inflammatory effects of the nicotinergic peptides SLURP −1 and SLURP −2 on human intestinal epithelial cells and immunocytes. BioMed. Res. Int. 2014;2014:1–8. doi: 10.1155/2014/609086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ying J., Longling L., Bin L., Yanhong Z., Qian C., Changqing L. Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat. PLoS One. 2014;7:e102342. doi: 10.1371/journal.pone.0102342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yeboah M.M., Xue X., Duan B., Ochani M., Tracey K.J., Susin M., Metz C.N. Cholinergic agonists attenuate renal ischemia injury in rats. Int. Soc. Nephrol. 2008;74:62–69. doi: 10.1038/ki.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeboah M.M., Xue X., Javdan M., Susin M., Metz C.N. Nicotinic acetylcholine receptor expression and regulation in the rat kidney after ischemia-reperfusion injury. Am. J. Physiol. Renal Physiol. 2008;295:F654–61. doi: 10.1152/ajprenal.90255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Orth S.R. Cigarette smoking: an important renal risk factor –far beyond carcinogenesis. Tob. Induc. Dis. 2002;1:137–155. doi: 10.1186/1617-9625-1-2-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsuyoshi C., Abe S.J., Sung S., Moscula J., Jankowski L., Huang Y., Hong D.L., Rosin P.G., Guyenet M.D. Okusa, Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through (7-nAchR+ splenocytes. J. Clin. Invest. 2016;126:1939–1952. doi: 10.1172/JCI83658. [DOI] [PMC free article] [PubMed] [Google Scholar]