Introduction
Somatic growth may be altered in patients with cryopyrin-associated periodic syndrome (CAPS), a rare dominantly inherited autoinflammatory disease prevalently driven by uncontrolled production of IL-1 and caused by missense mutations in the NLRP3 gene, which encodes cryopyrin, a protein chiefly involved in the processing of IL-1 (1). In particular, neonatal-onset multisystem inflammatory disease (NOMID) is the most severe clinical expression of CAPS. NOMID is characterized by the triad urticaria-like rash, chronic meningitis, and a distinctive osteo-arthropathy that begins soon after birth (2). While advances have been made in the treatment of CAPS following the introduction of specific IL-1 antagonists (3), the long-term benefits of this treatment on growth are unknown, specifically in NOMID patients. In addition, for children with NOMID there are no data regarding baseline plasma levels of GH and/or normal, subnormal, or paradoxical GH release in response to several secretagogues. Furthermore, there are no data regarding the occurrence of overt endocrine disorders in NOMID patients.
Case Report
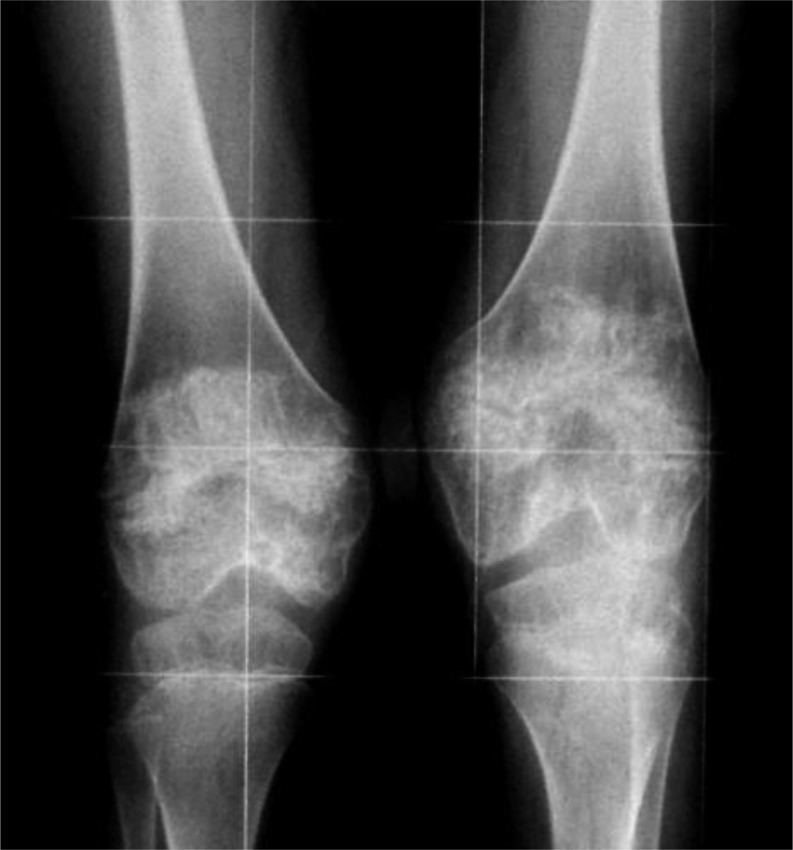
We report on a 20-yr-old Caucasian man born in 1997 at 36 wk of gestation from unrelated parents with a neonatal weight of 2.5 kg (–1.4 SD). He experienced recurrent bouts of fever beginning in the first month of life, despite antibiotic and corticosteroid treatments. Protruding patellae and severe migratory urticaria-like rashes were evident during childhood in association with a consistent elevation of the levels of inflammatory markers. Diagnosis of NOMID was made 3 yr after the detection of distal femurs with broad epiphyses and early ossification of patellae, and after genotype analysis (NLRP3 mutation: T1718C). At this time, he was completely unable to walk unaided. Treatment with the tumor necrosis factor antagonist etanercept (0.4 mg/kg, subcutaneously [sc] injected twice a week) was started at 4 yr of age with limited success on systemic manifestations (4). Treatment with the IL-1 receptor antagonist anakinra (1 mg/kg, sc injected every day) was started in combination with 0.1 mg/kg methylprednisolone on alternate days at 7 yr of age. After only 1 wk of anakinra treatment, dramatic improvement was evident in all clinical signs and laboratory findings, including urticaria-like rash and acute-phase reactants, which became normal for the first time in his life (5). Despite these improvements, growth was poor, with a height of 104 cm (–3.47 SD) and weight of 17.4 kg (–2.44 SD). NOMID-specific imaging of the knees revealed mass-producing lesions in the physis of both femora (Fig. 1).
Fig. 1.
Anteroposterior radiograph of the knees performed at 7 yr of age (before starting anakinra treatment). The radiograph displays the unique skeletal feature of NOMID, which is believed to be the expression of abnormal endochondral bone growth, mainly affecting large joints and long bones. Mass-like calcified lesions separated from the metaphysis by well-defined borders are visible in the physis of the distal femura.
The patient began to walk autonomously at 10 ys of age. The anakinra treatment at an adjusted dosage of 2 mg/kg/day, then rounded to 100 mg/day, combined with very low-dose methylprednisolone on alternate days, was continued for many years without substantial side effects. Nonetheless, growth was subnormal and seemed to worsen at approximately 13 yr of age, when NOMID-related neurologic symptoms were almost absent and inflammatory parameters were completely normal. Height and weight was 124.3 cm (–4.6 SD) and 39 kg (+1.39 SD), respectively, with Tanner stage G1-PH1 and a testicular volume of 3 ml. Growth velocity was far lower than the 3rd percentile for age, while bone age was significantly delayed, being equivalent to 9.8 yr of age at the patient’s biological age of 13 yr.
His parents insisted that further investigations of the growth and pubertal delays be done. Systemic causes of short stature other than the primary disease were excluded. To measure the secretion of GH, two provocative tests were performed (clonidine test and combined arginine + GH-release hormone test). The stimulated GH peak levels in the tests were 3.3 and 7.5 ng/ml, respectively, and GH deficiency (GHD) was diagnosed. On the basis of auxological, biochemical, and radiologic findings, recombinant human GH (rhGH) therapy was started at 13.5 yr of age (in November 2010), after magnetic resonance imaging revealed no change in the appearance of the brain, including the absence of mid-line defects, in comparison with previous imaging studies. At this time, the level of IGF-1 was 72 ng/ml (normal value for age and sex: 203–477 ng/ml) and rhGH was administered daily via sc injection at the initial dose of 20 μg/kg/day, with dose adjustment every 6 months on the basis of growth velocity and serum IGF-1 level. Three months later, the height velocity had sharply improved and peaked at a rate of 11 cm/yr after 19 mo. Puberty started at the age of 14 yr. At that time, NOMID was completely controlled by IL-1 blockade with the initial use of anakinra, then canakinumab at 17 yr of age (300 mg sc every 3–4 wk). Concurrently, very low-dose methylprednisolone was occasionally used by the patient to control neurologic symptoms such as vomiting (6).
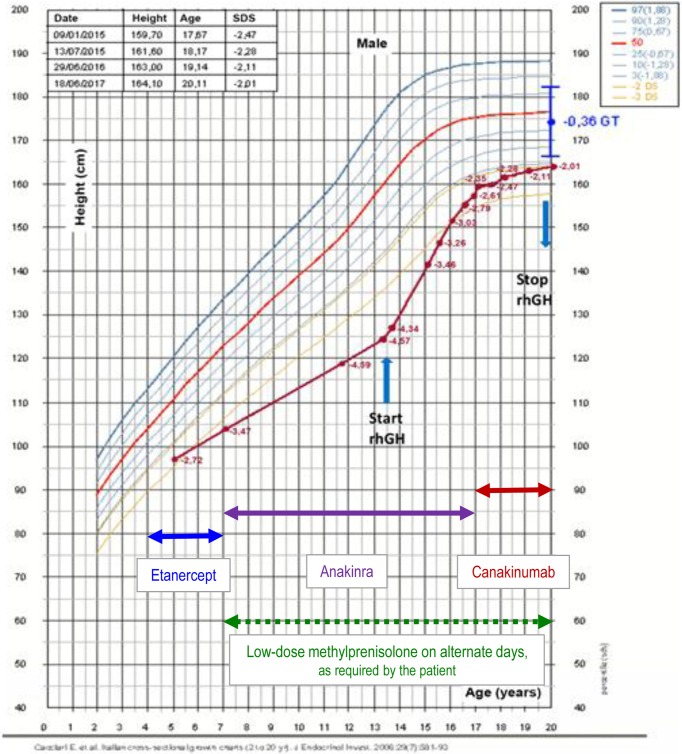
Auxometric data (height, weight, body mass index), results of IGF-1 assessments, and rhGH doses used are presented in Table 1. Therapy with rhGH was stopped at 20 yr of age (in May 2017). At that time, the growth velocity was less than 1 cm/yr and bone epiphyseal fusion had been achieved. Moreover, at this time the patient had attained his final height of 164.5 cm, which was the lower limit of his genetic target. However, he had gained more than 2.5 SD of his final height, with a significant catch-up growth during the first 3 yr of treatment (Fig. 2). No rhGH-related side effects were observed.
Table 1. Auxometric data (height, weight, body mass index) combined with results of IGF-1 assessments and rhGH doses administered.
Fig. 2.
Percentiles of height. The percentiles were determined as previously described (11). The red line denotes the patient’s growth. The blue arrows indicate the beginning and end of recombinant human GH (rhGH) therapy. The blue point is the patient’s genetic target (GT).
Discussion
A subverted repertoire of IL-1-mediated signalling pathways might not be the only pathogenetic system at the growth plate level to explain the baffling skeletal manifestations of NOMID. Constitutively activated NLRP3 inflammasome and IL-1 oversecretion in mouse chondrocytes lead to abnormal skeletal development, and prolonged hyperinflammatory status induces abnormal endochondral bone growth in NOMID patients (7). However, the exact relationship between IL-1 and GH is unknown, although the influence of IL-1 on the brain has been revealed by extensive data indicating its epileptogenic properties in mouse models (8). Several studies have also provided evidence that proinflammatory cytokines might have inhibitory effects on the skeleton through changes in the GH/IGF-I system, and that IL-1 is an important mediator of GH resistance during inflammation (9).
It is not known whether NOMID treatment with IL-1 blockade modulates systemic inflammation, which could potentially lead to secondary improvement of growth delay or hinder a potential IL-1-mediated GH resistance. Nevertheless, treatment with IL-1 antagonists as a first-line therapy in NOMID soon after diagnosis might thwart the occurrence of irreversible bone changes and improve disease effects on somatic growth, which might be affected by previous or concomitant less specific therapies. Indeed, deterioration of short-term growth might also depend on the use of corticosteroids and could occur through alterations in gonadal function at the level of the pituitary, or through direct effects on the gonads (10).
The present data simply indicate that stunted growth as part of the NOMID clinical scenery might be related to the significant skeletal picture of the disease itself and also to GHD, and that a substantial response to rhGH can be observed in such a complex disorder even if started late. Therefore, a full endocrinologic assessment to investigate hormone deficiencies should be considered in the management of NOMID patients. Further clinical studies are needed to establish the benefits of rhGH treatment in NOMID (in terms of height gain, metabolic changes, changes in quality of life) as well as the development of undesirable effects during and after discontinuation of treatment.
Conflict of Interest
The authors declare that they have no disclosures in relationship with the subject of this communication.
References
- 1.Rigante D. A systematic approach to autoinflammatory syndromes: a spelling booklet for the beginner. Expert Rev Clin Immunol 2017;13: 571–97. doi: 10.1080/1744666X.2017.1280396 [DOI] [PubMed] [Google Scholar]
- 2.Cantarini L, Lucherini OM, Frediani B, Brizi MG, Bartolomei B, Cimaz R, et al. Bridging the gap between the clinician and the patient with cryopyrin-associated periodic syndromes. Int J Immunopathol Pharmacol 2011;24: 827–36. doi: 10.1177/039463201102400402 [DOI] [PubMed] [Google Scholar]
- 3.Rigante D, Vitale A, Lucherini OM, Cantarini L. The hereditary autoinflammatory disorders uncovered. Autoimmun Rev 2014;13: 892–900. doi: 10.1016/j.autrev.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 4.Federico G, Rigante D, Pugliese AL, Ranno O, Catania S, Stabile A. Etanercept induces improvement of arthropathy in chronic infantile neurological cutaneous articular (CINCA) syndrome. Scand J Rheumatol 2003;32: 312–4. doi: 10.1080/03009740310003974 [DOI] [PubMed] [Google Scholar]
- 5.Rigante D, Ansuini V, Caldarelli M, Bertoni B, La Torraca I, Stabile A. Hydrocephalus in CINCA syndrome treated with anakinra. Childs Nerv Syst 2006;22: 334–7. doi: 10.1007/s00381-006-1280-3 [DOI] [PubMed] [Google Scholar]
- 6.Rigante D, Verrecchia E, Falsini B, Manna R. Switch from anakinra to canakinumab in a severe case of CINCA syndrome. Int J Rheum Dis 2016;19: 1354–6. doi: 10.1111/1756-185X.12800 [DOI] [PubMed] [Google Scholar]
- 7.Bonar SL, Brydges SD, Mueller JL, McGeough MD, Pena C, Chen D, et al. Constitutively activated NLRP3 inflammasome causes inflammation and abnormal skeletal development in mice. PLoS ONE 2012;7: e35979. doi: 10.1371/journal.pone.0035979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubé C, Vezzani A, Behrens M, Bartfai T, Baram TZ. Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann Neurol 2005;57: 152–5. doi: 10.1002/ana.20358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooney RN, Shumate M. The inhibitory effects of interleukin-1 on growth hormone action during catabolic illness. Vitam Horm 2006;74: 317–40. doi: 10.1016/S0083-6729(06)74013-4 [DOI] [PubMed] [Google Scholar]
- 10.Mushtaq T, Ahmed SF. The impact of corticosteroids on growth and bone health. Arch Dis Child 2002;87: 93–6. doi: 10.1136/adc.87.2.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cacciari E, Milani S, Balsamo A, Spada E, Bona G, Cavallo L, et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J Endocrinol Invest 2006;29: 581–93. doi: 10.1007/BF03344156 [DOI] [PubMed] [Google Scholar]