ABSTRACT
Mammalian sperm evolutionarily acquired complex mechanisms to regulate their behaviors, which are thought to be crucial in navigating through the female reproductive tract toward fertilization. However, all current knowledge of this process is largely extrapolated from in vitro and ex vivo studies, because in vivo analysis of sperm in their native fertilization environment has not been possible. Here, we report a functional optical coherence tomography approach that allows, for the first time, in vivo three-dimensional tracking of sperm behaviors in the mouse oviduct. Motile sperm are identified with their intrinsic dynamic characteristics. Sperm trajectories are reconstructed in three dimensions with a ∼5 µm spatial resolution, allowing for quantitative analysis of the sperm velocity and location relative to the oviduct. Using this method, we found different behavior patterns, including sperm collection by the oviduct epithelium, spatial dependence of sperm velocity, and sperm grouping and separation as the first in vivo evidence of sperm cooperation in the ampulla, the site of fertilization. This approach opens new avenues to study sperm-oviduct interactions in vivo toward a more complete understanding of fertility and reproductive disorders.
KEY WORDS: Sperm behavior, Optical coherence tomography, Mouse oviduct, In vivo, Three-dimensional tracking, Quantitative imaging
Summary: A novel three-dimensional sperm tracking approach based on functional optical coherence tomography using endogenous contrast reveals previously unseen sperm behaviors in the mouse oviduct ampulla in vivo.
INTRODUCTION
In 1677, using one of the first microscopes of his design, Antonie van Leeuwenhoek was the first scientist to describe the spermatozoa (Lewenhoeck, 1677). Over the subsequent 340 years, while great insight into sperm dynamics and regulation have been provided, studies of mammalian sperm activities have mainly relied on in vitro or ex vivo experiments (Bahat et al., 2003; Jikeli et al., 2015; Kantsler et al., 2014; Kölle et al., 2009; Moore et al., 2002; Oliveira et al., 1999; Su et al., 2012). As a result, most of our current knowledge regarding mammalian sperm behaviors during fertilization, such as sperm cooperation, is largely extrapolated and completely lacks in vivo evidence (Coy et al., 2012; Pizzari and Foster, 2008). Furthermore, owing to its unique biofluidic mechanics (Fauci and Dillon, 2005) and molecular modulation in response to gametes (Georgiou et al., 2007), accurate in vitro and ex vivo modeling of the native oviduct microenvironment is impossible. Thus, the lack of the ability to directly analyze sperm behaviors in the mammalian oviduct in vivo is a significant technical hurdle preventing advanced studies in reproductive biology (Coy et al., 2012; Eisenbach and Giojalas, 2006; Kölle, 2015).
Owing to the location of the oviduct deep inside the body cavity, visualization of sperm within the mammalian reproductive tract is challenging. As a step forward, fluorescent labeling of sperm has enabled the first generation of in vivo imaging (Druart et al., 2009; Ishikawa et al., 2016; Muro et al., 2016). Specifically, Druart et al. used confocal fluorescence microscopy with a fiber-optic probe to examine ram sperm motility through the ewe cervix to the utero-tubal junction after intra-uterine insemination (Druart et al., 2009). Ishikawa et al. employed epifluorescence microscopy to assess the effect of mouse oviduct contraction on sperm transportation in the isthmus (Ishikawa et al., 2016). Relying on different fluorescent labeling of the sperm acrosome and midpiece mitochondria, Muro et al. observed sperm behaviors throughout the mouse female reproductive tract at a number of time points after natural mating (Muro et al., 2016). With similar transgenic mice and an epifluorescence imaging setup, other studies have also investigated the change of the sperm acrosomal status at various anatomical locations of the reproductive tract over time post-coitus (Hino et al., 2016; La Spina et al., 2016). Such work revealed interesting findings on sperm activities in the mouse oviduct. However, this approach lacks depth-resolved information, which is unfavorable for sperm tracking in the oviduct – a dynamic three dimensional (3D) environment. This limitation prevents volumetric reconstruction of sperm movements within the oviduct, and therefore makes the method prone to errors in quantitative measurements (such as sperm velocity). Thus, high-resolution, high-speed, depth-resolved 3D imaging capable of capturing sperm dynamics in vivo in relation to the microstructure of the oviduct is lacking.
Although the ampulla is where fertilization occurs (Suarez and Pacey, 2006), surprisingly little is known about whether its environment impacts sperm behaviors. For example, it is widely acknowledged that sperm cells are bound to the epithelial cells in the isthmus, forming a sperm reservoir, and later become capacitated with detachment (Suarez, 2002); however, after these events, it is unknown how sperm behave and interact with the oviduct wall or mucosa fold in the intact ampulla in vivo. Only recently did Suarez's group begin to identify, through ex vivo and in vitro experiments, interesting flagellar movement patterns and sperm-epithelium bindings in the ampulla that are different from the ones in the isthmus (Ardon et al., 2016; Chang and Suarez, 2012). However, whether these activities of sperm are present in the in vivo context is not known. Many fundamental issues, such as what behaviors sperm exhibit prior to fertilization and what role the oviduct cilia play in regulating such sperm behaviors, still remain unresolved (Suarez, 2016).
To achieve a complete understanding of mammalian sperm motility and fertilization, we have developed a functional optical coherence tomography (OCT) method that enables, for the first time, in vivo 3D dynamic analyses of sperm behaviors within the living mouse oviduct in its native state. OCT is a low-coherence imaging modality that relies on backscattered light from tissue to produce 3D structural imaging with micro-scale spatial resolution, thus not requiring exogenous contrast agents (Huang et al., 1991), and is widely applied in research and clinical settings (Fercher et al., 2003). Taking advantage of the millimeter-level imaging depth of OCT and employing a surgical procedure that is similar to the one routinely used in transgenic mouse production (Fig. S1), we were able to perform time-lapse 3D imaging of the oviduct ampulla of a live female mouse, covering the entire lumen and oviduct wall. Although the large imaging field of view is ideal for tracking sperm, the morphology of mouse sperm cannot be resolved with traditional structural OCT imaging due to its small size, making it challenging to identify sperm in the oviduct lumen among other scatters. To overcome this limitation, we have developed a functional OCT method with a new parameter that distinguishes motile sperm based on its unique tortuous trajectory and endogenous contrast. Using this method, our in vivo experiments exposed a variety of 3D sperm trajectories in the ampulla, revealing their intriguing movement patterns. We have identified a sperm collection process by the ampulla epithelium that features a consistent reduction of sperm velocity, determined the influences of sperm-epithelium distance on the velocity of sperm in the near-epithelium region, documented multiple sperm grouping and separation events serving as the first in vivo evidence of sperm cooperation in the ampulla, observed linear and circular trajectories of sperm between adjacent mucosa folds, and shown sperm motility in the presence of the cumulus-oocyte complex. We expect that this new optical method for in vivo sperm analysis, together with previously developed approaches for resolving the cumulus-oocyte complex (Burton et al., 2015) and mapping cilia beat frequency (Wang et al., 2015a) in the mouse oviduct, will provide unprecedented visualization and functional analyses of mammalian fertilization. From this vantage point, we will be able to achieve a more complete understanding of the complex and dynamic interplay between sperm and the oviduct that leads up to the moment of conception.
RESULTS
Functional analysis of trajectory enables identification of motile sperm with endogenous contrast
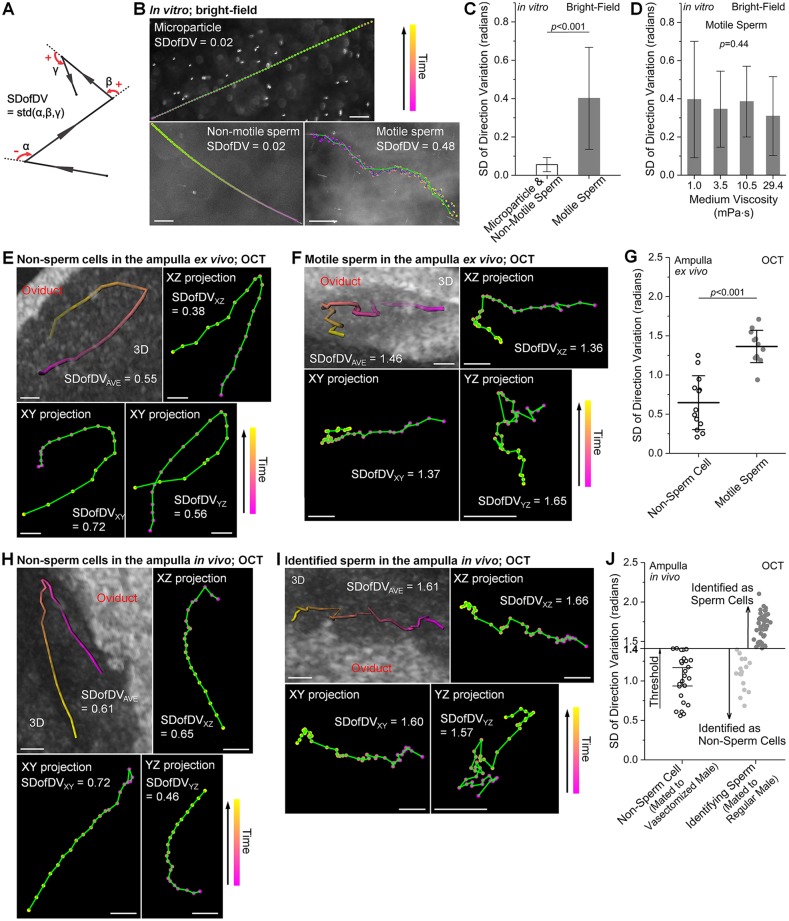
Spermatozoa have unique morphology and motion characteristics, which distinguish them from other cells (Phillips, 1972). Owing to limited spatial resolution of OCT, subcellular details of individual sperm, such as flagella, cannot be resolved. As a result, sperm cells in suspension are detected as bright spots in structural OCT images, which are indistinguishable from other cells in the oviduct. To distinguish motile sperm from non-sperm cells in the oviduct ampulla, we developed a quantitative sperm identification method relying on the tortuous trajectory and the constantly changing direction of the swimming sperm. Previous studies have characterized the movement features of mammalian sperm in vitro (Corkidi et al., 2008; Phillips, 1972; Su et al., 2012; Babcock et al., 2014), although not for identification purposes, revealing frequent and distinct variations in the direction of the moving sperm, which are used as a signature for identifying motile sperm in our method. To quantitatively assess this signature, we established a new parameter, the standard deviation of direction variation (SDofDV), to characterize the obtained trajectories. The direction variation is measured by the angle of the direction change in a 2D plane (Fig. 1A), in the range of (−π, +π], with the counterclockwise and clockwise rotations denoting positive and negative angles, respectively. The SD of these angles is calculated to reflect the dispersion of the direction change. Within the 3D time-lapse dataset acquired with OCT, both sperm and non-sperm cells were detected as bright spots (scatters). Volumetric trajectories for sperm identification were obtained by tracking the bright spots over time within the oviduct lumen (Fig. S2). Then the bulk movement correction was performed based on the oviduct wall or mucosa fold (Fig. S3), and the true velocity of the scatters, as well as its three components in the x, y and z axes were obtained. Calculation of the SDofDV was conducted for three 2D projections, x-z, x-y and y-z planes. The final SDofDV used for sperm identification is an average of three values from 2D projections.
Fig. 1.
The SDofDV as a dynamic signature of motile sperm. (A) An illustration for calculating the SDofDV from a trajectory in a 2D plane. (B) Representative trajectories of a microparticle, non-motile sperm and motile sperm in vitro in the Petri dish obtained with bright-field microscopy. (C) The SDofDV values are higher (statistically significant) for motile sperm than for microparticles and non-motile sperm in vitro in the Petri dish. Data are mean±s.d.; n=60 for microparticles and non-motile sperm; n=169 for motile sperm; P=4.7×10−38; t-test; effect size=1.3. (D) The SDofDV values of motile sperm do not show statistically significant difference over a wide range of media viscosities. Data are mean±s.d.; n=30 for motile sperm in each viscosity of medium; P=0.44, one-way ANOVA. (E,F) Representative 3D trajectories and 2D projections of (E) naturally existing non-sperm cells in the ampulla and (F) injected motile sperm in the washed ampulla, both ex vivo from virgin females, obtained with OCT. (G) The SDofDV values are higher (statistically significant) for motile sperm than for non-sperm cells in the ampulla ex vivo. Data are mean±s.d.; n=13 for both non-sperm cells and motile sperm; P=1.1×10−6; t-test; effect size=1.8. (H,I) Representative 3D trajectories and 2D projections of (H) non-sperm cells in the ampulla of the female mated to vasectomized male and (I) identified motile sperm in the ampulla after mating to regular male, obtained with OCT in vivo. (J) The SDofDV values obtained in vivo from females mated to vasectomized males define the threshold for in vivo sperm identification. Whiskers are c.i. (95%); n=26 for non-sperm cells in females mated to vasectomized males. n=16 and n=44 for identified non-sperm cells and sperm cells, respectively, in females mated to regular males. Green lines in B,E,F,H,I connect the positions with a 900 ms interval. Scale bars: 50 µm in B; 30 µm in E,F,H,I.
To determine whether the SDofDV can be applied for identification of motile sperm, we first performed in vitro analysis of sperm behaviors in a Petri dish using bright-field microscopy. The microparticles and non-motile sperm produced smooth trajectories (owing to bulk movements), whereas the motile sperm followed a tortuous path (Fig. 1B). This resulted in higher SDofDV values (statistically significant) for motile sperm in comparison with microparticles and non-motile sperm (Fig. 1C), indicating that the SDofDV can be used to distinguish the swimming sperm based on the trajectory of movement. To determine whether the SDofDV is affected by viscosity, we quantified the SDofDV of motile sperm within media containing different concentrations of methylcellulose. The experiment revealed no statistically significant differences (Fig. 1D), suggesting that the SDofDV of motile sperm is independent of the media viscosity over a wide range. These in vitro measurements are expected to differ from those in the ampulla, because the cilia beating in the ampulla lumen generates micro flows, making cell movements more complex than in the Petri dish. To determine whether the SDofDV can be used to distinguish motile sperm in the oviduct ampulla using OCT imaging, we performed experiments in the ampulla ex vivo. Measurements of the SDofDV were conducted on non-sperm cells naturally present in the ampulla of virgin females (Fig. 1E) in comparison with the injected sperm in the washed oviduct ampulla (Fig. 1F). The SDofDV featured higher values (statistically significant) from motile sperm than from non-sperm cells (Fig. 1G), validating it as an effective parameter to distinguish motile sperm in the ampulla. To define the SDofDV threshold for sperm identification in vivo, we performed analysis in females mated to vasectomized males (Fig. 1H). The movement of the non-sperm cells (Fig. 1H) and the identified sperm (Fig. 1I) in vivo in the ampulla exhibited similar characteristics to those in the ampulla ex vivo (Fig. 1E,F). The in vivo sperm identification results are presented in Fig. 1J, where the trajectories with the SDofDV higher than the threshold were identified as motile sperm. The time interval for measuring the SDofDV was kept consistent at 900 ms over all experiments.
Sperm collection by the epithelium of the ampulla features a reduction of sperm velocity
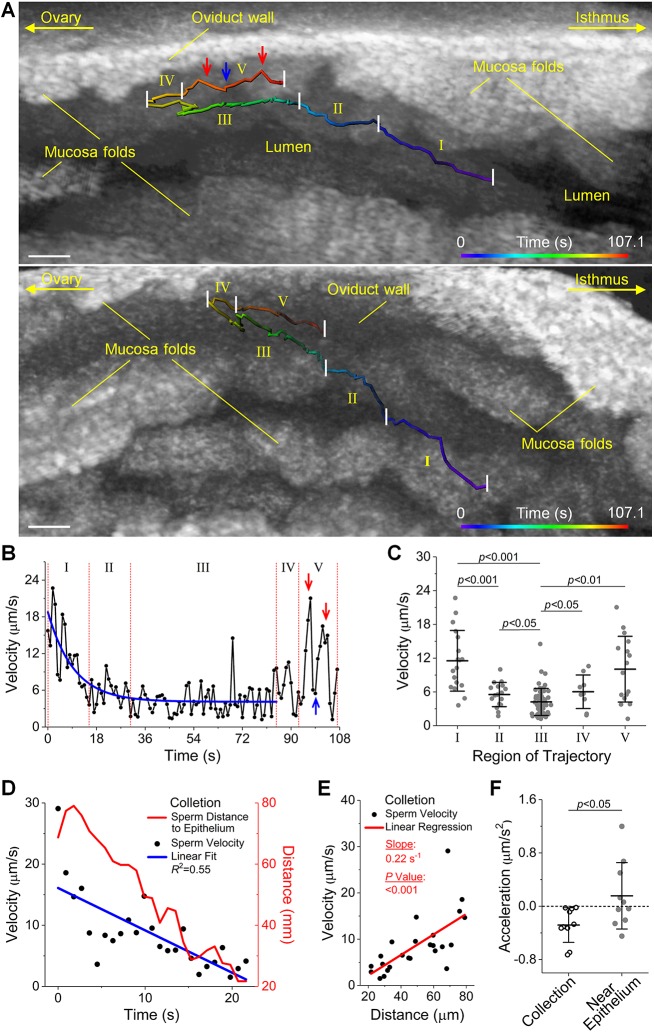
By analyses of sperm trajectories, it was observed that when sperm traveled through the luminal center of the ampulla in the isthmus-to-ampulla direction, they tended to move toward the ampulla epithelium (Fig. 2; Fig. S4A-C), which we defined as the process of sperm collection by the epithelium of the ampulla. In the representative trajectory (Fig. 2A; Movie 1), from the central lumen to the wall of the ampulla (indicated by parts I, II and III), the sperm velocity decreased over time (Fig. 2B). After reaching the near-epithelium region (the end of part III), the sperm switched direction by ∼180° and speeded up significantly close to the ampulla wall (Fig. 2C). For the velocity reduction, statistically significant differences were observed (Fig. 2C) between parts I and II, parts I and III, as well as parts II and III. To determine whether such a reduction of velocity is a unique feature of the sperm collection process, we performed comparative analysis with the velocity trends from a number of trajectories of the sperm traveling in the isthmus-to-ampulla direction, but already within the near-epithelium region (a representative trajectory shown in Fig. S4D,E). During the sperm collection process, we found a consistent decreasing trend of sperm velocity as the sperm move closer to the ampulla epithelium (Fig. 2D). This velocity change is in correlation with the sperm distance to the epithelium (Fig. 2E). To quantitatively evaluate such trends, we calculated the acceleration based on linear fit of the velocity over time. In comparison with the near-epithelium movement where the acceleration range covered both positive and negative values, the process of sperm collection featured consistently negative accelerations (Fig. 2G), indicating that the sperm slowed down while being collected by the ampulla epithelium.
Fig. 2.
Sperm collection by the epithelium of the ampulla featuring a trend of sperm velocity reduction. (A) A representative sperm trajectory in the ampulla in vivo visualized from two alternative angles shows the process of sperm collection by the luminal epithelium (Movie 1). The sperm swimming in the isthmus-to-ampulla direction through central lumen tend to go toward the ampulla epithelium (parts I, II and III) and then move in the near-epithelium region (parts IV and V). (B) Plot of the sperm velocity over time shows a velocity reduction (fit with an exponential decay function) when the sperm swim toward the ampulla epithelium, and an increase of velocity when the sperm enter the near-epithelium region. Red and blue arrows indicate corresponding positions on the velocity trend and the sperm trajectory in A. (C) Statistical analysis of the velocity between each part of the trajectory shows statistically significant changes of sperm velocity during the sperm collection process. Data are mean±s.d.; P=2.4×10−4 between parts I and II; P=2.1×10−5 between parts I and III; P=0.045 between parts II and III; P=0.045 between parts III and IV; P=0.0012 between parts III and V; t-test. (D) An overlay of sperm velocity and sperm distance to the epithelium versus time shows a trend of velocity decrease with the sperm getting closer to the ampulla epithelium in a representative sperm collection process. (E) Linear regression of the sperm velocity with respect to the sperm distance to the epithelium in the representative sperm collection process shows a statistically significant correlation. n=25 for distance-velocity data; P=2.3×10−4; t statistics for the slope. (F) The sperm collection process features a deceleration in comparison with the near-epithelium movement (in the same overall moving direction with the sperm collection). Data are mean±s.d.; n=10 for sperm in both collection process and near-epithelium movement; P=0.031; Wilcoxon rank sum test; effect size=0.5. Scale bars: 50 µm.
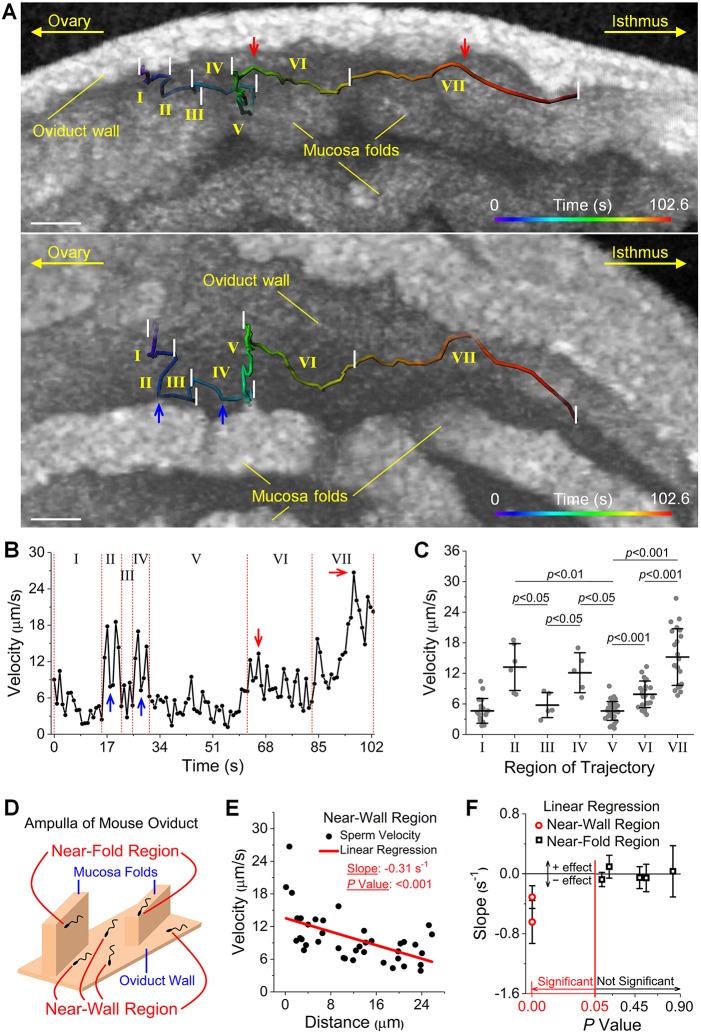
Sperm velocity is influenced by its distance to the ampulla wall in the near-wall region
After sperm were collected by the epithelium of the ampulla, major observed sperm activities were in the near-epithelium region, including the near-wall region and the near-fold region. An illustration of these two regions is shown in Fig. 3D. Specifically, the near-fold region refers to the location where sperm are close to the mucosa fold (Fig. S5A,B), but not close to the ampulla wall (Fig. S5B,C). The representative trajectory of sperm in the near-epithelium region (Fig. 3A; Movie 2) shows a frequent variation of sperm velocity (Fig. 3B). In parts II and IV, the sperm swam towards and along the mucosa fold with relatively higher velocities in comparison with parts III and V, where the sperm moved away from the fold (Fig. 3B). Such differences in velocity are statistically significant (Fig. 3C). In addition, in parts II and IV, when the sperm switched its direction to parallel with the fold (Fig. 3A, blue arrows), the velocity exhibited sudden decreases (Fig. 3B, blue arrows). Considering the distance of the sperm to the ampulla wall, from parts V to VI and to VII, the sperm came closer to the wall (Fig. 3A) and the overall velocity became higher (Fig. 3B), which is statistically significant (Fig. 3C). When the sperm were at their closest point to the ampulla wall in parts VI and VII (Fig. 3A, red arrows), the velocity reached peak values (Fig. 3B, red arrows). To statistically determine whether the sperm-epithelium distance plays a role in the sperm velocity, we quantified the sperm distance to the epithelium of the ampulla wall or the mucosa fold in 3D (Fig. S6) and conducted correlation analyses. Specifically, the velocity data were plotted with respect to the distance for sperm in the near-wall or near-fold region, and linear regression was performed to obtain the slope with its t statistics. In the near-wall region, our results show a statistically significant dependence of the velocity of sperm on its distance to the ampulla wall (Fig. 3E; Fig. S7), indicating that sperm moved faster when getting closer to the ampulla wall. In contrast, in the near-fold region, the distance of the sperm to the mucosa fold did not influence the sperm velocity (Fig. S6D), as summarized and plotted in Fig. 3F.
Fig. 3.
The velocity of the sperm in the near-wall region is influenced by the sperm-epithelium distance. (A) A representative sperm trajectory in the near-epithelium region of the ampulla in vivo visualized from two alternative angles (Movie 2). The trajectory is split into seven parts based on the sperm swimming directions or its distance to the oviduct wall. (B) Plot of the sperm velocity shows its dependence on sperm location relative to the oviduct wall and on its swimming direction relative to the mucosa fold. Blue arrows indicate sudden decreases of velocity that correspond to direction switches from towards the mucosa fold to parallel with the mucosa fold, as indicated in A. Red arrows indicate peak velocities corresponding to shorter distances to the epithelium of the oviduct wall in A. (C) Statistical analysis of the sperm velocity between each part of the trajectory shows statistically significant changes. Data are mean±s.d.; P=0.010 between parts II and III; P=0.0055 between parts II and V; P=0.015 between parts III and IV; P=0.012 between parts IV and V; P=5.7×10−7 between part V and VI; P=8.2×10−9 between part V and VII; P=4.4×10−6 between part VI and VII; t-test. (D) An illustration of the near-wall region and near-fold region in the ampulla. (E) Linear regression performed on sperm velocity versus sperm-epithelium distance in the near-wall region shows a statistically significant correlation. n=39 for distance-velocity data, P=1.8×10−4, t statistics for the slope. (F) Analysis over multiple sperm trajectories shows that the dependence of sperm velocity on sperm-epithelium distance exists in the near-wall region, but not the near-fold region. Data are mean±c.i. (95%). P values are from t statistics for the slope. Scale bars: 50 µm.
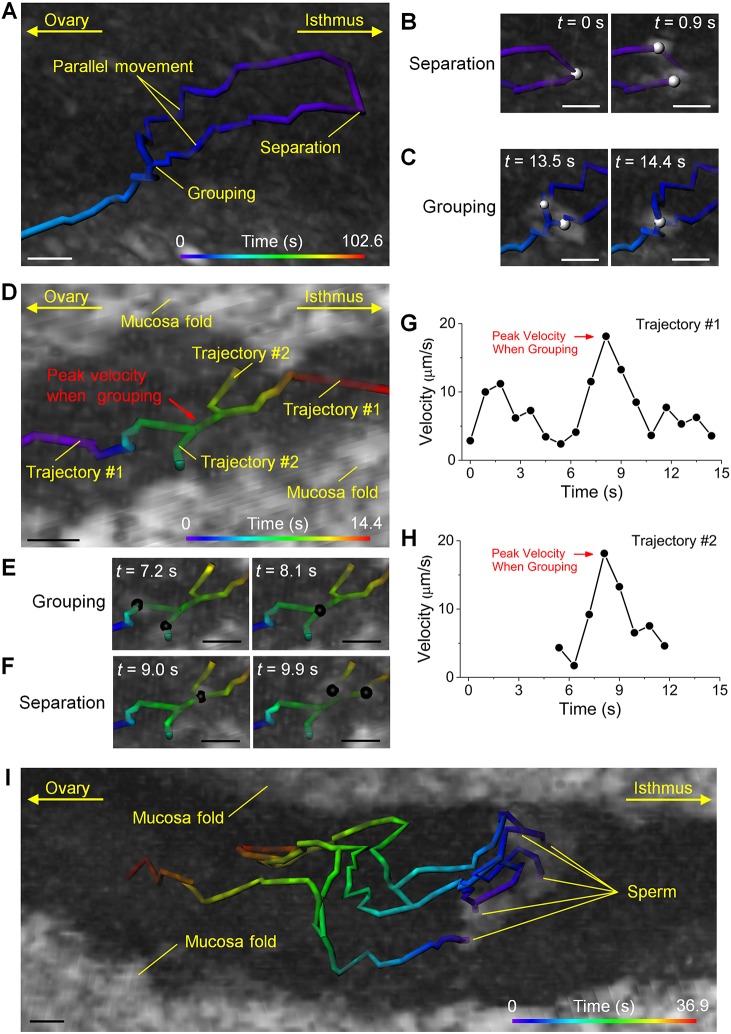
Sperm grouping and separation in the ampulla
Grouping of sperm and separation of sperm aggregates were observed at various locations in the oviduct ampulla (Fig. 4). Specifically, in the center of the ampulla lumen, after the separation of a sperm aggregate, the two subgroups of sperm moved in parallel in similar directions and ultimately re-grouped (Fig. 4A-C; Movie 3). This behavior suggests that, although separated, sub-groups of sperm can eventually return to the assemblage form for further movement. In another case, located deep between two mucosa folds (Fig. 4D; Movie 4), during the sperm traveling along the fold (Fig. 4D, trajectory #1), another sperm or aggregate joined in (Fig. 4E), traveled together transiently and then separated out (Fig. 4F), completing a short trip across the gap between the mucosa folds (Fig. 4D, trajectory #2). Interestingly, the velocity profiles measured from both trajectories over time clearly show velocity peaks at the time of grouping (Fig. 4D,G,H, red arrows). As separation took place, the velocity of subgroups dropped to a similar level to that prior to grouping (Fig. 4G,H). This suggests a possible velocity advantage of forming a sperm aggregate. However, such velocity changes were not observed in all trajectories of sperm grouping and separation (see, for example, Fig. S8 for trajectories in Fig. 4A), which might be due to an influence of the location of sperm on its velocity. More complex sperm interactions were also observed. Representative trajectories captured between two mucosa folds close to the center of the lumen are shown in Fig. 4I and Movie 5. Over the course of ∼37 s, separation and grouping activities occurred between multiple sperm aggregates. After three sperm groups were established with clear distances between them, splitting and inter-group joining activities still happened (Fig. 4I, middle to top and bottom to middle). This suggests that a relatively large number of sperm could act cooperatively during their movement in similar directions in the ampulla.
Fig. 4.
Sperm grouping and separation. (A) Separation of a sperm aggregate, followed by parallel movement of sub-groups and re-grouping observed around the central lumen of the ampulla (Movie 3). (B,C) The corresponding processes of (B) separation and (C) grouping are presented with balls labeling the sperm locations. (D) A process of sperm grouping, transient joint movement and separation between two mucosa folds in the ampulla (Movie 4). (E,F) The corresponding (E) grouping and (F) separation are presented with balls showing the sperm locations. (G,H) The transient joint movement upon sperm grouping features a sharp increase (red arrows) of sperm velocity that drops back as separation takes place. (I) Between two mucosa folds and close to the luminal center of the ampulla, a number of grouping and separation activities occur among multiple sperm aggregates (Movie 5). Scale bars: 20 µm.
Sperm motility between adjacent mucosa folds and in the presence of cumulus-oocyte complex
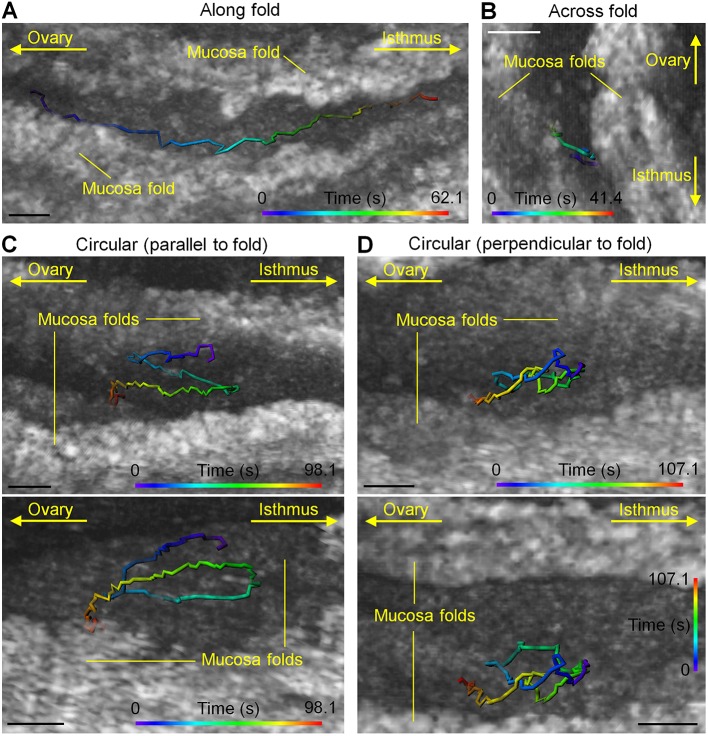
To further reveal the diversity of sperm behaviors in the ampulla, we present four examples of sperm activities between adjacent mucosa folds (Fig. 5). Sperm were seen staying consistently close to the ampulla wall and moving along the fold in an overall well-maintained direction (Fig. 5A; Movie 6). While crossing the gap between two folds (Fig. 5B; Movie 7), the sperm first had a continuous interaction with one of the folds (Fig. 5B, the right fold), then swam toward the other one and formed new interactions after reaching the target (Fig. 5B, the left fold). Such frequent sperm-epithelium interactions inside the ampulla in vivo confirm the previous in vitro finding that sperm can bind to the ampulla epithelium after leaving the reservoir inside the isthmus (Chang and Suarez, 2012). In addition to these relatively linear movements, two types of circling behaviors were also found at nearly identical locations between the folds. The circle path can be formed parallel to the fold, which is well visualized from the angle that shows the side of the mucosa fold (Fig. 5C; Movie 8). Circling perpendicular to the fold was also observed (Fig. 5D; Movie 9). Sperm were also tracked in the presence of the cumulus-oocyte complex in the oviduct ampulla (Fig. 6), suggesting the potential for sperm imaging in the native fertilization context.
Fig. 5.
Sperm behaviors between adjacent mucosa folds show a diversity of activities. (A-D) Representative trajectories show sperm swimming (A) along the mucosa folds (Movie 6), (B) across the gap between the mucosa folds (Movie 7), as well as circling (C) parallel and (D) perpendicular to the mucosa fold (Movies 8 and 9, respectively). Scale bars: 50 µm.
Fig. 6.
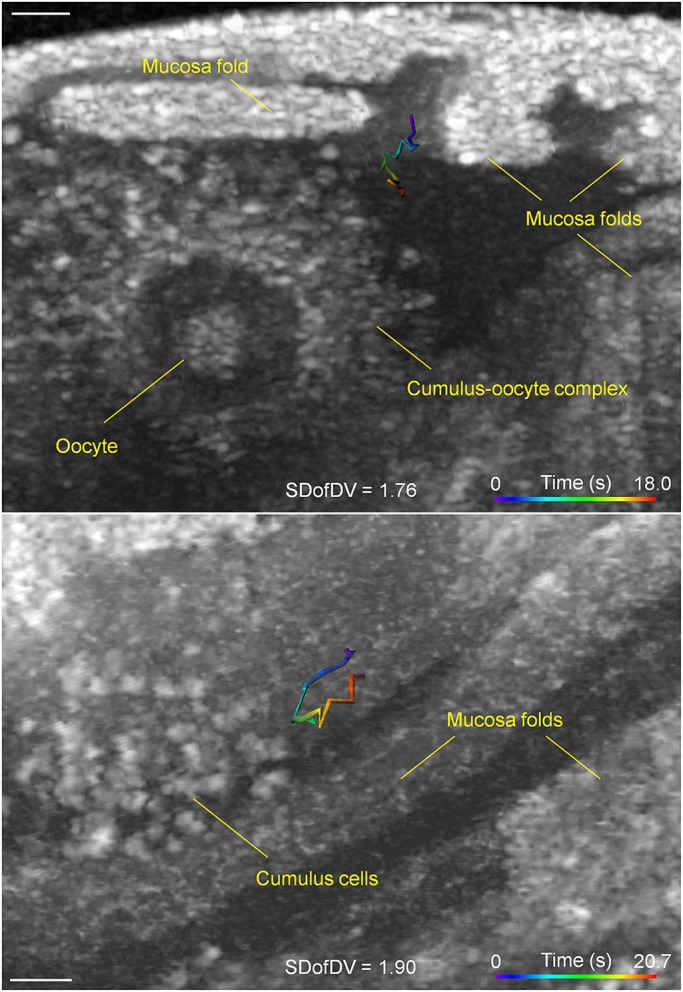
Simultaneous tracking of sperm and oocytes in the ampulla. Two sperm trajectories captured next to the cumulus-oocyte complexes in the ampulla. Scale bars: 50 μm.
DISCUSSION
Presented results demonstrate that the OCT-based in vivo functional imaging approach enables tracking of sperm in 3D in the living mouse oviduct and allows for quantitative analysis of sperm behaviors within their native fertilization context. Relying on the distinct movement characteristics of motile sperm and taking advantage of the unique imaging scale of OCT, we have identified, reconstructed and determined a number of in vivo 3D behavior patterns of the sperm in the oviduct ampulla, which have never been seen before. The SDofDV was demonstrated as an effective parameter for distinguishing motile sperm with endogenous contrast. One of the advantages of using this movement-based parameter for sperm identification is that no extra data need to be acquired in addition to the 3D sperm trajectory. Thus, it has superior efficiency for analyses of sperm behaviors using OCT. As a disadvantage, because the calculation of SD requires three or more angles of change in direction, identification based on individual or very few time points (two or three) is not feasible. It is worth noting that the SDofDV could be dependent on the time steps in the time-lapse imaging. In this study, a step of 900 ms was used, which allows motile sperm standing out significantly from non-sperm cells. Shorter time intervals are preferred as they might provide a better separation between motile sperm and non-sperm cells based on the SDofDV, but demand faster 3D OCT imaging, which will need hardware improvements or the sacrifice of the transverse imaging field of view.
In comparison with the fluorescence imaging methods (Druart et al., 2009; Hino et al., 2016; Ishikawa et al., 2016; La Spina et al., 2016; Muro et al., 2016), this OCT-based imaging approach has a number of advantages that we believe will take in vivo sperm imaging analysis to an unprecedented level. First, the 3D depth-resolved capability provides accurate delineation (∼5 µm resolution) of the sperm location relative to the oviduct epithelium, which enables quantitative analysis of sperm activities. Second, because the intrinsic dynamic feature of the trajectory is employed for identification of motile sperm, no fluorescent labels or exogenous contrast agents are required. Third, at the same time of sperm tracking, the microstructure of oviduct tissue is well resolved, allowing for convenient analysis of sperm-oviduct interaction. Fourth, the volumetric OCT imaging is based on laser scanning and, thus, the imaging field of view and the 3D imaging speed are adaptive to the need of particular experiments. These important features of the presented in vivo imaging approach are currently difficult to achieve with either the epifluorescence (Hino et al., 2016; Ishikawa et al., 2016; La Spina et al., 2016; Muro et al., 2016) or the fibered confocal methods (Druart et al., 2009). However, fluorescence imaging has the advantage of providing cell type and subcellular labeling specificity, which allows the study of structural and functional changes of sperm during their activities, such as acrosomal exocytosis (La Spina et al., 2016). Owing to the insufficient spatial resolution, the OCT imaging currently does not allow discrimination between individual sperm. Therefore, defining the number of sperm in each aggregate or distinguishing between an individual sperm and an aggregate is currently not feasible. This limitation can potentially be solved by using higher-resolution optical coherence microscopy (Karnowski et al., 2017) at the expense of effective focus range and employing a laser source with a larger bandwidth that provides a better axial resolution (Liu et al., 2011).
The tendency of mammalian sperm (bull and human) to swim from the center towards boundaries was previously observed in vitro in microfluidic channels (El-Sherry et al., 2014; Kantsler et al., 2014). Our results present the first in vivo observation of such sperm behaviors in the natural fertilization environment and indicate a unique velocity-reduction trend during the sperm collection by the epithelium of the ampulla. Previous in vitro experiments suggested that the rheotaxis guides sperm to swim upstream (Kantsler et al., 2014; Miki and Clapham, 2013), and by swimming towards the luminal edge, sperm move under the weakest effect of the shear fluid flow (Kantsler et al., 2014). Thus, given that the oviduct fluid in the ampulla largely flows in the ampulla-to-isthmus direction to deliver the oocytes to the fertilization site, the observed in vivo process of sperm collection by the ampulla epithelium supports the hypothesis that the rheotaxis is a factor guiding the sperm behavior in the ampulla. Our results also show a direction switch of sperm entering the near-epithelium region, suggesting that the ampulla fluid flow close to the epithelium might be in an opposite direction in comparison with the fluid flow in the central lumen. Such bi-directional flows could be generated by the beat of motile cilia, which was shown by Jonas et al. in the ciliated surface of Xenopus tropicalis embryos (Jonas et al., 2011). Furthermore, a study by Ishikawa et al. concluded that the oviduct muscle contraction plays an important role for mouse sperm transportation through the isthmus, but not inside the ampulla (Ishikawa et al., 2016). Therefore, our in vivo data suggest that ciliary dynamics in the ampulla may produce the necessary mechanical cues that regulate sperm behaviors.
Previous work on sperm rheotaxis has shown that the swimming velocity of sperm increases in response to an increase of the fluid flow velocity within a certain range (El-Sherry et al., 2014). The distance-dependent sperm velocity in the near-wall region revealed from our data might also be contributed to by the rheotaxis, as the cilia-driven fluid flow in the near field could span different magnitudes (higher magnitude closer to surface), as shown with Xenopus epithelium in vitro (Huang et al., 2015). The absence of such a distance-related influence in the near-fold region may be due to the fact that the mucosa folds in the ampulla are tall and usually probe into the central part of the lumen (Burton et al., 2015; Wang et al., 2015a), which can result in a complex field of flow, thus creating different effects on sperm velocity through the rheotaxis. It is worth noting that other possible taxes, such as the chemotaxis and thermotaxis, may also contribute to the observed sperm behaviors, though dissociating these factors can be challenging based on the current knowledge and state of imaging technologies. Future work will focus on mapping the fields of fluid flow inside the oviduct ampulla in vivo with OCT imaging of non-sperm cells to explore the rheotaxis. As a complementary approach to the current analysis, tracking methods similar to the recently demonstrated particle streak velocimetry OCT (Zhou et al., 2016) can be used. Mapping of cilia beat frequency in the oviduct (Wang et al., 2015a) will be integrated to determine the interplay between sperm and cilia in the ampulla.
To the best of our knowledge, the results of sperm grouping provide the first in vivo evidence of mammalian sperm cooperation in the ampulla (Moore et al., 2002), the site of fertilization where the number of sperm is significantly reduced (Eisenbach and Giojalas, 2006). Such fascinating cooperative behaviors of sperm are believed to be driven by sperm competition as an evolutionary response (Fisher et al., 2014; Fisher and Hoekstra, 2010). There are still several unresolved issues with respect to sperm cooperation, including the discrimination mechanism (Foster and Pizzari, 2010), genetic determination (Immler, 2008) and possible altruism (Foster and Pizzari, 2010). Previous work found sperm aggregation on the way toward the ampulla (Ishikawa et al., 2016). Our in vivo imaging data show that, in addition to the isthmus, inside the ampulla where fertilization occurs, sperm also cooperate by forming groups. This suggests that the cooperative behaviors may persist until fertilization. Recent fluorescence imaging work (La Spina et al., 2016; Muro et al., 2016) studied sperm interactions with oocytes, revealing that fertilization takes place one by one and spans a relatively long time, and that spermatozoa are very efficient in this process. Such observations raise questions about the necessity of sperm grouping in the ampulla. It is generally considered that grouping allows sperm to move faster (Fisher et al., 2014), which we also observed in some of our experiments. However, as a very limited number of spermatozoa enter the site of fertilization, faster movement may no longer be crucial due to lack of competition, though it could still be favorable for reaching the cumulus-oocyte complex earlier. Parallel movement of sperm after group separation was another interesting type of behavior observed in our study, which could be a response to similar stimuli. The overall diversity of the observed sperm behaviors suggests multiple factors in the ampulla guiding sperm towards successful fertilization with possible barriers to polyspermy. Future studies of a large number of sperm trajectories will likely reveal new observations and will establish the percentage of sperm exhibiting each type of behavior. The exact reason and the benefits of such sperm behavior in the oviduct ampulla are currently unknown and deserve further investigations.
This study covers a variety of locations in the oviduct ampulla, and results suggest that after leaving the isthmus reservoir, as sperm head into the ampulla through the lumen, they are collected by the ampulla epithelium and start swimming in the near-epithelium region, where different sperm activities take place and the behaviors of sperm can be influenced by their distance to the ampulla wall. Sperm cooperation (in the forms of grouping and parallel movement) occurs throughout this entire process. Prolonged data acquisition and a larger field of view are required to follow individual sperm or sperm aggregate to confirm and enrich this model of sperm behaviors. To achieve this, the OCT system with a MHz-level A-scan rate can be employed (Wang et al., 2015b), which will allow the transverse field of view covering larger area but with the spatial sampling interval still within the resolution. For a longer duration of OCT imaging, an intravital imaging window (Bochner et al., 2015) might be useful to minimize any possible physiological alterations from long-term exposure of the tissues to the air.
In summary, we have developed and demonstrated a novel functional OCT method for in vivo 3D tracking and quantitative analysis of sperm behaviors in the mouse oviduct ampulla. This method provides a tool to study cellular interactions between sperm and oviduct epithelium, as well as to investigate regulatory mechanisms guiding sperm toward fertilization, which were previously inaccessible. It can also be used as an in vivo platform for screening sperm and oviductal abnormalities. In addition, the method could provide new opportunities to study inter-species sperm-oviduct interactions. Ultimately, such knowledge will contribute to the understanding of normal mammalian reproduction and human reproductive disorders.
MATERIALS AND METHODS
OCT system and data acquisition
An in-house built spectral domain OCT system was used in this study. The schematic of the system is shown in Fig. S1A. The details of the system can be found in our previous work (Burton et al., 2015; Wang et al., 2015a). The OCT system consisted of a titanium sapphire laser with a ∼810 nm central wavelength and a ∼110 nm bandwidth, a sample arm equipped with a 5× scan lens, a reference arm that contained a dispersion compensator to improve the axial resolution over depth, and a high-resolution spectrometer with transmission gratings (1800 lines/mm) and a line-field CMOS camera (4096 pixels). The light beam coming out of the laser was coupled into a single-mode fiber that was connected to a 50/50 fiber coupler to split light to the sample and reference arms as well as collect light back for interference, which was resolved by the spectrometer. Fast Fourier transform was performed on the interpolated equally k-spaced fringes and the amplitude spectrum was used as the OCT intensity profile over depth (A-scan), which was presented in dB units. The OCT system provided an axial resolution of ∼5 µm in tissue (refractive index assumed as 1.4) and a transverse resolution of ∼4 µm, and is able to reveal detailed structures of the mouse oviduct and provide high-precision localization of spermatozoa inside the oviduct. A total of 2048 pixels are available in the depthwise direction with a pixel scale of ∼2 µm in tissue (refractive index assumed as 1.4). The A-scan rate of the system was set as ∼68 kHz. With an imaging depth covering the whole lumen of the mouse oviduct ampulla, the sensitivity was over 90 dB. The scan lens in the sample arm provided a large working distance of ∼25 mm, convenient for live-mouse imaging. In this study, time-lapse 3D OCT imaging with a temporal resolution of 900 ms was performed. The transverse scan of the imaging beam was obtained through a set of two galvanometer mirrors, featuring programmable movements. This allowed for a high flexibility of adjusting the number of pixels and the scanning distance in each of the two transverse directions. Although a number of different settings were employed in the experiments, in the transverse plane, the total number of pixels (A-scans) was kept as 5400 (such as 360×150, 450×120 and 300×180), and the pixel scale was kept smaller than 4 µm in both directions. This resulted in a transverse field of view ranging between 0.4 mm2 and 0.7 mm2.
Animal procedures for in vivo imaging
Timed matings were set between adult CD-1 males and females. Vaginal plugs were checked in the morning. In vivo imaging experiments were performed on the day of finding a plug. The setup for in vivo imaging of the mouse oviduct has previously been described in detail (Wang et al., 2015a) and is illustrated in Fig. S1B. The surgical procedure is similar to the one that is routinely used for zygote injection in generation of the transgenic mouse (Conner, 2001), which is not expected to cause complications associated with the reproductive tract and preimplantation embryos (Ittner and Götz, 2007). The anesthetized female was placed on a heating pad to maintain the body temperature. The hair was removed on the right dorsal side above the leg, which was followed by skin disinfection with 70% ethanol. An incision of ∼1 cm long was then made to the skin and muscle layers. After locating the reproductive organs, a pair of blunt forceps was used to grab the fat pad associated with the ovary and gently pull the ovary, oviduct and part of the uterus out of the body cavity. A surgical clamp was then used to hold the fat pad and stabilize the reproductive organs for the imaging experiments with OCT. Solution of 1× phosphate-buffered saline at 37°C was periodically added to the tissue to avoid dehydration. All the surgical tools were pre-warmed to avoid heat dissipation from the tissue. Vasectomized males (CD-1) were purchased from Charles River. Successful vasectomy was verified by confirming that no embryos were present in the post-coital uterus at post-implantation stages. All the procedures employed in this study were approved by the Institutional Animal Care and Use Committee at the Baylor College of Medicine. All experiments were conducted following the approved protocols.
Mouse sperm collection
Mouse sperm for in vitro and ex vivo experiments were extracted following the established protocols (Chang and Suarez, 2011; Duselis and Vrana, 2007). Briefly, the caudal epididymides were harvested from the adult CD-1 male right after euthanization. The dissected caudal epididymides were then placed in the 37°C culturing medium that contains HEPES-buffered human tubal fluid with EDTA and glutamine (InVitroCare). The associated fat and blood were carefully cleared out and the caudal epididymides were transferred to another dish with clean 37°C culturing medium for sperm extraction. Two to three cuts were made to each caudal epididymis and the concentrated sperm were pushed out gently from the cuts with a pair of blunt forceps. The sperm were transferred to a fresh culturing medium inside a pre-heated 37°C incubator to allow sperm dispersal in the medium for about 10 min.
Bright-field microscopic imaging
In vitro experiments to assess the sperm movement characteristics were performed using an upright bright-field microscope (Zeiss Axio Zoom V16). With a digital camera (Zeiss AxioCam HRm), time-lapse 2D imaging at ∼0.7 µm resolution and a 150 ms time interval was obtained to capture the dynamics of sperm. Sperm was imaged at 37°C in HEPES-buffered human tubal fluid with EDTA and glutamine. Polystyrene microparticles of ∼10 µm diameter were used as the control. Gentle fluid flows were created using a transferring pipette to imitate bulk movement. Media with different viscosities were obtained by adding methylcellulose (M0512, Sigma-Aldrich) at concentrations of 0%, 0.2%, 0.4% and 0.6% (weight/volume), which had measured viscosities of 1.0, 3.5, 10.5 and 29.4 mPa·s, respectively. The kinematic viscosity of the media was measured using a glass capillary viscometer; the dynamic viscosity was obtained by multiplying the kinematic viscosity by the density of the media.
Experiments performed in the mouse oviduct ex vivo
Adult virgin females (CD-1) were monitored for estrous cycle through an established protocol (Caligioni, 2009). At the estrous phase, the oviduct was dissected and placed in the 37°C medium containing HEPES-buffered human tubal fluid with EDTA and glutamine (InVitroCare). Imaging of the naturally existing cells in the ampulla was immediately performed. For imaging of injected motile sperm in the ex vivo ampulla, naturally existing cells and debris were gently washed out form the oviduct lumen, which was confirmed by OCT 3D structural imaging. Then motile sperm collected from the CD-1 male were injected into the ampulla; the imaging was conducted immediately.
Sperm tracking in 3D in the oviduct ampulla
The Imaris software (Bitplane) was used for 3D rendering and volumetric processing of OCT data. The 3D sperm tracking was conducted using built-in spot and tracking functions in the Imaris. Specifically, within a manually selected 3D volume, an intensity threshold was set to detect spots, which was tracked over time (Fig. S2), forming a 3D trajectory. Spatial position and velocity amplitude of the trajectory were exported for further processing.
Compensation for bulk movement of the oviduct
The compensation was achieved by subtracting the bulk movement velocity from the scatter raw velocity in 3D. An illustration is shown in Fig. S3. Specifically, the bulk movement of the oviduct ampulla was tracked based on a high-intensity spot from the oviduct wall or the mucosa fold that is close to the targeted scatter. The 3D position (xb, yb, zb) and the amplitude of velocity (vb) of the bulk movement spot, as well as the 3D position (xs, ys, zs) and the amplitude of raw velocity (vs,r) of the scatter were directly exported from the Imaris software. To get the 3D direction of velocity vector (Vxyz) at the nth time point, two angles selected from two planes were used, including the angle (θxy-z) between the x-y plane and the Vxyz, as well as the angle (θx-y) between the x axis and the velocity component Vxy in the x-y plane. For the bulk movement, these two angles for the nth time point were calculated as
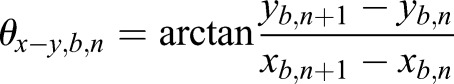 |
and
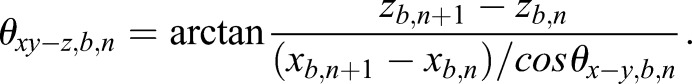 |
Similarly, for the scatter movement, the angles at the nth time point were
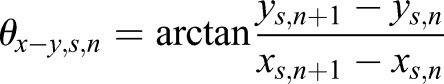 |
and
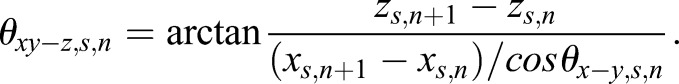 |
With these angles, the three components (vx,s,f, vy,s,f and vz,s,f) of the final compensated scatter velocity were calculated as
and
Thus, the final scatter velocity (vs,f) of each time point can be obtained as
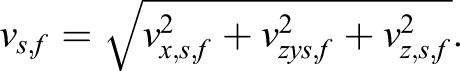 |
In the three 2D projections, x-y plane, y-z plane and x-z plane, the in-plane angles of the directional changes at the nth time point from the final compensated scatter trajectory were calculated as
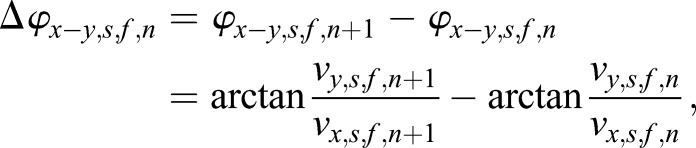 |
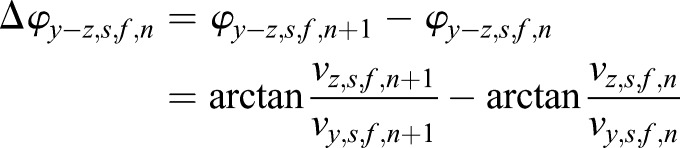 |
and
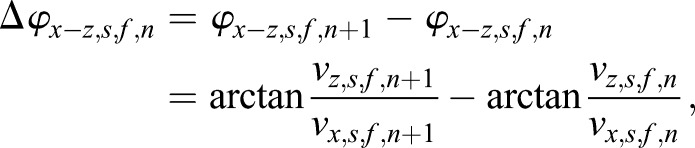 |
which were used to assess the scatter movement characteristics (the SDofDV). During the above calculations, the angle range was set as (−π, +π]. The angles of −π/2, 0, π/2 and π were processed separately. The angles of direction variations, Δφx-y, Δφx-y and Δφx-y, were adjusted to the range of (−π, +π]. All sperm velocity values presented in this study are the final sperm velocities with the oviduct bulk movement compensated.
Measurement of distance between sperm and the epithelium of the oviduct ampulla
The measurement of distance between the sperm and the ampulla epithelium was performed in 3D using the Imaris software. The measurement method is shown in Fig. S6. At each time point, a 3D region was first identified containing the targeted sperm and the oviduct tissue (Fig. S6A). The surface of the oviduct tissue, including the oviduct wall and mucosa fold, was created through a built-in isosurface function in the Imaris based on designated intensity threshold (Fig. S6B). The surface was smoothed with a 10 µm spatial cube. A built-in spot function in the Imaris was used to create a spot at the sperm location with an adjustable diameter (Fig. S6B). Thus, the distance can be easily measured with adjusting the spot diameter, and when the spot was just touching the surface (Fig. S6C), the radius of the spot was recorded as the distance between the sperm and the ampulla epithelium. To determine the just-touching status, 3D visualization can be accessed from the front and the back (Fig. S6D). Because from the back the over-sized spot can be clearly seen (Fig. S6E), the measurement started from oversizing and gradually reducing the size of the spot until the spot was just not seen from the back.
Statistics
For all comparative studies, one-sample Kolmogorov–Smirnov (K–S) test (α=0.05) was first performed on the data from each group to determine whether the data follow the normal distribution. For Figs 1C,D,G, 2C and 3C, the P values of the K–S tests were less than 0.05, indicating normal distribution and, therefore, the two-tailed two-sample t-test (α=0.05) or the one-way ANOVA (α=0.05) was employed to measure the statistical significance levels for these comparisons. For Fig. 2F, data in the ‘Near Epithelium’ group did not follow the normal distribution (P>0.05 from K–S test), and, thus, two-tailed Wilcoxon rank sum test (α=0.05) was used to statistically establish the difference. Data variation within each group was estimated and we presented the mean±s.d. as error bars to show the data dispersion (Figs 1C,D,G, 2C,F and 3C). Between the groups being compared, if the variance of one group is no more than four times the variance of the other group, equal variances were assumed for the t-test. Otherwise, unequal variances were assumed and Satterthwaite's approximation for the effective degrees of freedom was used. For the one-way ANOVA, Levene's test was conducted to confirm the equal variance between groups. The exact P values of the t-tests, the one-way ANOVA and the Wilcoxon rank sum test are reported in the figure legends. For regression analyses to determine a possible correlation, t statistics (α=0.05) of the slope from linear regressions were used to test the significance with the exact P values reported in the figure legends.
In studies involving different trajectories with the t-test (Fig. 1C,G), we ensured the sample size to be sufficient for an adequate power (>0.80) to detect the hypothesized difference. For the one-way ANOVA (Fig. 1D) and non-normal distribution with the Wilcoxon rank sum test (Fig. 2F), we chose the sample size with which we did not expect the s.d. to be significantly changed when adding new data points. When a statistically significant difference was detected, the effect size of comparison was calculated and presented in the figure legends to further reveal the difference between groups. For the t-test, the effect size was calculated as the Cohen's d. For the Wilcoxon rank sum test, the effect size was calculated as 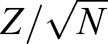 , where Z is the z value of the Wilcoxon rank sum test and N is the total number of samples from the two groups (Pallant, 2007). In studies focusing on individual sperm trajectories (Figs 2C, 3C and 3F), all available data points within the trajectory were used. The numbers of samples or data points are reported in the figure legends. No method of randomization was used in the experiments. No data were excluded from the statistical analyses. Group allocations of samples were conducted without considering the target parameters that were quantified through automatic programs to maximize blinding and avoid bias in data analyses.
, where Z is the z value of the Wilcoxon rank sum test and N is the total number of samples from the two groups (Pallant, 2007). In studies focusing on individual sperm trajectories (Figs 2C, 3C and 3F), all available data points within the trajectory were used. The numbers of samples or data points are reported in the figure legends. No method of randomization was used in the experiments. No data were excluded from the statistical analyses. Group allocations of samples were conducted without considering the target parameters that were quantified through automatic programs to maximize blinding and avoid bias in data analyses.
Supplementary Material
Acknowledgements
We gratefully acknowledge Prof. Richard R. Behringer (The University of Texas M.D. Anderson Cancer Center, USA) and Prof. Ross A. Poché (Baylor College of Medicine, Houston, TX, USA) for helpful discussions of this study and critiques of the manuscript. We thank the Optical Imaging and Vital Microscopy Core at the Baylor College of Medicine for technical support.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: S.W., I.V.L.; Methodology: S.W., I.V.L.; Software: S.W.; Validation: S.W.; Formal analysis: S.W.; Investigation: S.W., I.V.L.; Resources: I.V.L.; Data curation: S.W., I.V.L.; Writing - original draft: S.W., I.V.L.; Writing - review & editing: S.W., I.V.L.; Visualization: S.W.; Supervision: I.V.L.; Project administration: I.V.L.; Funding acquisition: S.W., I.V.L.
Funding
This work was supported by the National Institutes of Health (R01HL120140 to I.V.L.) and the American Heart Association (16POST30990070 to S.W.]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.157685.supplemental
References
- Ardon F., Markello R. D., Hu L., Deutsch Z. I., Tung C.-K., Wu M. and Suarez S. S. (2016). Dynamics of bovine sperm interaction with epithelium differ between oviductal isthmus and ampulla. Biol. Reprod. 95, 90 10.1095/biolreprod.116.140632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock D. F., Wandernoth P. M. and Wennemuth G. (2014). Episodic rolling and transient attachments create diversity in sperm swimming behavior. BMC Biol.12, 67 10.1186/s12915-014-0067-3 [DOI] [PMC free article] [PubMed]
- Bahat A., Tur-Kaspa I., Gakamsky A., Giojalas L. C., Breitbart H. and Eisenbach M. (2003). Thermotaxis of mammalian sperm cells: a potential navigation mechanism in the female genital tract. Nat. Med. 9, 149-150. 10.1038/nm0203-149 [DOI] [PubMed] [Google Scholar]
- Bochner F., Fellus-Alyagor L., Kalchenko V., Shinar S. and Neeman M. (2015). A novel intravital imaging window for longitudinal microscopy of the mouse ovary. Sci. Rep. 5, 12446 10.1038/srep12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton J. C., Wang S., Stewart C. A., Behringer R. R. and Larina I. V. (2015). High-resolution three-dimensional in vivo imaging of mouse oviduct using optical coherence tomography. Biomed. Opt. Express 6, 2713-2723. 10.1364/BOE.6.002713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligioni C. (2009). Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. 10.1002/0471142301.nsa04is48 [DOI]
- Chang H. and Suarez S. S. (2011). Two distinct Ca(2+) signaling pathways modulate sperm flagellar beating patterns in mice. Biol. Reprod. 85, 296-305. 10.1095/biolreprod.110.089789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. and Suarez S. S. (2012). Unexpected flagellar movement patterns and epithelial binding behavior of mouse sperm in the oviduct. Biol. Reprod. 86, 140, 1-8 10.1095/biolreprod.111.096578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner D. A. (2001). Transgenic mouse production by zygote injection. In Current Protocols in Molecular Biology. John Wiley & Sons; [DOI] [PubMed] [Google Scholar]
- Corkidi G., Taboada B., Wood C. D., Guerrero A. and Darszon A. (2008). Tracking sperm in three-dimensions. Biochem. Biophys. Res. Commun. 373, 125-129. 10.1016/j.bbrc.2008.05.189 [DOI] [PubMed] [Google Scholar]
- Coy P., García-Vázquez F. A., Visconti P. E. and Avilés M. (2012). Roles of the oviduct in mammalian fertilization. Reproduction 144, 649-660. 10.1530/REP-12-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druart X., Cognie J., Baril G., Clement F., Dacheux J.-L. and Gatti J.-L. (2009). In vivo imaging of in situ motility of fresh and liquid stored ram spermatozoa in the ewe genital tract. Reproduction 138, 45-53. 10.1530/REP-09-0108 [DOI] [PubMed] [Google Scholar]
- Duselis A. R. and Vrana P. B. (2007). Harvesting sperm and artificial insemination of mice. J. Vis. Exp., e184 10.3791/184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbach M. and Giojalas L. C. (2006). Sperm guidance in mammals - an unpaved road to the egg. Nat. Rev. Mol. Cell Biol. 7, 276-285. 10.1038/nrm1893 [DOI] [PubMed] [Google Scholar]
- El-Sherry T. M., Elsayed M., Abdelhafez H. K. and Abdelgawad M. (2014). Characterization of rheotaxis of bull sperm using microfluidics. Integr. Biol. 6, 1111-1121. 10.1039/C4IB00196F [DOI] [PubMed] [Google Scholar]
- Fauci L. J. and Dillon R. (2005). Biofluidmechanics of reproduction. Annu. Rev. Fluid Mech. 38, 371-394. 10.1146/annurev.fluid.37.061903.175725 [DOI] [Google Scholar]
- Fercher A. F., Drexler W., Hitzenberger C. K. and Lasser T. (2003). Optical coherence tomography - principles and applications. Rep. Prog. Phys. 66, 239 10.1088/0034-4885/66/2/204 [DOI] [Google Scholar]
- Fisher H. S. and Hoekstra H. E. (2010). Competition drives cooperation among closely related sperm of deer mice. Nature 463, 801-803. 10.1038/nature08736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher H. S., Giomi L., Hoekstra H. E. and Mahadevan L. (2014). The dynamics of sperm cooperation in a competitive environment. Proc. R. Soc. B 281 10.1098/rspb.2014.0296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K. R. and Pizzari T. (2010). Cooperation: the secret society of sperm. Curr. Biol. 20, R314-R316. 10.1016/j.cub.2010.02.015 [DOI] [PubMed] [Google Scholar]
- Georgiou A. S., Snijders A. P. L., Sostaric E., Aflatoonian R., Vazquez J. L., Vazquez J. M., Roca J., Martinez E. A., Wright P. C. and Fazeli A. (2007). Modulation of the oviductal environment by gametes. J. Proteome Res. 6, 4656-4666. 10.1021/pr070349m [DOI] [PubMed] [Google Scholar]
- Hino T., Muro Y., Tamura-Nakano M., Okabe M., Tateno H. and Yanagimachi R. (2016). The behavior and acrosomal status of mouse spermatozoa in vitro, and within the oviduct during fertilization after natural mating. Biol. Reprod. 95, 50, 51-11-50, 51-11. [DOI] [PubMed] [Google Scholar]
- Huang D., Swanson E. A., Lin C. P., Schuman J. S., Stinson W. G., Chang W., Hee M. R., Flotte T., Gregory K., Puliafito C. A. et al. (1991). Optical coherence tomography. Science 254, 1178-1181. 10.1126/science.1957169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B. K., Gamm U. A., Bhandari V., Khokha M. K. and Choma M. A. (2015). Three-dimensional, three-vector-component velocimetry of cilia-driven fluid flow using correlation-based approaches in optical coherence tomography. Biomed. Opt. Express 6, 3515-3538. 10.1364/BOE.6.003515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immler S. (2008). Sperm competition and sperm cooperation: the potential role of diploid and haploid expression. Reproduction 135, 275-283. 10.1530/REP-07-0482 [DOI] [PubMed] [Google Scholar]
- Ishikawa Y., Usui T., Yamashita M., Kanemori Y. and Baba T. (2016). Surfing and swimming of ejaculated sperm in the mouse oviduct. Biol. Reprod. 94, 89, 1-9 10.1095/biolreprod.115.135418 [DOI] [PubMed] [Google Scholar]
- Ittner L. M. and Götz J. (2007). Pronuclear injection for the production of transgenic mice. Nat. Protoc. 2, 1206-1215. 10.1038/nprot.2007.145 [DOI] [PubMed] [Google Scholar]
- Jikeli J. F., Alvarez L., Friedrich B. M., Wilson L. G., Pascal R., Colin R., Pichlo M., Rennhack A., Brenker C. and Kaupp U. B. (2015). Sperm navigation along helical paths in 3D chemoattractant landscapes. Nat. Commun. 6, 7985 10.1038/ncomms8985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas S., Bhattacharya D., Khokha M. K. and Choma M. A. (2011). Microfluidic characterization of cilia-driven fluid flow using optical coherence tomography-based particle tracking velocimetry. Biomed. Opt. Express 2, 2022-2034. 10.1364/BOE.2.002022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantsler V., Dunkel J., Blayney M. and Goldstein R. E. (2014). Rheotaxis facilitates upstream navigation of mammalian sperm cells. eLife 3, e02403 10.7554/eLife.02403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnowski K., Ajduk A., Wieloch B., Tamborski S., Krawiec K., Wojtkowski M. and Szkulmowski M. (2017). Optical coherence microscopy as a novel, non-invasive method for the 4D live imaging of early mammalian embryos. Scientific Rep.7, 4165 10.1038/s41598-017-04220-8 [DOI] [PMC free article] [PubMed]
- Kölle S. (2015). Transport, distribution and elimination of mammalian sperm following natural mating and insemination. Reprod. Domestic Animals 50 Suppl. 3, 2-6. 10.1111/rda.12576 [DOI] [PubMed] [Google Scholar]
- Kölle S., Dubielzig S., Reese S., Wehrend A., König P. and Kummer W. (2009). Ciliary transport, gamete interaction, and effects of the early embryo in the oviduct: ex vivo analyses using a new digital videomicroscopic system in the cow. Biol. Reprod. 81, 267-274. 10.1095/biolreprod.108.073874 [DOI] [PubMed] [Google Scholar]
- La Spina F. A., Molina L. C. P., Romarowski A., Vitale A. M., Falzone T. L., Krapf D., Hirohashi N. and Buffone M. G. (2016). Mouse sperm begin to undergo acrosomal exocytosis in the upper isthmus of the oviduct. Dev. Biol. 411, 172-182. 10.1016/j.ydbio.2016.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewenhoeck D. A. (1677). Observationes D. Anthonii Lewenhoeck, De Natis'e Semine Genitali Animalculis. Philos. Trans. 12, 1040-1046. 10.1098/rstl.1677.0068 [DOI] [Google Scholar]
- Liu L., Gardecki J. A., Nadkarni S. K., Toussaint J. D., Yagi Y., Bouma B. E. and Tearney G. J. (2011). Imaging the subcellular structure of human coronary atherosclerosis using micro-optical coherence tomography. Nat. Med.17, 1010 10.1038/nm.2409 [DOI] [PMC free article] [PubMed]
- Miki K. and Clapham D. E. (2013). Rheotaxis guides mammalian sperm. Curr. Biol. 23, 443-452. 10.1016/j.cub.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H., Dvoráková K., Jenkins N. and Breed W. (2002). Exceptional sperm cooperation in the wood mouse. Nature 418, 174-177. 10.1038/nature00832 [DOI] [PubMed] [Google Scholar]
- Muro Y., Hasuwa H., Isotani A., Miyata H., Yamagata K., Ikawa M., Yanagimachi R. and Okabe M. (2016). Behavior of mouse spermatozoa in the female reproductive tract from soon after mating to the beginning of fertilization. Biol. Reprod. 94, 80, 1-7 10.1095/biolreprod.115.135368 [DOI] [PubMed] [Google Scholar]
- Oliveira R. G., Tomasi L., Rovasio R. A. and Giojalas L. C. (1999). Increased velocity and induction of chemotactic response in mouse spermatozoa by follicular and oviductal fluids. J. Reprod. Fertil. 115, 23-27. 10.1530/jrf.0.1150023 [DOI] [PubMed] [Google Scholar]
- Pallant J. (2007). SPSS Survival Manual: A Step by Step Guide to Data Analysis Using SPSS for Windows. Milton Keynes, UK: Open University Press. [Google Scholar]
- Phillips D. M. (1972). Comparative analysis of mammalian sperm motility. J. Cell Biol. 53, 561-573. 10.1083/jcb.53.2.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzari T. and Foster K. R. (2008). Sperm sociality: cooperation, altruism, and spite. PLoS Biol. 6, e130 10.1371/journal.pbio.0060130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T.-W., Xue L. and Ozcan A. (2012). High-throughput lensfree 3D tracking of human sperms reveals rare statistics of helical trajectories. Proc. Natl Acad. Sci. USA 109, 16018-16022. 10.1073/pnas.1212506109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez S. S. (2002). Formation of a reservoir of sperm in the oviduct. Reprod. Domest. Anim. 37, 140-143. 10.1046/j.1439-0531.2002.00346.x [DOI] [PubMed] [Google Scholar]
- Suarez S. S. (2016). Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res. 363, 185-194. 10.1007/s00441-015-2244-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez S. S. and Pacey A. A. (2006). Sperm transport in the female reproductive tract. Hum. Reprod. Update 12, 23-37. 10.1093/humupd/dmi047 [DOI] [PubMed] [Google Scholar]
- Wang S., Burton J. C., Behringer R. R. and Larina I. V. (2015a). In vivo micro-scale tomography of ciliary behavior in the mammalian oviduct. Sci. Rep. 5, 13216 10.1038/srep13216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Singh M., Lopez A. L., Wu C., Raghunathan R., Schill A., Li J., Larin K. V. and Larina I. V. (2015b). Direct four-dimensional structural and functional imaging of cardiovascular dynamics in mouse embryos with 1.5 MHz optical coherence tomography. Opt. Lett. 40, 4791-4794. 10.1364/OL.40.004791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K. C., Huang B. K., Gamm U. A., Bhandari V., Khokha M. K. and Choma M. A. (2016). Particle streak velocimetry-optical coherence tomography: a novel method for multidimensional imaging of microscale fluid flows. Biomed. Opt. Express 7, 1590-1603. 10.1364/BOE.7.001590 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.