Abstract
Who should take on risky tasks in an age-heterogeneous society? Life-history theory predicts that, in social insects, riskier tasks should be undertaken by sterile individuals with a shorter life expectancy. The loss of individuals with shorter life expectancy is less costly for colony reproductive success than the loss of individuals with longer life expectancy. Termite colonies have a sterile soldier caste, specialized defenders engaged in the most risky tasks. Here we show that termite soldiers exhibit age-dependent polyethism, as old soldiers are engaged in front-line defence more than young soldiers. Our nest defence experiment showed that old soldiers went to the front line and blocked the nest opening against approaching predatory ants more often than young soldiers. We also found that young soldiers were more biased toward choosing central nest defence as royal guards than old soldiers. These results demonstrate that termite soldiers have age-based task allocation, by which ageing predisposes soldiers to switch to more dangerous tasks. This age-dependent soldier task allocation increases the life expectancy of soldiers, allowing them to promote their lifetime contribution to colony reproductive success.
Keywords: task allocation, division of labour, ageing, social insects, life-history strategy
1. Introduction
Division of labour among colony members based on age, i.e. age polyethism, evolved in all major groups of eusocial insects [1]. Colony-level selection favours adaptive colony demography in terms of age structure so as to promote the colony's reproductive success [2]. A theoretical study of age polyethism in social insects demonstrated that age polyethism is profitable when safer tasks are performed earlier in life and when associated with higher ageing-related mortality or programmed senescence [2–4]. Honeybee workers display an elaborate division of labour by age, usually progressing from inside-nest to outside-nest labour [5]. This age polyethism schedule is evidently adaptive because it postpones the most risky labour to the latest stage of workers' lives, increasing their life expectancy [5,6]. Age-related division of labour has also been investigated in termite workers [7], and typically seen in the neotropical termite Neocapritermes taracua, in which older workers, rather than young workers, perform self-sacrificing defence [8].
The termite soldier caste is morphologically and behaviourally specialized for defence [2]. Because soldier is a terminal caste that differentiates from workers, soldier production imposes an opportunity cost on the colony [2]. Therefore, it would be adaptive for the colony to position soldiers of different age classes so as to increase their life expectancy. Nevertheless, termite soldiers have never been studied from the viewpoint of age polyethism [8]. Mortality risk for soldiers differs among defence positions in the nest, as soldiers on the front line of defence must take more risks than royal guards deep inside the nest (electronic supplementary material, figure S1). For instance, in the subterranean termite Reticulitermes speratus, entrance-guard and royal-guard soldiers are located in different areas of the nest and perform distinct modes of defence (electronic supplementary material, text S1). We can reasonably assume age-related mortality increase if life expectancy is not intrinsically eternal or accumulation of extrinsic damage, including self-inflicted injury, promotes mortality [9]. Hence, we reasonably predict age-based task allocation in termite soldiers.
In this study, we investigated age-dependent task allocation in the soldier caste of a subterranean termite, Reticulitermes speratus. In Reticulitermes termites, soldiers live at least five years after the final molt from workers [10]. Differentiation of new soldiers occurs only in early summer [11], which provides an ideal opportunity to distinguish new recruits from veteran soldiers. We compared the defence behaviours of young and old soldiers in experimental nests in the presence of the termite predatory ant Brachyponera chinensis. We also investigated sex differences in soldier defence behaviour, as the termite soldier caste consists of both sexes, in contrast to the female-dominated societies of the eusocial Hymenoptera.
2. Material and methods
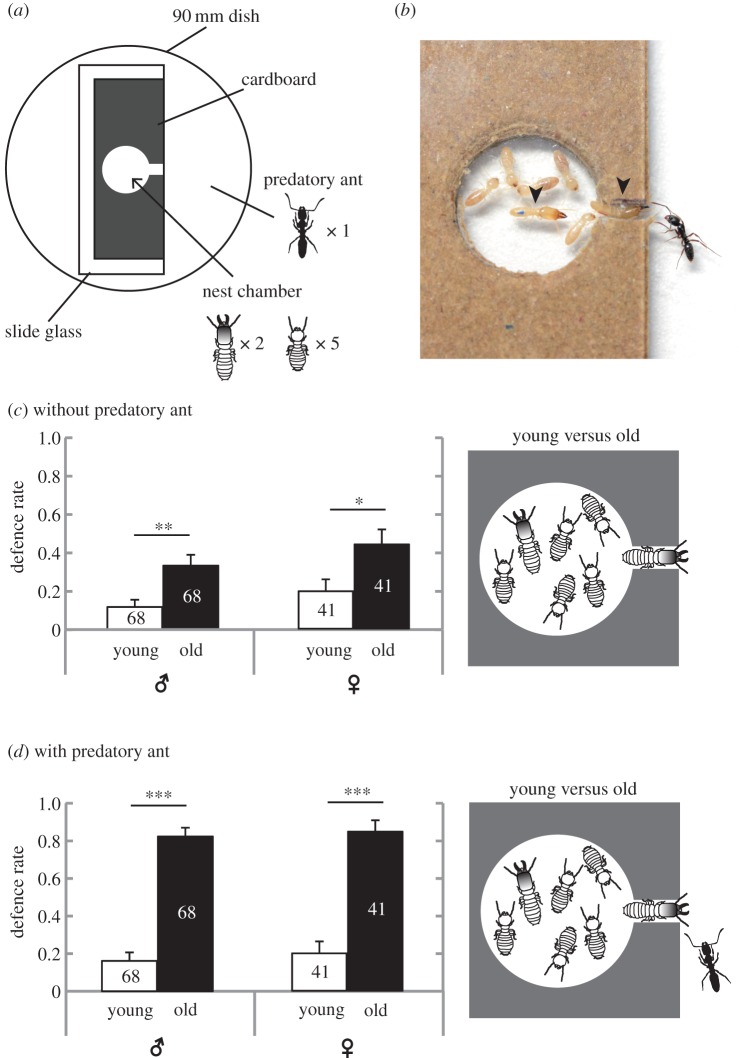
To observe soldier defence behaviour, we prepared experimental cardboard nests with a cylindrical chamber and an entrance [12] (figure 1a). The experimental nest was placed in the centre of a Petri dish, and then two soldiers and five workers were placed in the nest chamber (figure 1b). One hour later, we recorded the position of each soldier. Thereafter, a single predatory ant, i.e. the termite-hunting needle ant Brachyponera chinensis, was placed in the Petri dish. Subsequent termite defence and ant predation behaviour was recorded. We compared the defensive rates (number of soldiers defending at the nest opening per number of trials) between old and young soldiers of each sex, and between male and female soldiers of each age class. We performed five to twenty replicates (depending on soldier availability from each colony) for each treatment, for each colony. This experiment was repeated using the five colonies. See electronic supplementary material for details (text S2).
Figure 1.
Comparison of defence behaviour between young and old soldiers. (a) Diagram illustrating the experimental setup from above. (b) A close-up photo image of the nest chamber with an approaching predatory ant. Arrowheads indicate soldiers. (c) Comparison of defence rates between young and old soldiers (left: male, right: female) in the absence of predatory ants. (d) Comparison of defence rates in the presence of predatory ants. Values denote the means ± s.e.; n is indicated on each bar. *p < 0.05, **p < 0.01, ***p < 0.001, generalized linear mixed model (GLMM). (Online version in colour.)
3. Results
Old soldiers of both sexes showed defence behaviour at the nest entrance more often than young soldiers, even in the absence of predatory ants (likelihood ratio test, males: 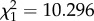 , p = 0.001; females:
, p = 0.001; females: 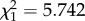 , p = 0.017; figure 1c). Then we introduced a single predatory ant in each Petri dish. When a predatory ant approached the nest entrance, a soldier fiercely attacked the ant (electronic supplementary material, movie S2). Introduction of the predatory ant greatly increased the age-related difference in defence behaviour, as old soldiers blocked the nest opening and fought against the approaching ants much more frequently than young soldiers (likelihood ratio test, males:
, p = 0.017; figure 1c). Then we introduced a single predatory ant in each Petri dish. When a predatory ant approached the nest entrance, a soldier fiercely attacked the ant (electronic supplementary material, movie S2). Introduction of the predatory ant greatly increased the age-related difference in defence behaviour, as old soldiers blocked the nest opening and fought against the approaching ants much more frequently than young soldiers (likelihood ratio test, males: 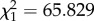 , p < 0.001; females:
, p < 0.001; females: 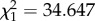 , p < 0.001, figure 1d). When an old male and an old female soldier were placed in an experimental nest, females engaged in front-line defence more often than males (likelihood ratio test, ant absent:
, p < 0.001, figure 1d). When an old male and an old female soldier were placed in an experimental nest, females engaged in front-line defence more often than males (likelihood ratio test, ant absent: 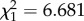 , p = 0.010; ant present:
, p = 0.010; ant present: 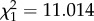 , p < 0.001; electronic supplementary material, figure S3). When a young male and a young female soldier were placed in a nest, young soldiers themselves performed defence behaviour as well as old soldiers (41 of 41 trials with predatory ants approaching the nest entrance). There was no significant difference in defence behaviour between young male and young female soldiers (likelihood ratio test, ant absent:
, p < 0.001; electronic supplementary material, figure S3). When a young male and a young female soldier were placed in a nest, young soldiers themselves performed defence behaviour as well as old soldiers (41 of 41 trials with predatory ants approaching the nest entrance). There was no significant difference in defence behaviour between young male and young female soldiers (likelihood ratio test, ant absent: 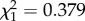 , p = 0.538; ant present:
, p = 0.538; ant present: 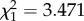 , p = 0.062).
, p = 0.062).
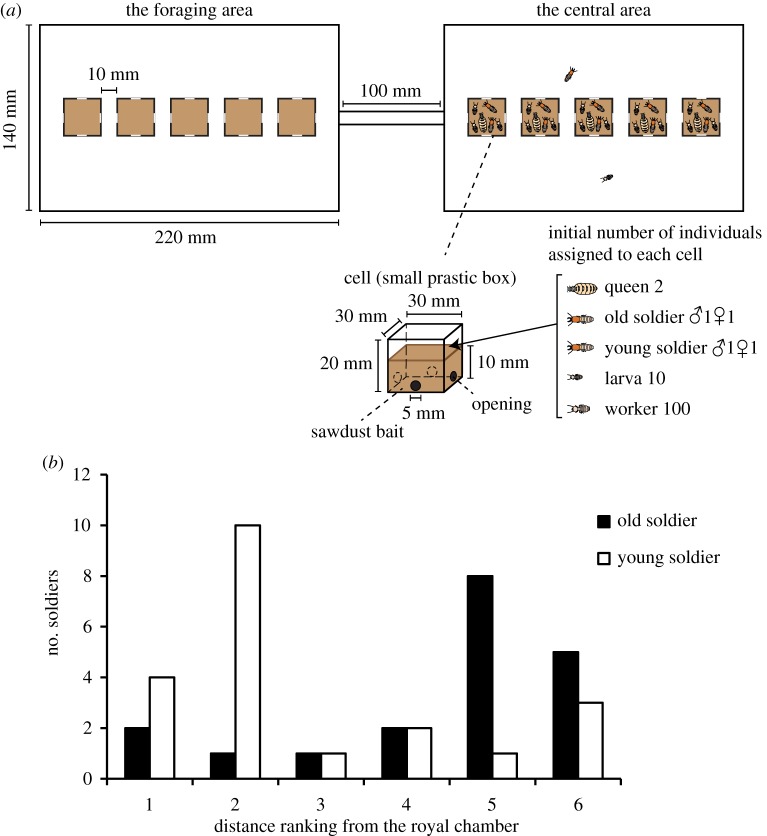
Comparison of survivorship under starvation conditions showed significantly higher survivorship of young soldiers than old soldiers (Wald test, z = −3.403, p < 0.001). There was a significant difference between the distribution of young and old soldiers in the nest, as young soldiers were located around the royal chamber and old soldiers guarded outer nest parts (likelihood ratio test, 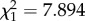 , p = 0.005; figure 2b). Sex and colony had no significant effects on the soldier distribution pattern (likelihood ratio test, sex:
, p = 0.005; figure 2b). Sex and colony had no significant effects on the soldier distribution pattern (likelihood ratio test, sex: 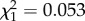 , p = 0.817; colony:
, p = 0.817; colony: 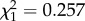 , p = 0.612).
, p = 0.612).
Figure 2.
Distribution of old and young soldiers in an artificial nest. (a) Diagram of an artificial nest imitating two nest logs (large plastic cases) connected by a gallery (acrylic tube). Five small plastic cases, imitating nest chambers in a log, were arranged in each large plastic case. Small openings on each side of the small cases allow termites to pass through. (b) Comparison of the distribution of young and old soldiers in terms of the distance from the royal chamber, in which queens are harboured. Young soldiers were located around the royal chamber more often than old soldiers (see electronic supplementary information for distance ranking). (Online version in colour.)
4. Discussion
Soldiers are completely sterile in R. speratus (see electronic supplementary material, text S1 for details), while reproductive soldiers are reported in a basal termite Zootermopsis nevadensis [13]. Selection would act on the age polyethism of sterile termite soldiers through colony-level performance and thus through colony productivity, as demonstrated in sterile ant workers [14]. Here, we showed that old soldiers go to the front line of nest defence more often than young soldiers (figure 1c). A marked behavioural difference was observed when predatory ants were introduced to the test arena (figure 1d). We also revealed that young soldiers are more biased toward choosing central nest defence as royal guards (figure 2). In addition, old soldiers showed significantly higher mortality than young soldiers under starvation conditions, although an increase in intrinsic mortality with age remains to be explored. These results demonstrate that termite soldiers exhibit age-based task allocation, which is consistent with the life-history theory that age polyethism is profitable when safer tasks are performed earlier in life and when associated with higher ageing-related mortality [3].
It has been shown that as workers age, they engage in more diverse tasks, many of which involve activities at the nest periphery or outside the nest, that are associated with higher risk in eusocial Hymenoptera [1,5,15] and termites [7]. Because workers engage in a variety of tasks throughout their lives including brood care, grooming, nest construction, foraging and anti-predatory defence, chronological task schedules have resulted from age-related increase in task performance or repertoire expansion [16] due to physical maturation [17] and neurophysiological development [18]. In termite workers, especially, age and size polyethism are inter-linked because of hemimetabolous development. Therefore, it has remained unclear whether life expectancy is an ultimate factor in allocating risk-related tasks in social insects (see also electronic supplementary material, text S3).
In the present study, we used a study system of termite soldiers, a physical caste specialized to nest defence, by which we separated the effects of size and age. Our results cannot be explained by age-related increase of task performance because phragmotic soldiers act as living plugs blocking the nest openings with their heads, where young and old plugs seem unlikely to differ in their defence performance. Indeed, both young and old soldiers perfectly protected the nests against predatory ants when either only young soldiers or old soldiers were used in the experiment (young soldiers: 41/41; old soldiers 81/81). In addition, life-long resilience, i.e. no age-related decline in task performance [18], may make phragmotic soldiers valuable to colony defence as long as they live. Therefore, our results most explicitly suggest that life expectancy and the risk associated with each task should be taken into account as an ultimate factor of age polyethism in social insects.
Supplementary Material
Supplementary Material
Acknowledgements
We thank M. Takata and T. Fujita for help with termite sampling, S. Dobata and other members of the Laboratory of Insect Ecology, Kyoto University for helpful discussion. We thank James F.A. Traniello and an anonymous referee for their constructive comments.
Ethics
The research adhered to the ASAB/ABS Guidelines for the Use of Animals in Research.
Data accessibility
Data are available as electronic supplementary material.
Authors' contributions
K.M., S.Y. and W.S. designed the study. All authors collected termites and performed the experiments. K.M., S.Y., W.S. and Y.M. wrote the manuscript, and all authors are accountable for the content and approved the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
This work was funded by the Japan Society for the Promotion of Science to K.M. (Kiban Kenkyu S: 25221206).
References
- 1.Hölldobler B, Wilson EO. 2009. The superorganism: the beauty, elegance, and strangeness of insect societies. New York, NY: WW Norton & Company. [Google Scholar]
- 2.Oster GF, Wilson EO. 1978. Caste and ecology in the social insects. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 3.Tofilski A. 2002. Influence of age polyethism on longevity of workers in social insects. Behav. Ecol. Sociobiol. 51, 234–237. ( 10.1007/s00265-001-0429-z) [DOI] [Google Scholar]
- 4.Giraldo YM, Traniello JFA. 2014. Worker senescence and the sociobiology of aging in ants. Behav. Ecol. Sociobiol. 68, 1901–1919. ( 10.1007/s00265-014-1826-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seeley TD. 1982. Adaptive significance of the age polyethism schedule in honeybee colonies. Behav. Ecol. Sociobiol. 11, 287–293. ( 10.1007/BF00299306) [DOI] [Google Scholar]
- 6.Wakano JY, Nakata K, Yamamura N. 1998. Dynamic model of optimal age polyethism in social insects under stable and fluctuating environments. J. Theor. Biol. 193, 153–165. ( 10.1006/jtbi.1998.0697) [DOI] [Google Scholar]
- 7.Crosland MWJ, Lok CM, Wong TC, Shakarad M, Traniello JFA. 1997. Division of labour in a lower termite: the majority of tasks are performed by older workers. Anim. Behav. 54, 999–1012. ( 10.1006/anbe.1997.0509) [DOI] [PubMed] [Google Scholar]
- 8.Šobotník J, et al. 2012. Explosive backpacks in old termite workers. Science 337, 436 ( 10.1126/science.1219129) [DOI] [PubMed] [Google Scholar]
- 9.Lane SM, Briffa M. 2017. The price of attack: rethinking damage costs in animal contests. Anim. Behav. 126, 23–29. ( 10.1016/j.anbehav.2017.01.015) [DOI] [Google Scholar]
- 10.Buchli HR. 1958. L'origine des castes et les potentialités ontogénétiques des termites européens du genre Reticulitermes Holmgren. Ann. Sci. Nat. Zool. 20, 261–429. [Google Scholar]
- 11.Matsuura K. 2002. Sociobiology of the termite Reticulitermes speratus. Doctoral thesis, Kyoto University, Kyoto, Japan. [Google Scholar]
- 12.Matsuura K. 2002. Colony-level stabilization of soldier head width for head-plug defense in the termite Reticulitermes speratus (Isoptera: Rhinotermitidae). Behav. Ecol. Sociobiol. 51, 172–179. ( 10.1007/s00265-001-0426-2) [DOI] [Google Scholar]
- 13.Thorne BL, Breisch NL, Muscedere ML. 2003. Evolution of eusociality and the soldier caste in termites: influence of intraspecific competition and accelerated inheritance. Proc. Natl Acad. Sci. USA 100, 12 808–12 813. ( 10.1073/pnas.2133530100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giehr J, Heinze J, Schrempf A. 2017. Group demography affects ant colony performance and individual speed of queen and worker aging. BMC Evol. Biol. 17, 173 ( 10.1186/s12862-017-1026-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Traniello JFA. 1989. Foraging strategies of ants. Annu. Rev. Entomol. 34, 191–210. ( 10.1146/annurev.en.34.010189.001203) [DOI] [Google Scholar]
- 16.Seid MA, Traniello JFA. 2006. Age-related repertoire expansion and division of labor in Pheidole dentata (Hymenoptera: Formicidae): a new perspective on temporal polyethism and behavioral plasticity in ants. Behav. Ecol. Sociobiol. 60, 631–644. ( 10.1007/s00265-006-0207-z) [DOI] [Google Scholar]
- 17.Muscedere ML, Traniello JFA, Gronenberg W. 2011. Coming of age in an ant colony: cephalic muscle maturation accompanies behavioral development in Pheidole dentata. Naturwissenschaften 98, 783 ( 10.1007/s00114-011-0828-6) [DOI] [PubMed] [Google Scholar]
- 18.Giraldo YM, et al. 2016. Lifespan behavioural and neural resilience in a social insect. Proc. R. Soc. B 283, 20152603 ( 10.1098/rspb.2015.2603) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available as electronic supplementary material.