Abstract
Cooperatively breeding common marmosets show substantial variation in the amount of help they provide. Pay-to-stay and social prestige models of helping attribute this variation to audience effects, i.e. that individuals help more if group members can witness their interactions with immatures, whereas models of kin selection, group augmentation or those stressing the need to gain parenting experience do not predict any audience effects. We quantified the readiness of adult marmosets to share food in the presence or absence of other group members. Contrary to both predictions, we found a reverse audience effect on food-sharing behaviour: marmosets would systematically share more food with immatures when no audience was present. Thus, helping in common marmosets, at least in related family groups, does not support the pay-to-stay or the social prestige model, and helpers do not take advantage of the opportunity to engage in reputation management. Rather, the results appear to reflect a genuine concern for the immatures' well-being, which seems particularly strong when solely responsible for the immatures.
Keywords: marmosets, food sharing, audience effects, social prestige, pay to stay, diffusion of responsibility
1. Introduction
Callitrichid monkeys, including common marmosets, show the highest levels of allomaternal care in non-human primates [1]. They typically live in extended family groups with a breeding pair and sexually mature helpers who may be more or less closely related [2,3]. All group members contribute to rearing offspring, mostly by carrying infants and sharing food with them. Food sharing can be reactive (i.e. in response to begging by immatures), but also proactive (i.e. initiated by the food possessor, in the absence of begging) [4,5].
The timing and intensity of help provided by different group members can vary substantially (e.g. [6–9]), but the factors underlying this variability are poorly understood [3]. A better understanding of these factors is crucial because this allows evaluation of different evolutionary scenarios of cooperative breeding. Likely ultimate explanations for cooperative infant care [3,10] are kin selection, gaining parenting experience, group augmentation, social prestige and pay-to-stay. Kin selection must have played a crucial role via indirect fitness benefits. Nevertheless, it is unlikely to explain the full range of variation in helping effort because equally related helpers can differ considerably in their contribution, and to date there are no data available showing that more closely related helpers help more [3]. Thus, in addition to these indirect fitness benefits, direct benefits may also contribute, such as gaining parenting experience and group augmentation effects. Furthermore, helpers may pay dominant breeders for the opportunity to stay in the group until a breeding opportunity becomes available (i.e. pay-to-stay models). Finally, helpers may signal individual quality (i.e. increase their social prestige) to potential breeding partners by helping, and so improve future mating success.
Audience effects on helping can contribute to evaluate these evolutionary scenarios for cooperative breeding. Kin selection, group augmentation and parenting experience models predict that helping effort is not influenced by the presence of an audience. By contrast, strategic pay-to-stay and social prestige models predict more helping by helpers in the presence of a relevant audience, and thus reputation management.
The goal of this study was to test these predictions, by assessing audience effects on helping in common marmosets. We quantified the propensity of adult marmosets to share food with immatures in two conditions: a group condition (GC), when the whole family group was present versus a solo condition (SC), when alone with the immatures (see supplementary material for details). If marmosets engaged in reputation management, the following predictions ensue: when an audience was present, helpers, but not breeders, should (1) share more with immatures, (2) be less likely to refuse to share when immatures were begging and (3) emit fewer aggressive chatter vocalizations towards immatures. Furthermore, any effects should not be attributable to (4) the behaviour of immatures (different begging rates) and thus (5) also be specific to adult-initiated proactive sharing.
2. Material and methods
We tested 45 common marmosets (Callithrix jacchus, 31 adults and 14 immatures, from six family groups). The adults were tested on average four times per week, during the period of immature age of 7–25 weeks, alternately in the GC or the SC. In each condition and on each test day, the readiness to share food was quantified with the access-bias-free method [11], in three consecutive trials. This method consists of handing over a cricket to a focal adult and recording whether the food is shared and, if so, how (proactively, i.e. initiated by the helper, or reactively, i.e. initiated by the immature). Furthermore, we also recorded all food-related social behaviours. The marmosets were neither food- nor water-deprived for any of the experiments.
Trials of the GC were conducted in a partition of the home enclosure with all group members present. Trials of the SC were conducted in the same partition of the home cage, but with only the helper and the family's immature twins present, whereas the rest of the group was visually separated in an adjacent enclosure.
All food sharing types, vocalizations and begging were coded as binary response variables and analysed with GLMMs (random effect: focal nested in the family group). A detailed description of the experimental procedures, housing conditions and data analysis procedures is provided in the electronic supplementary materials.
3. Results
We analysed 2581 trials, 51.1% from the GC and 48.9% from the SC. The majority of all crickets were shared with immatures (66.8% in the GC; 85.2% in the SC).
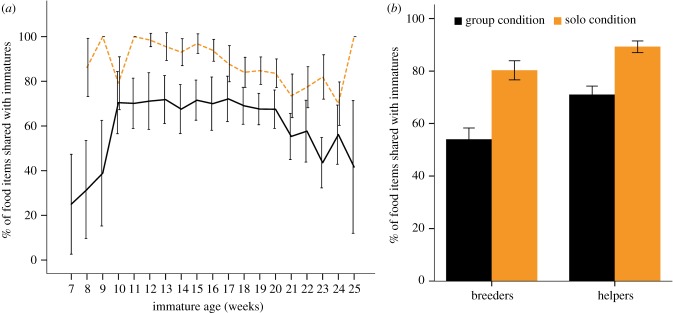
Contrary to the predictions, marmosets shared overall more food during the SC compared to the GC and they did so over the entire testing period (figure 1a). In fact, food sharing rates in the SC were very high and reached almost 100% during weeks 11–15 of immature age. Accordingly, model 1 (table 1) explained the data better compared to the null model (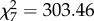 , p < 0.001) and revealed a significant effect of condition, age, the number of crickets consumed by the immature in previous trials of the same session and family size. We found no effect of the social status of the donor or the interaction between social status*condition (figure 1b), and all classes of animals shared more in the SC (electronic supplementary material, figure S2).
, p < 0.001) and revealed a significant effect of condition, age, the number of crickets consumed by the immature in previous trials of the same session and family size. We found no effect of the social status of the donor or the interaction between social status*condition (figure 1b), and all classes of animals shared more in the SC (electronic supplementary material, figure S2).
Figure 1.
(a) Percentage of crickets shared by adults with immatures (error bars: s.e.m. of the means). (b) Percentage of shared crickets split up by donor status and condition (error bars: s.e.m. of the means). Orange: solo condition (no audience); black: group condition (audience present). (Online version in colour.)
Table 1.
Overview of GLMMs on different dependent variables. Italics: p < 0.05.
| model | dependent variable | fixed factor | estimate (s.e.) | odds ratio | 95% CI | z-value | p-value |
|---|---|---|---|---|---|---|---|
| 1 | total adult immature food sharing | condition (solo) | 1.39 (0.17) | 4.02 | 2.86; 5.65 | 8.01 | 1.11 × 10−15*** |
| crickets eaten by immatures | 0.09 (0.02) | 1.09 | 1.06; 1.13 | 5.64 | 1.70 × 10−08*** | ||
| immatures’ age in weeks | 0.47 (0.10) | 1.60 | 1.33; 1.93 | 4.86 | 1.16 × 10−06*** | ||
| squared age | −0.02 (0.00) | 0.98 | 0.98; 0.99 | −5.70 | 1.24 × 10−08*** | ||
| family size | 0.75 (0.27) | 2.12 | 1.26; 3.59 | 2.81 | 4.96 × 10−03** | ||
| donor status (helper) | 0.36 (0.58) | 1.43 | 0.46; 4.43 | 0.62 | 0.54 | ||
| donor status (helper) * condition (solo) | 0.29 (0.24) | 1.34 | 0.83; 2.16 | 1.21 | 0.23 | ||
| 2 | refusals despite immature begging | condition (solo) | −1.01 (0.16) | 0.36 | 0.27; 0.50 | −6.47 | 9.98 × 10−11*** |
| crickets eaten by immatures | −0.07 (0.01) | 0.93 | 0.91; 0.96 | −4.89 | 1.03 × 10−06*** | ||
| immatures’ age in weeks | 0.14 (0.02) | 1.15 | 1.10; 1.19 | 7.07 | 1.54 × 10−12*** | ||
| family size | −0.04 (0.17) | 0.96 | 0.69; 1.33 | −0.23 | 0.82 | ||
| donor status (helper) | −0.16 (0.41) | 0.85 | 0.39; 1.90 | −0.39 | 0.70 | ||
| donor status (helper) * condition (solo) | −0.29 (0.21) | 0.75 | 0.49; 1.14 | −1.35 | 0.18 | ||
| 3 | chatter calls toward immatures | condition (solo) | −1.57 (0.25) | 0.21 | 0.13; 0.34 | −6.25 | 4.15 × 10−10*** |
| crickets eaten by immatures | −0.08 (0.02) | 0.93 | 0.89; 0.97 | −3.49 | 4.86 × 10−04*** | ||
| immatures’ age in weeks | 0.15 (0.03) | 1.17 | 1.11; 1.23 | 5.59 | 2.34 × 10−08*** | ||
| family size | 1.55 (0.41) | 4.70 | 2.11; 10.50 | 3.79 | 1.52 × 10−04*** | ||
| donor status (helper) | −1.02 (0.63) | 0.36 | 0.11;1.23 | −1.62 | 0.10 | ||
| donor status (helper) * condition (solo) | 0.42 (0.33) | 1.52 | 0.80; 2.89 | 1.29 | 0.20 | ||
| 4 | immature begging | condition | −1.14 (0.16) | 0.32 | 0.23; 0.44 | −7.08 | 1.48 × 10−12*** |
| crickets eaten by immatures | −0.05 (0.01) | 0.96 | 0.93; 0.98 | −3.56 | 3.66 × 10−04*** | ||
| immatures’ age in weeks | 0.15 (0.02) | 1.17 | 1.13; 1.21 | 8.69 | <2 × 10−16*** | ||
| family size | −0.92 (0.16) | 0.40 | 0.29; 0.54 | −5.83 | 5.59 × 10−09*** | ||
| donor status (helper) | 0.15 (0.32) | 1.16 | 0.61; 2.18 | 0.45 | 0.65 | ||
| donor status (helper) * condition (solo) | −0.62 (0.21) | 0.54 | 0.36; 0.81 | −3.00 | 2.73 × 10−03** | ||
| 5 | proactive adult immature food sharing | condition (solo) | 1.66 (0.19) | 5.27 | 3.66; 7.58 | 8.93 | <2 × 10−16*** |
| crickets eaten by immatures | 0.07 (0.01) | 1.08 | 1.05; 1.10 | 5.23 | 1.68 × 10−07*** | ||
| immatures’ age in weeks | −0.15 (0.02) | 0.86 | 0.83; 0.90 | −7.50 | 6.21 × 10−14*** | ||
| family size | 1.42 (0.27) | 4.16 | 2.46; 7.01 | 5.34 | 9.33 × 10−08*** | ||
| donor status (helper) | 0.23 (0.41) | 1.26 | 0.56; 2.83 | 0.57 | 0.57 | ||
| donor status (helper) * condition (solo) | 0.56 (0.23) | 1.76 | 1.11; 2.77 | 2.42 | 0.02* |
Although both helpers and breeders shared consistently more in the SC compared to the GC, it could still be that marmosets engaged in reputation management by performing fewer uncooperative, socio-negative behaviours in the presence of an audience, such as refusing a begging immature, or emitting aggressive chatter vocalizations towards begging immatures. The results showed that this was not the case, and consistent with the previous finding, the marmosets used aggressive behaviours more, rather than less, often when the audience was present (refusing begging immatures: 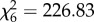 , p < 0.001, model 2 in table 1; emitting chatters towards begging immatures:
, p < 0.001, model 2 in table 1; emitting chatters towards begging immatures: 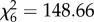 , p < 0.001, model 3 in table 1 and figure 2).
, p < 0.001, model 3 in table 1 and figure 2).
Figure 2.
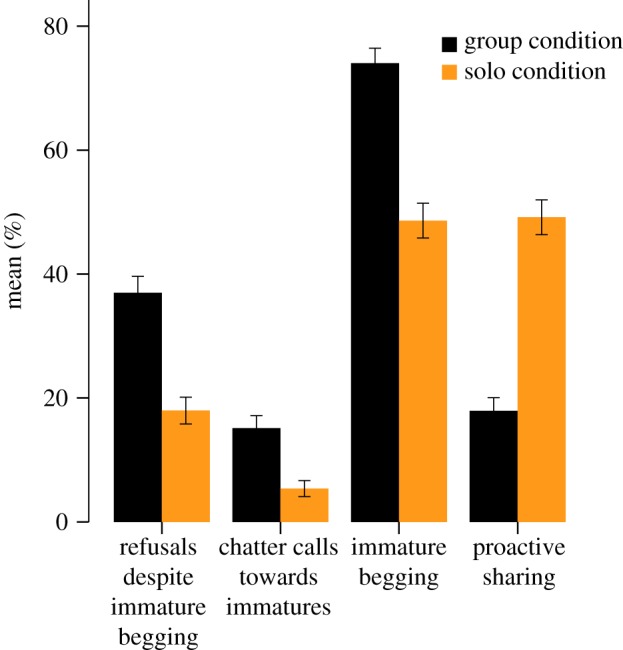
Mean percentage of trials with refusal, chatter, immature begging and proactive food sharing in the GC and the SC (error bars: s.e.m. of the means). Orange: solo condition, black: group condition. (Online version in colour.)
It thus appears that marmosets are more willing to share with immatures when they are alone with them. An alternative explanation is that this pattern of results does not reflect a higher willingness to share, but that immatures are more likely to beg in the SC, whereas adults respond with the same level of tolerance in both conditions. To exclude this possibility, we also analysed begging rates by immatures and proactive food sharing (i.e. the subset of all sharing events that were initiated by the adult rather than the immature). Contrary to this alternative explanation, immatures actually begged more in the GC (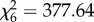 , p < 0.001, model 4, table 1 and figure 2), and proactive sharing was again higher in the SC (
, p < 0.001, model 4, table 1 and figure 2), and proactive sharing was again higher in the SC (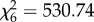 , p < 0.001, model 5, table 1 and figure 2). In both analyses, the full model also contained a significant interaction effect between condition and status, indicating that helpers showed a stronger increase in sharing when alone with the immatures than breeders, and that immatures showed a stronger decrease in begging when alone with a helper than when alone with a breeder (electronic supplementary material, figure S3).
, p < 0.001, model 5, table 1 and figure 2). In both analyses, the full model also contained a significant interaction effect between condition and status, indicating that helpers showed a stronger increase in sharing when alone with the immatures than breeders, and that immatures showed a stronger decrease in begging when alone with a helper than when alone with a breeder (electronic supplementary material, figure S3).
4. Discussion
We investigated audience effects on food sharing in marmosets and found that they did not share more when an audience was present. Thus our results do not support the pay-to-stay and the social prestige models of helping in marmosets, at least in related family groups. It remains to be established, however, whether paying-to-stay and social prestige could be important for non-related helpers, which we did not test in the present study.
Our findings nevertheless revealed sensitivity to the audience, namely a strong reverse audience effect, in that all adults shared more when they were alone with immatures. This increase in sharing in the SC reflected a higher willingness to share by the adults: immatures did not beg more when alone with the adult, and adults strongly increased proactive food sharing. All other suggested predictions were therefore also not met. Both kin selection and group augmentation suggest a genuine interest in the well-being of the immatures, and therefore predict no difference between the SC and the GC. Overall and proactive sharing increased in helpers and breeders, but helpers showed a stronger increase in proactive sharing than breeders. This could suggest that the helpers were keen to gain parenting experience and took advantage of the SC because they did not get enough access to the immatures while together in the whole group [12]. However, this explanation cannot account for the full pattern of results. First, it cannot explain the increase in sharing, including proactive sharing, in breeders. Second, the increase was detectable until week 25. Because immature marmosets are usually carried only until week 11 [9], it is unclear what kind of parenting skills helpers could acquire with immatures that old.
Why then do we find a reverse audience effect? The marmoset pattern of results calls to mind the so-called diffusion-of-responsibility effect which is well established in humans [13,14]: people are less likely to help in the presence of a larger number of bystanders, but feel more responsible when alone with a needy individual. This effect is fully compatible with kin selection and group augmentation having played a role for the evolution of helping, but also shows that helping is further fine-tuned according to situational conditions in marmosets. Future research will have to establish to what extent our findings show similarities and differences with the human diffusion-of-responsibility effect, and whether such an effect is more likely in cooperatively breeding primates, such as callitrichids and humans, than in independently breeding primates, such as capuchin monkeys or chimpanzees [15].
In conclusion, our results show that helping in common marmosets is not driven by reputation management or punishment avoidance. Rather it is driven by an intrinsic motivation to help that is more strongly expressed when individuals are alone with offspring.
Supplementary Material
Acknowledgements
We thank Erik Willems for statistical support and Carel van Schaik for useful comments on earlier drafts of this paper.
Ethics
We performed our study in accordance with the national laws. The study was approved by the Kantonales Veterinäramt, licence number 183/13.
Data accessibility
Data can be accessed from the Dryad Digital Repository [16] (doi:10.5061/dryad.6455c).
Authors' contributions
R.K.B. and J.M.B. designed the study, analysed the data and wrote the manuscript. R.K.B. and T.K.S. collected the data. All the authors commented on the manuscript and gave their final approval for publication. All the authors agree to be held accountable for the content of this publication.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by a grant of the Janggen Poehn Stiftung to R.K.B. and SNF grant nos. 105312-114107 and 310030_130383 to J.M.B.
References
- 1.van Schaik CP. 2016. Parenting and reproductive investment. In The primate origins of human nature, pp. 233–250. New York, NY: Wiley. [Google Scholar]
- 2.Digby LJ, Ferrari SF, Saltzman W. 2007. Callitrichines: the role of competition in cooperatively breeding species. In Primates in perspective (eds Campbell C, Fuentes A, MacKinnon K, Bearder S, Stumpf R), pp. 85–106. New York, NY: Oxford University Press. [Google Scholar]
- 3.Erb WM, Porter LM. 2017. Mother's little helpers: what we know (and don't know) about cooperative infant care in callitrichines. Evol. Anthropol. 26, 25–37. ( 10.1002/evan.21516) [DOI] [PubMed] [Google Scholar]
- 4.Brown GR, Almond REA, van Bergen Y. 2004. Begging, stealing, and offering: food transfer in non-human primates. Adv. Study Behav. 34, 265–295. ( 10.1016/S0065-3454(04)34007-6) [DOI] [Google Scholar]
- 5.Jaeggi AV, Gurven M. 2013. Reciprocity explains food sharing in humans and other primates independent of kin selection and tolerated scrounging: a phylogenetic meta-analysis. Proc. R. Soc. B 280, 20131615 ( 10.1098/rspb.2013.1615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tardif SD, Carson RL, Gangaware BL. 1990. Infant-care behavior of mothers and fathers in a communal-care primate, the cotton-top tamarin (Saguinus oedipus). Am. J. Primatol. 22, 73–85. ( 10.1002/ajp.1350220202) [DOI] [PubMed] [Google Scholar]
- 7.Ferrari SF. 1992. The care of infants in a wild marmoset (Callithrix flaviceps) group. Am. J. Primatol. 26, 109–118. ( 10.1002/ajp.1350260205) [DOI] [PubMed] [Google Scholar]
- 8.Huck M, Löttker P, Heymann EW. 2004. Proximate mechanisms of reproductive monopolization in male moustached tamarins (Saguinus mystax). Am. J. Primatol. 64, 39–56. ( 10.1002/ajp.20060) [DOI] [PubMed] [Google Scholar]
- 9.Finkenwirth C, Martins E, Deschner T, Burkart JM. 2016. Oxytocin is associated with infant-care behavior and motivation in cooperatively breeding marmoset monkeys. Horm. Behav. 80, 10–18. ( 10.1016/j.yhbeh.2016.01.008) [DOI] [PubMed] [Google Scholar]
- 10.Wong M, Balshine S. 2011. The evolution of cooperative breeding in the african cichlid fish, Neolamprologus pulcher. Biol. Rev. 86, 511–530. ( 10.1111/j.1469-185X.2010.00158.x) [DOI] [PubMed] [Google Scholar]
- 11.Guerreiro Martins E, Moura A, Finkenwirth C, Burkart JM. Submitted. Food sharing in three species of callitrichid monkeys: individual differences and interspecific variation. J. Comp. Psychol. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto ME, Araujo A, Arruda MdeF, Lima AKM, Siqueira Jd O, Hattori WT. 2014. Male and female breeding strategies in a cooperative primate. Behav. Processes 109, 27–33. ( 10.1016/j.beproc.2014.06.009) [DOI] [PubMed] [Google Scholar]
- 13.Latané B, Nida S. 1981. Ten years of research on group size and helping. Psychol. Bull. 89, 308–324. ( 10.1037/0033-2909.89.2.308) [DOI] [Google Scholar]
- 14.Bierhoff H-W, Rohmann E. 2017. Diffusion von Verantwortung. In Handbuch verantwortung (eds Heidbrink L, Langbehn C, Loh J), pp. 911–931. Berlin, Germany: Springer. [Google Scholar]
- 15.Burkart JM, van Schaik CP. 2016. Revisiting the consequences of cooperative breeding. J. Zool. 299, 77–83. ( 10.1111/jzo.12322) [DOI] [Google Scholar]
- 16.Brügger RK, Kappeler-Schmalzriedt T, Burkart JM. 2018. Data from: Reverse audience effects on helping in cooperatively breeding marmoset monkeys Dryad Digital Repository. ( 10.5061/dryad.6455c) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Brügger RK, Kappeler-Schmalzriedt T, Burkart JM. 2018. Data from: Reverse audience effects on helping in cooperatively breeding marmoset monkeys Dryad Digital Repository. ( 10.5061/dryad.6455c) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data can be accessed from the Dryad Digital Repository [16] (doi:10.5061/dryad.6455c).