Abstract
Urbanization, one of the most extreme human-induced environmental changes, represents a major challenge for many organisms. Anthropogenic habitats can have opposing effects on different fitness components, for example, by decreasing starvation risk but also health status. Assessment of the net fitness effect of anthropogenic habitats is therefore difficult. Telomere length is associated with phenotypic quality and mortality rate in many species, and the rate of telomere shortening is considered an integrative measure of the ‘life stress’ experienced by an individual. This makes telomere length a promising candidate for examining the effects of urbanization on the health status of individuals. We investigated whether telomere length differed between urban and forest-dwelling common blackbirds (Turdus merula). Using the terminal restriction fragment assay, we analysed telomere length in yearlings and older adults from five population dyads (urban versus forest) across Europe. In both age classes, urban blackbirds had significantly shorter telomeres (547 bp) than blackbirds in natural habitats, indicating lower health status in urban blackbirds. We propose several potential hypotheses to explain our results. Our findings show that even successful city dwellers such as blackbirds pay a price for living in these anthropogenic habitats.
Keywords: birds, human-induced environmental change, telomeres, urbanization
1. Introduction
Urbanization is an extreme form of human-induced environmental alteration [1] that can have both positive and negative effects on organisms [2]. On the one hand, urban habitats may offer safety from predators and starvation, increasing lifespan. On the other hand, various pollutants (chemical, light and noise) may have a negative impact on health, decreasing survival prospects. Previous studies have investigated urbanization effects on health using different physiological biomarkers such as hormones and oxidative stress (e.g. [3,4]). However, interpretation of variation in biomarkers is difficult as there is usually no clear-cut association between physiological markers and fitness components, and therefore, the biomarkers used so far may well be reversible traits falling into the regulatory and acclimatory responses as defined by McDonnell & Hahs [5]. This impedes an assessment of potential net health differences between individuals in urban and natural habitats. Hence, there is a need for biomarkers that can be interpreted unambiguously to capture the net effect of urbanization on the individual status of organisms.
Telomeres, nucleoprotein structures at the end of chromosomes promoting genome stability [6], are excellent candidates for this purpose. There is evidence associating telomere length with disease, mortality rate and lifespan in different organisms (e.g. [7–10]) and supporting its use as a biomarker of ageing and phenotypic quality (e.g. [11]). Furthermore, environmental changes inherent to urbanization, such as noise pollution and diseases, are known to affect telomere length [10,12], and living in cities is thought to modify animals' oxidative balance [4], which seems the main physiological mechanism responsible for the shortening of telomeres (e.g. [13]). Therefore, we would expect shorter telomeres in organisms living in urban areas. In line with this expectation, a recent cross-fostering experiment with great tit (Parus major) nestlings found that being raised in an urban environment significantly shortened their telomeres [14], although the selective disappearance of individuals with short telomeres after fledging annulled this difference in the adult population [15]. Note, however, that this study relied on a single urban–rural comparison in Sweden, and that a similar study of great tit nestlings in France could not replicate this finding [16]. This highlights that multiple geographical replicates are required to reveal general effects of urbanization on organisms.
We tested whether urban areas are associated with telomere length in European populations of adult common blackbirds (Turdus merula). We sampled five pairs of urban versus forest populations encompassing a large geographical range (electronic supplementary material, figure S1). Blackbirds are particularly interesting in this context, because urban populations hold a larger proportion of older individuals than non-urban populations [17], indicating a higher adult survival rate in cities. This suggests that age-corrected telomere length may be longer in urban blackbirds. Furthermore, experimental exposure to stressors significantly shortened telomeres in adult blackbirds, confirming that telomeres are indicative of health in this species [11].
2. Material and methods
(a). Study design and sampling
Urban capture sites consisted of parks and gardens located in the core area of cities. Forest capture sites were in protected forested areas, with minimum human activity. The habitat differences were confirmed with a commonly used urbanization index (electronic supplementary material, table S1).
Blackbirds were captured using mist nets in March–July 2015 and classified as either yearlings or older birds (≥2 years old) based on their plumage. Immediately after capture, blood samples (350–450 µl) were taken from the brachial vein, kept at 4°C for up to 5 h until centrifugation (15 min at 5000g), after which the red blood cells were frozen at −80°C. Telomere length was measured in yearlings from all locations (N = 62, 32 males and 30 females), and as part of another study, in a subset of older (≥2 years old) birds from three locations (N = 42, males only).
(b). Telomere length assays
Telomere length was analysed in erythrocytes using telomere restriction fragment assays, as previously described [9]. In every gel, we included randomly selected samples from all locations, habitats and sexes to avoid confounding effects of gel identity. Telomere length was quantified through densitometry using ImageJ v. 1.38x. The lower limit of the measurement was lane-specifically set at the point with the lowest signal on the part of the distribution representing the small telomeres, while the upper limit was set at the length of 48 000 bp. A lane-specific background, selected outside the telomeric region as a mean value calculated between 60 000 and 70 000 bp, was subtracted from the optical density measurements. Between-gel coefficient of variation of a standard sample (blood of the same individual) run on all gels was 2.5% (yearlings) and 3.9% (older adults).
(c). Statistical analyses
We analysed our data using linear mixed models with the R package ‘lme4’ [18]. Because telomeres shorten with age [7,9], which may be habitat-dependent (see below), and because yearlings and older birds were measured in different batches, we first analysed yearlings only. The starting model in this case included habitat type (urban or forest), sex and their interaction as fixed factors, while gel identity and location nested within dyad were considered as random factors. However, telomere length was independent of sex and hence sex was removed from the models (electronic supplementary material, table S2). In a second analysis, we tested for an urbanization effect among birds older than yearlings. Finally, we also compared the age ratio in urban and forest areas using the same starting model previously described.
3. Results
Average (±s.d.) telomere length was 11.3 ± 1.0 kb for yearlings and 10.8 ± 1.2 kb for older individuals. Telomere length of urban yearlings was significantly shorter than that of forest yearlings (figure 1; difference ± s.e.: 547 ± 224 bp; F1,56.9 = 5.94, p = 0.017) and this pattern was observed in all five dyads (electronic supplementary material, figure S2). In blackbirds older than yearlings, the difference between urban and forest blackbirds was even larger (922 ± 332 bp; F1,47.0 = 7.73, p = 0.008; figure 1) though less consistent across locations (electronic supplementary material, figure S3). In agreement with previous results [17], we found a higher proportion of adults older than yearlings in cities for our studied populations (Z = 3.50, p = 0.0005; N = 263; figure 2; electronic supplementary material, table S4).
Figure 1.
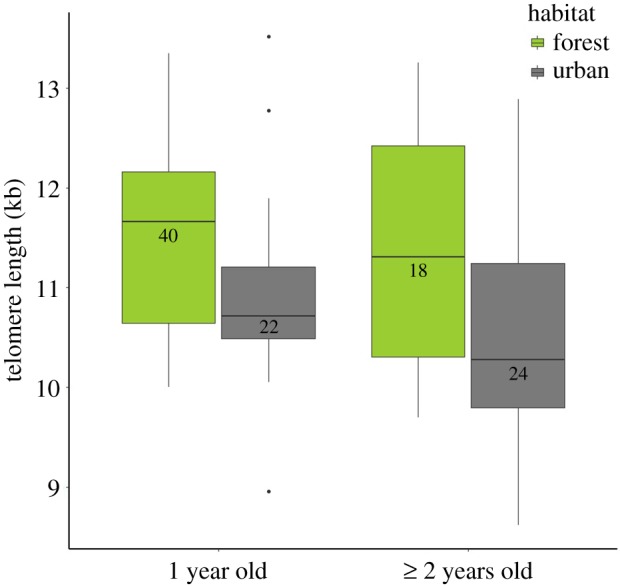
Telomere length of blackbirds for each habitat in all study locations combined. Box plots show the median, upper and lower quartiles, maximum and minimum values, and outliers. (Online version in colour.)
Figure 2.
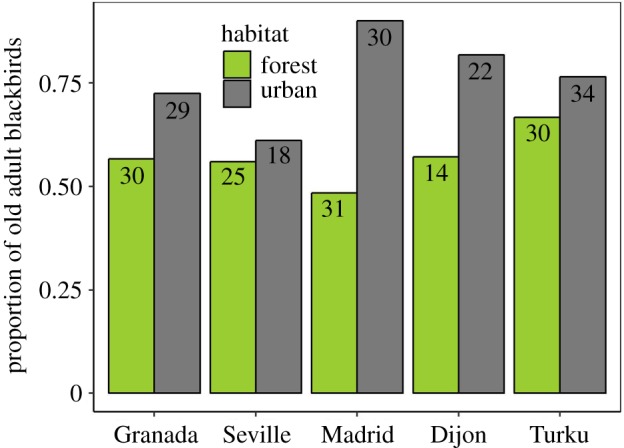
Proportion of old adult blackbirds (≥2 years old) in urban and forest habitats for each location. Numbers within each bar represents sample sizes. (Online version in colour.)
4. Discussion
Adult urban blackbirds have shorter telomeres than blackbirds in forests (figure 1) independent of age or location (figure 1; electronic supplementary material, figure S2), indicating it to be a robust effect. Our findings suggest that urban blackbirds are in a poorer state, given that shorter telomeres in blackbirds are indicators of poor phenotypic quality [11]. Anthropogenic landscape change causes a global loss of biodiversity [19], which highlights the relevance of our results to species, like the blackbird, that do persist in these environments. However, our findings contrast with the apparently higher survival rates of urban blackbirds ([17]; figure 2), indicating that the poorer phenotypic state of urban blackbirds is more than offset by other factors (i.e. predator absence) with respect to survival. The differences between our results and those recently found in adult great tits for a Swedish urban–rural comparison [15] could be explained by (i) species-specific effects of urbanization or (ii) local versus general patterns. Interestingly, the habitat effect in our study was larger in Turku than in Dijon (electronic supplementary material, figures S2 and S3), matching the contrasting findings between French [16] and Swedish nestling great tits [14]. Future studies should target disentangling the causes of such geographical variation.
Different mechanisms, not mutually exclusive, may have caused the habitat effect on telomere length. (i) The origin may be genetic, given that heritability of telomere length is often high [20], and that cities can select for specific genes [21]. Under this hypothesis, we would expect the habitat effect to be already present at conception. (ii) Telomere attrition during the first year of life may be higher in urban habitat, either in the nest [14] or thereafter. (iii) Selection on telomere length may be habitat-dependent [15], with stronger selection against short telomeres in forests. Hypotheses (ii) and (iii) can both be tested using longitudinal data on survival and telomere dynamics. (iv) Habitat selection may depend on telomere length, with individuals with short telomeres being more likely to colonize urban habitats [22]. (v) Urban blackbirds are older than forest blackbirds ([17]; this study), and because paternal age affects offspring telomere length in several species [23], this can potentially explain the habitat effect on telomere length. Habitat-associated variation in age structure could also explain our findings in older birds, but not in yearlings, for which within-group variation in age is minimal.
Our investigation provides the first large-scale evidence showing that telomere length is habitat-dependent in free-living populations. Long-term individual-based studies are now needed to unravel how the habitat effect on telomere length arises and whether other species or taxonomic groups show similar patterns.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank F. Espinosa, I. Hernandez, A. Pastoriza, J. Martínez de la Puente and M. Vazquez for their help capturing blackbirds and E. Mulder for her guidance during lab analyses. C. Bauch, B. Jimeno and O. Sanllorente made very useful suggestions to improve the study.
Ethics
Our study was approved by the Ethical Committee of the Consejo Superior de Investigaciones Científicas (CSIC) with the reference number 258/2015.
Data accessibility
The dataset is included in the electronic supplementary material.
Authors' contributions
J.D.I. conceived the study. J.D.I., R.L.T., J.I.A., A.D., J.F. and B.F. collected data. J.P. and S.V. performed lab analyses. J.D.I. and J.P. analysed data. J.D.I. wrote the first draft of the manuscript. All authors edited the manuscript. All authors agree to be held accountable for the content of the manuscript and approve its final version.
Competing interests
We have no competing interests.
Funding
J.D.I. was funded by a postdoctoral contract (TAHUB-104) from the program ‘Andalucía Talent Hub’ (Marie Skłodowska-Curie actions—COFUND). A.D. was funded by the ‘Severo Ochoa’ program from MICINN (Spain). J.P. was funded by a grant from the Universidad Complutense de Madrid (CT45/15-CT46/15).
References
- 1.Foley JA, et al. 2005. Global consequences of land use. Science 309, 570–574. ( 10.1126/science.1111772) [DOI] [PubMed] [Google Scholar]
- 2.Gaston K. 2010. Urban ecology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Bonier F. 2012. Hormones in the city: endocrine ecology of urban birds. Horm. Behav. 61, 763–772. ( 10.1016/j.yhbeh.2012.03.016) [DOI] [PubMed] [Google Scholar]
- 4.Hutton P, McGraw KJ. 2016. Urban impacts on oxidative balance and animal signals. Front. Ecol. Evol. 4, 54 ( 10.3389/fevo.2016.00054) [DOI] [Google Scholar]
- 5.McDonnell MJ, Hahs AK. 2015. Adaptation and adaptedness of organisms to urban environments. Annu. Rev. Ecol. Evol. Syst. 46, 261–280. ( 10.1146/annurev-ecolsys-112414-054258) [DOI] [Google Scholar]
- 6.Blackburn EH. 1991. Structure and function of telomeres. Nature 350, 569–573. ( 10.1038/350569a0) [DOI] [PubMed] [Google Scholar]
- 7.Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748. ( 10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauch C, Becker PH, Verhulst S. 2014. Within the genome, long telomeres are more informative than short telomeres with respect to fitness components in a long-lived seabird. Mol. Ecol. 23, 300–310. ( 10.1111/mec.12602) [DOI] [PubMed] [Google Scholar]
- 9.Salomons HM, Mulder GA, van de Zande L, Haussmann MF, Linskens MHK, Verhulst S. 2009. Telomere shortening and survival in free-living corvids. Proc. R. Soc. B 276, 3157–3165. ( 10.1098/rspb.2009.0517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S. 2015. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347, 436–438. ( 10.1126/science.1261121) [DOI] [PubMed] [Google Scholar]
- 11.Hau M, Haussmann MF, Greives TJ, Matlack C, Costantini D, Quetting M, Adelman JS, Miranda A, Partecke J. 2015. Repeated stressors in adulthood increase the rate of biological ageing. Front. Zool. 12, 4 ( 10.1186/s12983-015-0095-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meillere A, Brischoux F, Ribout C, Angelier F. 2015. Traffic noise exposure affects telomere length in nestling house sparrows. Biol. Lett. 11, 1–5. ( 10.1098/rsbl.2015.0559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badás EP, Martínez J, Rivero de Aguilar Cachafeiro J, Miranda F, Figuerola J, Merino S. 2015. Ageing and reproduction: antioxidant supplementation alleviates telomere loss in wild birds. J. Evol. Biol. 28, 896–905. ( 10.1111/jeb.12615) [DOI] [PubMed] [Google Scholar]
- 14.Salmón P, Nilsson JF, Nord A, Bensch S, Isaksson C.. 2016. Urban environment shortens telomere length in nestling great tits, Parus major. Biol. Lett. 12, 254–260. ( 10.1098/rsbl.2016.0155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmón P, Nilsson JF, Watson H, Bensch S, Isaksson C.. 2017. Selective disappearance of great tits with short telomeres in urban areas. Proc. R. Soc. B 284, 20171349 ( 10.1098/rspb.2017.1349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biard C, Brischoux F, Meillère A, Michaud B, Nivière M, Ruault S, Vaugoyeau M, Angelier F. 2017. Growing in cities: an urban penalty for wild birds? A study of phenotypic differences between urban and rural great tit chicks (Parus major). Front. Ecol. Evol. 5, 1–14. ( 10.3389/fevo.2017.00079) [DOI] [Google Scholar]
- 17.Evans KL, Gaston KJ, Sharp SP, McGowan A, Simeoni M, Hatchwell BJ. 2009. Effects of urbanisation on disease prevalence and age structure in blackbird Turdus merula populations. Oikos 118, 774–782. ( 10.1111/j.1600-0706.2008.17226.x) [DOI] [Google Scholar]
- 18.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 19.Ibáñez-Álamo JD, Rubio E, Benedetti Y, Morelli F. 2016. Global loss of avian evolutionary uniqueness in urban areas. Glob. Change Biol. 23, 2990–2998. ( 10.1111/gcb.13567) [DOI] [PubMed] [Google Scholar]
- 20.Atema E, Mulder E, Dugdale HL, Briga M, van Noordwijk AJ, Verhulst S. 2015. Heritability of telomere length in the zebra finch. J. Ornithol. 156, 1113–1123. ( 10.1007/s10336-015-1212-7) [DOI] [Google Scholar]
- 21.Mueller JC, Partecke J, Hatchwell BJ, Gaston KJ, Evans KL. 2013. Candidate gene polymorphisms for behavioural adaptations during urbanization in blackbirds. Mol. Ecol. 22, 3629–3637. ( 10.1111/mec.12288) [DOI] [PubMed] [Google Scholar]
- 22.Verhulst S, Perrins CM, Riddington R. 1997. Natal dispersal of great tits in a patchy environment. Ecology 78, 864–872. ( 10.1890/0012-9658(1997)078[0864:NDOGTI]2.0.CO;2) [DOI] [Google Scholar]
- 23.Eisenberg DTA, Kuzawa CW. 2018. The paternal age at conception effect on offspring telomere length: mechanistic, comparative and adaptive perspectives. Phil. Trans. R. Soc. B 373, 20160442 ( 10.1098/rstb.2016.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset is included in the electronic supplementary material.


