Abstract
Animal movements can facilitate important ecological processes, and wide-ranging marine predators, such as sharks, potentially contribute significantly towards nutrient transfer between habitats. We applied network theory to 4 years of acoustic telemetry data for grey reef sharks (Carcharhinus amblyrhynchos) at Palmyra, an unfished atoll, to assess their potential role in nutrient dynamics throughout this remote ecosystem. We evaluated the dynamics of habitat connectivity and used network metrics to quantify shark-mediated nutrient distribution. Predator movements were consistent within year, but differed between years and by sex. Females used higher numbers of routes throughout the system, distributing nutrients over a larger proportion of the atoll. Extrapolations of tagged sharks to the population level suggest that prey consumption and subsequent egestion leads to the heterogeneous deposition of 94.5 kg d−1 of nitrogen around the atoll, with approximately 86% of this probably derived from pelagic resources. These results suggest that sharks may contribute substantially to nutrient transfer from offshore waters to near-shore reefs, subsidies that are important for coral reef health.
Keywords: acoustic telemetry, grey reef sharks, Carcharhinus amblyrhynchos, network theory, nitrogen cycle, Palmyra Atoll
1. Introduction
The movement patterns of animals can be highly complex, being influenced by both their social and physical environments. Consequently, understanding and accurately measuring population dynamics can be challenging [1–3]. Understanding movement patterns, however, is vital for identifying habitats critical for population connectivity or migration [4], for predicting how stochastic or future environmental conditions will affect populations [5] and for evaluating the effectiveness of protected areas [6]. It cannot simply be assumed that the habitat in which a population is most commonly observed, is that which provides a critical function (a source of food or location for reproduction; e.g. [7]). Therefore, to develop effective conservation approaches, it is essential to understand how a species's behaviour and movement varies across and between habitats [8,9].
In both terrestrial and marine environments, movements of predators can directly and indirectly influence ecological processes such as nutrient cycling and trophic interactions [10,11]. Indirectly, predators can affect nutrient cycling through interactions with prey species; for example, the foraging activities of grazing amphipods and isopods were shifted in response to the presence of predatory blue crabs (Callinectes sapidus), contributing to increases in labile organic matter within sea grass ecosystems [12]. More directly, animals can act as nutrient and organic matter vectors, by egesting material within the same habitats in which the food was consumed, or across habitat boundaries (translocation [10,13,14]). In Alaska, freshwater and/or marine-derived nutrients released by brown bears facilitate growth in white spruce up to 1 km from riparian zones [15]. By foraging at depth and then excreting faeces within the euphotic zone, marine mammals such as humpback and fin whales have been found to replenish nitrogen concentrations at the ocean's surface, thereby enhancing primary productivity (termed the upwards ‘whale pump’ [16]). Wide-ranging predators such as some whales and sharks also have the potential to contribute significantly to the horizonal transport of nutrients between habitats within marine ecosystems [9,17].
Measuring nutrient transfer between areas and assessing the stability of such flow, however, is non-trivial. There is substantial evidence that the disruption of animal movements can negatively impact productivity through the loss of certain species, posing considerable threat to an ecosystem's long-term resilience [11,18,19]. For example, in the Aleutian archipelago, seabirds act as vectors, transporting nutrients from the ocean to land [20]. However, since the introduction of arctic foxes (Alopex lagopus), which have preyed upon seabirds and thus reduced this important nutrient supply, plant communities have been transformed and productivity has decreased [20]. Understanding how predators link habitats and transport nutrients through their environment is, therefore, crucial for ecosystem management.
Palmyra Atoll is a remote, relatively undisturbed coral reef ecosystem, and is part of a US National Wildlife Refuge within the central Pacific Ocean [21,22]. Owing to its protected status, Palmyra has a healthy predator population, with grey reef sharks (Carcharhinus amblyrhynchos) being the most abundant predator on the fore-reefs [23,24]. The grey reef shark population at Palmyra is probably at carrying capacity [24], and may play a significant role in the transportation and flow of nutrients onto the reef and throughout the atoll. Grey reef sharks are often detected on (and suggested to favour) outer-reef slopes and drop-off habitats, but on occasion are detected within lagoons [25–27]. Previous research has also highlighted sexual segregation in some grey reef shark populations, which suggests that males and females may connect habitats differently and thus transfer nutrients in differing quantities [25]. Stable isotope analyses at Palmyra have demonstrated that grey reef sharks acquire resources from different habitats, including from pelagic and near-shore environments [7]. However, the use of these habitats for foraging is uneven, with around 86% of grey reef shark biomass being derived from pelagic resources [7]. Mobile species that transport nutrients between habitats have the potential to impact new primary productivity and contribute to the modification of the physical environment [28]. How mobile marine predators such as sharks facilitate this nutrient transport, how much they contribute and how this is subsequently distributed across shallow, productive reef habitats remain unexplored [25,29]. Thus, by transporting materials onto reef habitats that were produced elsewhere, grey reef sharks may generate important linkages between ecosystems and possibly play an ecologically important role in nutrient connectivity. Grey reef sharks show quite strong residency to core areas of the reef, and low rates of movement between reef habitats [27]. However, they are probably transporting pelagic nutrients to fore-reef and potentially back-reef habitats.
Using acoustic telemetry and network analyses, we measure the connectivity generated by the intra- and inter-habitat movements of predatory grey reef sharks. We then quantify estimates of potential nitrogen transport onto the reef by this species at Palmyra Atoll to understand how nitrogen is probably distributed along different routes of the movement network. We use recent population estimates [24] to extrapolate to the population level in order to assess how significant this nutrient subsidy is likely to be to reef productivity. Owing to previous evidence of sexual segregation in this species in Palmyra Atoll (Y. Papastamatiou and D. Bradley 2012–2014, personal observation), we hypothesize that male and female sharks will have a different influence on nutrient dynamics.
2. Method
(a). Study site and species
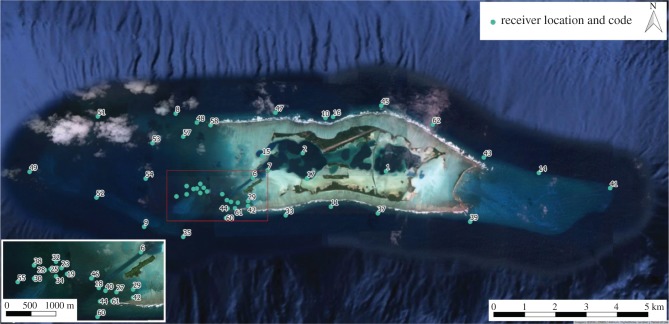
Palmyra Atoll (5°53′ N, 162°05′ W) is situated in the northern Line Islands in the central Pacific Ocean (figure 1). In 2001, the atoll became a US National Wildlife Refuge, prohibiting take of marine organisms. Since 2001, the only inhabitants have been small (less than 20) groups of researchers and refuge staff [21]. Within the wildlife refuge there is a spatial array of 65 VR2W acoustic receivers (Vemco, Halifax, Nova Scotia, Canada; figure 1). Receivers detect animal-borne, acoustic transmitters at an approximate range of 250–300 m; each time transmitters are detected, the identification number, date and time are recorded by the receiver. Receiver habitat was broadly classified by its geographical zone (lagoon, back-reef or fore-reef).
Figure 1.
Spatial array of acoustic receivers at Palmyra Atoll; only receivers included within the analyses performed in this study are shown. Satellite image from Google Earth. (Online version in colour.)
Detections were recorded from 41 grey reef sharks, comprising approximately 0.49% of the grey reef shark population at Palmyra Atoll [24]. These sharks were tagged with acoustic transmitters (Vemco V16 and V13 coded transmitters) that had been surgically inserted into their body cavities (for details on the method of shark capture and tagging, see [21]). Shark tagging took place on 10 days between 2010 and 2013 at various locations around the atoll. For each individual tagged, we recorded sex and size. Weekly sea surface temperature readings from Palmyra Atoll were obtained from the Coral Reef Ecosystem Program, National Oceanic and Atmospheric Administration (NOAA; electronic supplementary material, S1).
(b). Network analyses
Applying network theory to acoustic telemetry data allows the movement of sharks to be viewed as a system of connections, in which acoustic receivers are linked by shark movements (for further details on network theory and how it is applied to telemetry data, see [30]). This technique offers insight into how species move between and thus connect habitats [6].
To construct the movement networks, data were initially filtered to include only detections relating to movements of individuals between receivers (i.e. departures and arrivals). However, residency patterns of sharks at each receiver location were calculated from the full dataset (see below for details on residency). To limit transmitters being detected by more than one receiver at the same time (due to some overlap in detection range for a few receivers), the receivers with the greatest overlap were removed from analyses, ensuring that, within the same habitat classification, no two receivers were closer than 150 m (detection distance determined during range testing). Following data filtering, detections from 47 acoustic receivers between January 2011 and December 2014 (1461 days) were included in the analyses. Network theory was employed to analyse these detections, where movement networks measure the relationship between nodes (the acoustic receivers), which are linked by edges (shark movements) [30]. A key temporal measure associated with an edge is its duration: the time between an individual's last detection at one receiver and its first detection at a different receiver (time taken to make the movement [3]). As we were interested in movements that potentially led to the transfer of nutrients around Palmyra Atoll, we filtered the data to only include movements within ≤110 h time windows. This duration represents the length of time, post-feeding, that lemon sharks (Negaprion brevirostris) have been observed to continue producing faeces following prey consumption [31]. Lemon sharks are the only species, to our knowledge, for which faecal production time has been measured, and this quantity has been used in previous studies to filter edge duration when focusing on nutrient transfer by marine predators (e.g. [9]). From January 2011 to December 2014, tagged grey reef sharks were detected 848 100 times by the 47 acoustic receivers; this included 99 752 movements between receivers of which 99 342 were ≤110 h apart (table 1). To explore temporal dynamics, the data were divided into four ‘seasons’ by examining sea surface temperature data to determine thermally similar three-month periods (December–February, March–May, June–August and September–November).
Table 1.
Summary of the tagged grey reef sharks and their movements detected by the acoustic receivers in Palmyra Atoll; mean values displayed in the table are presented with one standard deviation.
| all sharks | female | male | |
|---|---|---|---|
| number of individuals detected | |||
| 2011 | 27 | 20 | 7 |
| 2012 | 38 | 25 | 13 |
| 2013 | 33 | 22 | 11 |
| 2014 | 30 | 20 | 10 |
| total | 41 | 28 | 13 |
| number of movements ≤110 h | |||
| 2011 | 16 665 | 13 559 | 3106 |
| 2012 | 21 750 | 17 177 | 4573 |
| 2013 | 29 639 | 24 648 | 4991 |
| 2014 | 31 288 | 24 933 | 6355 |
| total | 99 342 | 80 317 | 19 025 |
| days at liberty between 2011 and 2014a | |||
| min | 21 | 21 | 40 |
| max | 1439 | 1437 | 1439 |
| mean (s.d.) | 985 (396) | 977 (410) | 1001 (380) |
| fork length (m) | |||
| min | 0.86b | 0.86b | 1.03 |
| max | 1.47b | 1.47b | 1.31 |
| mean (s.d.) | 1.18 (0.15)b | 1.21 (0.17)b | 1.13 (0.10) |
aDays at liberty are the number of days between an individual's first and last detection in the dataset.
bThe fork length for one female was missing, thus for the measurements of all sharks, n = 40, and for females, n = 27.
(i). Connectivity within the network
To assess the connectivity within the reef ecosystem generated by grey reef shark movements, monthly network edge densities were extracted for each sex for every month of the study. Edge density is the proportion of edges (movements connecting receivers) existing in the network, out of the total number of edges possible for that network (if all receivers were linked by movements to every other receiver [30]). Movement networks with higher edge densities are more densely connected and, thus, individuals have a greater number of routes they can choose from to move through the system [2]. Analyses of variance were used to explore whether network edge densities (the dependent variable) differed between sexes, seasons and years (the independent variables).
(ii). Estimating nitrogen transfer throughout the atoll
To estimate shark-mediated nutrient flow and highlight areas important to nutrient connectivity, we calculated the potential quantity of nitrogen (N) that these predators may distribute within Palmyra Atoll during the ≤110 h filtered movement network. Length–weight relationships from Wetherbee et al. [32] were used to estimate the weight of each tagged individual (for all but one female for which total length was not recorded). Egestion rates of N for each individual within Palmyra Atoll per day were then calculated by using the upper limit of 2% (for carcharhinid sharks) of body weight ingested per day [33]. Absorption efficiency was set at 76% for organic matter based on estimates from lemon sharks [31]. We estimated N transfer using the method described by Nelson et al. [34], where the total egested kg N per day is the product of the biomass ingested by an individual shark, the biomass egested, the absorption efficiency and the per cent N found in grey reef shark tissue at Palmyra Atoll (14.84 ± 0.065% N mean ± s.e. [7]). For every tagged individual, we multiplied their estimated daily egested N (kg) by their residency time within the array, to give a cumulative estimate over the entire study period. To extrapolate to the population level, we took the average length of a male (138.7 cm) and female (146 cm) grey reef shark from [35], and the species abundance and sex ratio estimates from [24]. For all equations used, see electronic supplementary material S2.
Finally, the estimates of daily egested N by tagged sharks were then mapped spatially to explore which areas around Palmyra Atoll were likely to experience the largest influx of shark-derived N. To incorporate the movement of the grey reef sharks with the time they spent in different areas of the atoll, we calculated a sex-dependent dynamic residency score for each receiver. This score incorporated a residency index (the proportion of days the receiver detected a male/female shark over the study period), and the receiver's node strength (which combines the number of connections a node had (i.e. weighted degree) and the relative frequency with which those connections were used). Estimates of N distribution by the tagged male and female sharks were then spatially mapped according to the relative dynamic residency score of each receiver; see electronic supplementary material, S2 for further information. Statistical analyses were completed in R [36] and mapping in QGIS v. 2.14.0 [37].
3. Results
(a). Connectivity within the network
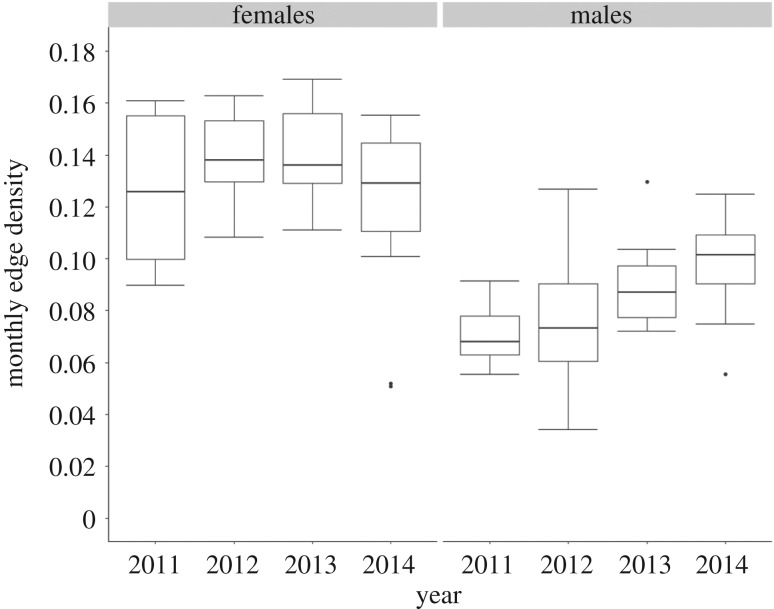
Across the study period, combining movements of both sexes, just under half of all edges possible in the network were present each year (edge density per year, mean (s.d.) = 0.477 (0.014); electronic supplementary material, S3). Females used a larger proportion of possible routes within the atoll than males, indicated by the female's significantly higher monthly edge density (F1,94 = 98.8, p < 0.01; figure 2). Unlike females, the monthly edge density of the male network differed significantly between years (females, F1,46 = 0.358, p = 0.55; males, F1,46 = 17.3, p < 0.01; figure 2), suggesting that, over the study period, males were less consistent in the linkages generated across the atoll. Between seasons, there was no significant difference in monthly edge density for either sex (females, F3,44 = 0.920, p = 0.44; males, F3,44 = 0.960, p = 0.42).
Figure 2.
The monthly edge densities of movement networks for female and male grey reef sharks (Carcharhinus amblyrhynchos) over each year of the study period; these differed significantly between the sexes (p < 0.01); the boxplots present the median and quartile values, the circles denote outliers.
(b). Estimating nitrogen transfer throughout the atoll
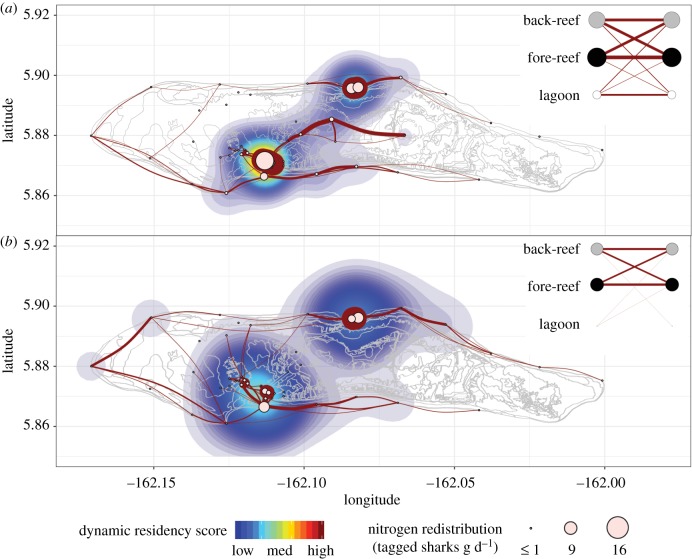
In total, over the 4-year duration of the study, tagged male (n = 13) and female (n = 27) grey reef sharks were estimated to have egested 42.11 ± 0.19 and 119.05 ± 0.52 kg, respectively, of N across the atoll and near-shore ecosystem. Given that 86% of biomass consumed by grey reef sharks at Palmyra is thought to be derived from pelagic resources [7], we predict that over the study period (1461 days) there was a maximum potential biomass subsidy of 138.60 ± 0.61 kg N transported onto Palmyra Atoll reefs by the tagged individuals. Based on the frequency with which the tagged sharks moved between and spent time at specific receiver locations, derived from our sex-specific movement networks, we then predict where the nitrogen is likely to be deposited (table 2; figure 3). We also visualize the relative frequency of shark movements between each geographical zone (back-reef, fore-reef and lagoon), to further explore the relative fluxes of N between different habitats (figure 3).
Table 2.
The five nodes around which the greatest quantity of nitrogen (N) is estimated to have been distributed by the tagged female or male grey reef sharks per day, based on the dynamic residency score of each node; see figure 1 for location of nodes.
| acoustic receiver (geographical zonea) | node strength | residency index (%) | dynamic residency score | quantity of nitrogen potentially distributed by the tagged grey reef sharks (g d−1) | |
|---|---|---|---|---|---|
| females | |||||
| 18 (FR) | 11 674 | 89.73 | 10 474.62 | 15.66 | |
| 40 (BR) | 9023 | 81.23 | 7329.64 | 10.96 | |
| 16 (FR) | 7360 | 84.11 | 6190.47 | 9.26 | |
| 10 (FR) | 7122 | 79.66 | 5673.21 | 8.48 | |
| 60 (FR) | 4094 | 92.19 | 3774.33 | 5.64 | |
| males | |||||
| 16 (FR) | 1704 | 82.26 | 1401.72 | 5.24 | |
| 60 (FR) | 1702 | 75.55 | 1285.83 | 4.80 | |
| 10 (FR) | 1567 | 55.34 | 867.22 | 3.24 | |
| 18 (FR) | 1727 | 39.11 | 675.43 | 2.52 | |
| 40 (BR) | 1413 | 32.26 | 455.84 | 1.70 | |
aGeographical zones include the fore-reef (FR), back-reef (BR) and lagoon.
Figure 3.
The 4-year movement networks of (a) female (n = 28) and (b) male (n = 13) grey reef sharks overlaid on kernel densities that represent dynamic residency at each receiver. Networks include only movements that took ≤110 h and have been filtered to show the 75 most frequently used routes by each sex. Edge thickness represents the frequency of movements (male range = 36–2711; female range = 129–13 131). The dynamic residency score was calculated as the node strength (Si) of each receiver divided by 100 and multiplied by a standard residency index, R (male range = 1–1401; female range = 2–10 474). The size of each node represents the potential N redistribution by the tagged grey reef sharks (table 2). The inset habitat networks illustrate the relative frequency of shark movements within and between geographical zones, with the size of the nodes representing the relative detection frequencies in each habitat; the left-hand nodes represent the zone the sharks moved into after last being detected in the habitat depicted by the right-hand node. The base map of Palmyra Atoll was acquired from NOAA National Ocean Service, National Centers for Coastal Ocean Science [38]. (Online version in colour.)
Using the mean length of male and female grey reef sharks sampled by Bradley et al. [35], an average male and female individual was estimated to egest as much as 0.008 ± 0.00004 and 0.011 ± 0.00005 kg d−1 of pelagic nitrogen in Palmyra Atoll, respectively. Taking recent population estimates and sex ratios (8344 individuals, 44% male, 56% female) from Bradley et al. [24], we extrapolate to the population, estimating a total biomass transfer of 94.52 ± 0.42 kg N d−1, of which as much as 81.28 ± 0.36 kg N d−1 is a subsidy from pelagic resources brought to the reef by grey reef sharks.
4. Discussion
Reef sharks transfer a significant amount of nitrogen to and within an isolated atoll, but the degree of connectivity differs between the sexes, with females using a higher number of routes throughout the near-shore ecosystem. Thus, in doing so, females, which are also typically larger than males, transfer nutrients more broadly across the atoll. Enhancing the understanding of these habitat linkages within reef ecosystems is critical to assist management and conservation strategies, protect movement corridors and respond to potential changes in nutrient dynamics [7].
Higher coverage of N distribution across the atoll by female sharks is probably due in part to female grey reef sharks being larger than males [35], as well as having higher movement rates within near-shore waters. By calculating kernel utilization distributions of acoustically tagged grey reef sharks at Palmyra Atoll, Bradley et al. [24] found that, compared with females, the activity space of male sharks was slightly larger, which is supported here by the distribution of dynamic residency scores (figure 3). Along with our results, this suggests that males may disperse more than females, potentially spending more time offshore, beyond the receiver array. Sexual segregation in the near-shore and offshore environment has been recorded in other populations of grey reef sharks, as well as other carcharhinids, and is suggested to be due to differential reproductive or foraging strategies [8]. The difference in routes used between sexes and their differing role in nutrient transfer needs to be incorporated into conservation plans, so areas important to or preferred by males and females are managed appropriately, ensuring each sex maintains their role in fostering connections throughout the ecosystem. Although male sharks used fewer routes, they also increased the proportion of routes they used over the years, even though the number of males detected decreased after 2012. This suggests the movement corridors used are not consistent over time for subsets of the population. This also demonstrates that measuring the efficacy of management strategies such as marine protected areas will require ongoing monitoring, because as animal movement patterns change, spatial strategies may need to be modified to ensure movement corridors remain protected. This may become even more important as marine ecosystems experience rapid effects of climate change.
There were no differences between seasons in the proportion of routes used. Grey reef shark movements on the Great Barrier Reef were not driven by environmental factors such as water temperature, rainfall or wind speed, and more probably related to biotic factors such as reproduction [39]. These results either reflect a resilience to change in environmental conditions within the movement network, or that environmental conditions experienced in these tropical systems were not variable enough to have an impact (average temperature for each season ranged from 27.2°C in March–May 2012 to 29.5°C in September–November 2014). Owing to reef sharks potentially being isolated from alternative suitable habitat, they may have higher tolerances to the range in local environmental conditions to avoid changing movement patterns [39].
Owing to an extensive 8-year tag–recapture programme at Palmyra that has led to accurate estimates of population size [24], we were in a unique position to be able to quantify population-level estimates of N distribution. The within-geographical-zone movements are potentially assisting nutrient recycling, as sharks may be egesting nutrients in the same habitat in which they were consumed [28]. For instance, from our analyses it can be seen that, in some cases, there was a high level of connectivity between nearby receivers (such as between acoustic receivers 10 and 16); this is also reflected in the high proportion of within-geographical-zone movements (figure 3). Moreover, it has recently been shown that grey reef sharks demonstrate strong residency within specific areas at the sub-habitat level [27]. In addition, grey reef sharks demonstrate vertical movement [27,40]; thus, the predators may be transporting nutrients vertically within habitats also [9]. Some routes and receiver locations along which the largest inputs of N were estimated to have occurred by the tagged sharks crossed between reef zones, demonstrating the potential for grey reef sharks to contribute to nutrient translocation. For example, just over 35% of movements by the tagged grey reef sharks that were recorded by the receiver array occurred between the fore-reef and back-reef (figure 3).
With approximately 86% of grey reef shark biomass derived from pelagic resources [7], these sharks may be distributing large quantities of nutrients onto the reef that could not have been produced within the atoll itself. Coral reefs are located in nutrient-limited oceanic waters, yet often support very high biodiversity and productivity [41]. While previous focus has been on tight nutrient cycling, research has shown that within coral reefs, fish are an important nutrient reservoir; both coral growth and primary production are enhanced by fish storing nutrients (in biomass) and egesting them [42–44]. New research indicates that, within reef systems, these fish-derived nutrients may play an important role in the maintenance of ecosystem dynamics [45]. Just how important might these shark-derived nutrient subsidies be in Palmyra? Palmyra Atoll has been recorded to have an average of 1.75 µM of dissolved inorganic nitrogen (ammonium, nitrates and nitrites) [46], which corresponds to between 32 and 109 µg of nitrogen-containing compounds per litre of water. In addition, during in situ nutrient sampling at locations around Palmyra Atoll (from 2006 to 2012) of nitrate and nitrite, a combined maximum of 15.21 µM was recorded (n = 125, range 0.08–15.21 µM [47]), corresponding to 942 µg of nitrate and nitrite per litre of water. Therefore, our estimations of an average-sized individual male and female grey reef shark subsidizing the reef with as much as 0.008 ± 0.00004 and 0.011 ± 0.00005 kg d−1, respectively, of pelagic-derived N into the atoll potentially provides a substantial contribution to reef primary productivity. While the precise effects of this nutrient subsidy on Palmyra's benthic communities remain to be explored, changes in grey reef shark population size will probably lead to disruptions in nutrient transport dynamics on this typical, nutrient-limited coral reef. Interspecific interactions between grey reef sharks and blacktip reef sharks may also alter nutrient dynamics, due to strong spatial partitioning between the two species [27]. Removal or reduction of one species may change the degree of among-habitat movements by the other, potentially altering nutrient deposition. For example, a loss of blacktip reef sharks may cause increased deposition of pelagic N into the lagoons by the grey reef sharks [27].
We recognize that these results should be interpreted with caution due to the fact that we do not know exactly where sharks go once they leave one receiver and arrive at another, and that not all egested material will be deposited within Palmyra Atoll; hence, we stress that these are estimates of potential nutrient flow. However, this is the first study to explicitly attempt to measure shark-derived nutrient transfer using a model that incorporates both the movement dynamics and residency patterns of free-ranging sharks. With the current available data and limited knowledge on shark daily rations, absorption and faecal production rates, this study's method enhances our understanding of the role grey reef sharks may play in nutrient connectivity.
Acoustic telemetry data and network theory are emerging as particularly useful tools for exploring habitat use and animal movements [6]. However, acoustic telemetry does have limitations. For example, here, as in many telemetry studies, the number of individuals with active tags was not consistent over the entire study period. This was partly due to some individuals being tagged after the beginning of 2011. In addition, by focusing on movements between different receivers, if sharks left Palmyra Atoll's fore-reef to feed within pelagic waters and then were next detected on the fore-reef by the same receiver (i.e. self-loops), the movement would not have been included within the analyses. Therefore, the number of movements made by the tagged individuals is likely to be on the conservative side. In addition, acoustic tagging of sharks was spatially non-uniform due to weather-dependent access to sampling sites. This will not affect the quantitative estimates of total N transferred, but it needs to be stressed that the visual representation of N redistribution (figure 3) is a spatial estimation for our tagged sharks only, not the population. Despite the limitations, acoustic telemetry can serve as a powerful instrument to quantify the movements of marine predators, particularly in remote or challenging environments, as well as over large areas [3,6,48].
In light of the fundamental influence that marine predators have on the functioning of ecosystems, understanding how these animals foster within- and cross-system connections is crucial to produce effective conservation and management strategies [7,49]. Palmyra Atoll, one of a limited number of near-pristine atolls, offered a valuable opportunity to assess unrestricted within-system connectivity fostered by grey reef shark movements and their potential role in nutrient transport [7,23]. This study offers a useful comparison for assessments of predator-initiated connections within exploited reefs, to predict the effects of exploitation on undisturbed reefs [50]. Further, it extends our understanding of grey reef shark movements across various reef systems, which is crucial for developing effective conservation approaches and species vulnerability assessments. Finally, it provides the first quantitative estimate, to our knowledge, of population-level nutrient transport in marine predators with implications for the long-term resilience of coral reef ecosystems.
Supplementary Material
Acknowledgements
Thanks are expressed to Kevin Weng for major contributions to the telemetry array at Palmyra, and Chris Lowe and Alan Friedlander for shark-tagging assistance. We thank the staff of The Nature Conservancy and the Palmyra Atoll National Wildlife Refuge (US Fish and Wildlife Service, Department of the Interior) for access to Palmyra Atoll. In addition, we thank the staff of the National Oceanic and Atmospheric Administration (NOAA) who produced the benthic map of Palmyra Atoll. Thanks are expressed also to Marcus Rowcliffe for his valuable feedback on earlier drafts of this manuscript, to James Nelson for his assistance with the nutrient flux calculations and to Douglas McCauley for sharing raw stable isotope data from [7]. Furthermore, thanks are expressed to Gareth Williams and Jamie Gove for sharing nutrient sample data from Palmyra Atoll and to the Ecosystem Sciences Division at NOAA's Pacific Islands Fisheries Science Centre for their efforts towards the collection of these samples. Finally, we thank two anonymous reviewers for their helpful feedback on an earlier version of the work. This is publication number PARC-0143 from the Palmyra Atoll Research Consortium (PARC).
Ethics
This project has been certified by the Institutional Animal Care and Use Committee (IACUC), University of California, Santa Barbara, Protocol no. 856. Sharks were captured at Palmyra Atoll, which has been a US National Wildlife Refuge since 2001 and part of the Pacific Remote Islands Marine National Monument since 2009, under US Fish and Wildlife Service special use permits (permit nos 12533-14011, 12533-13011, 12533-12011, 12533-11007, 12533-10011, 12533-09010, 12533-08011 and 12533-07006).
Data accessibility
Raw acoustic telemetry data can be found at https://github.com/JJWilliams24/Palmyra_Atoll.
Authors' contributions
J.J.W., Y.P.P. and D.M.P.J. conceived the project. J.J.W. and D.M.P.J. analysed the telemetry data and wrote the manuscript, with D.B. conducting the nitrogen flux analyses. Y.P.P., J.E.C. and D.B. collected the data. All the authors contributed to the revision of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by a Bertarelli Programme for Marine Science research grant to D.M.P.J. J.E.C., D.B. and Y.P.P. were supported by the Marisla Foundation.
References
- 1.Patterson TA, Thomas L, Wilcox C, Ovaskainen O, Matthiopoulos J. 2008. State-space models of individual animal movement. Trends Ecol. Evol. 23, 87–94. ( 10.1016/j.tree.2007.10.009) [DOI] [PubMed] [Google Scholar]
- 2.Lédée EJI, Heupel MR, Tobin AJ, Knip DM, Simpfendorfer CA. 2015. A comparison between traditional kernel-based methods and network analysis: an example from two nearshore shark species. Anim. Behav. 103, 17–28. ( 10.1016/j.anbehav.2015.01.039) [DOI] [Google Scholar]
- 3.Jacoby DMP, Freeman R. 2016. Emerging network-based tools in movement ecology. Trends Ecol. Evol. 31, 301–314. ( 10.1016/j.tree.2016.01.011) [DOI] [PubMed] [Google Scholar]
- 4.Heupel MR, Simpfendorfer CA, Espinoza M, Smoothey AF, Tobin A, Peddemors V. 2015. Conservation challenges of sharks with continental scale migrations. Front. Mar. Sci. 2, 1–7. ( 10.3389/fmars.2015.00012) [DOI] [Google Scholar]
- 5.Fortuna MA, Gomez-Rodriguez C, Bascompte J. 2006. Spatial network structure and amphibian persistence in stochastic environments. Proc. R. Soc. B 273, 1429–1434. ( 10.1098/rspb.2005.3448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinoza M, Lédée EJI, Simpfendorfer CA, Tobin AJ, Heupel MR. 2015. Contrasting movements and connectivity of reef-associated sharks using acoustic telemetry: implications for management. Ecol. Appl. 25, 2101–2118. ( 10.1890/14-2293.1) [DOI] [PubMed] [Google Scholar]
- 7.McCauley DJ, Young HS, Dunbar RB, Estes JA, Semmens BX, Micheli F. 2012. Assessing the effects of large mobile predators on ecosystem connectivity. Ecol. Appl. 22, 1711–1717. ( 10.1890/11-1653.1) [DOI] [PubMed] [Google Scholar]
- 8.Espinoza M, Heupel MR, Tobin AJ, Simpfendorfer CA. 2015. Residency patterns and movements of grey reef sharks (Carcharhinus amblyrhynchos) in semi-isolated coral reef habitats. Mar. Biol. 162, 343–358. ( 10.1007/s00227-014-2572-x) [DOI] [Google Scholar]
- 9.Papastamatiou YP, Meyer CG, Kosaki RK, Wallsgrove NJ, Popp BN. 2015. Movements and foraging of predators associated with mesophotic coral reefs and their potential for linking ecological habitats. Mar. Ecol. Prog. Ser. 521, 155–170. ( 10.3354/meps11110) [DOI] [Google Scholar]
- 10.Schmitz OJ, Hawlena D, Trussell GC. 2010. Predator control of ecosystem nutrient dynamics. Ecol. Lett. 13, 1199–1209. ( 10.1111/j.1461-0248.2010.01511.x) [DOI] [PubMed] [Google Scholar]
- 11.Saunders M, Brown C, Foley MM, Febria CM, Albright R, Mehling MG, Kavanaugh MT, Burfeind DD. 2015. Human impacts on connectivity in marine and freshwater ecosystems assessed using graph theory: a review. Mar. Freshw. Res. 67, 277–290. ( 10.1071/MF14358) [DOI] [Google Scholar]
- 12.Canuel EA, Spivak AC, Waterson EJ, Duffy JE. 2007. Biodiversity and food web structure influence short-term accumulation of sediment organic matter in an experimental seagrass system. Limnol. Oceanogr. 52, 590–602. ( 10.4319/lo.2007.52.2.0590) [DOI] [Google Scholar]
- 13.Jardine TD, Pusey BJ, Hamilton SK, Pettit NE, Davies PM, Douglas MM, Sinnamon V, Halliday IA, Bunn SE. 2012. Fish mediate high food web connectivity in the lower reaches of a tropical floodplain river. Oecologia 168, 829–838. ( 10.1007/s00442-011-2148-0) [DOI] [PubMed] [Google Scholar]
- 14.Hübner L, Pennings SC, Zimmer M. 2015. Sex- and habitat-specific movement of an omnivorous semi-terrestrial crab controls habitat connectivity and subsidies: a multi-parameter approach. Oecologia 178, 999–1015. ( 10.1007/s00442-015-3271-0) [DOI] [PubMed] [Google Scholar]
- 15.Hilderbrand GV, Hanley TA, Robbins CT, Schwartz CC. 1999. Role of brown bears (Ursus arctos) in the flow of marine nitrogen into a terrestrial ecosystem. Oecologia 121, 546–550. ( 10.1007/s004420050961) [DOI] [PubMed] [Google Scholar]
- 16.Roman J, McCarthy JJ. 2010. The whale pump: Marine mammals enhance primary productivity in a coastal basin. PLoS ONE 5, e13255 ( 10.1371/journal.pone.0013255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roman J, et al. 2014. Whales as marine ecosystem engineers. Front. Ecol. Environ. 12, 377–385. ( 10.1890/130220) [DOI] [Google Scholar]
- 18.Doughty CE, Wolf A, Malhi Y. 2013. The legacy of the Pleistocene megafauna extinctions on nutrient availability in Amazonia. Nat. Geosci. 6, 761–764. ( 10.1038/ngeo1895) [DOI] [Google Scholar]
- 19.Momigliano P, Harcourt R, Robbins WD, Stow A. 2015. Connectivity in grey reef sharks (Carcharhinus amblyrhynchos) determined using empirical and simulated genetic data. Sci. Rep. 5, 13229 ( 10.1038/srep13229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Croll D, Maron J, Estes JA, Danner E, Byrd G. 2005. Introduced predators transform subarctic islands from grassland to tundra. Science. 307, 1959–1961. ( 10.1126/science.1108485) [DOI] [PubMed] [Google Scholar]
- 21.Papastamatiou YP, Friedlander AM, Caselle JE, Lowe CG. 2010. Long-term movement patterns and trophic ecology of blacktip reef sharks (Carcharhinus melanopterus) at Palmyra Atoll. J. Exp. Mar. Bio. Ecol. 386, 94–102. ( 10.1016/j.jembe.2010.02.009) [DOI] [Google Scholar]
- 22.Davis K, Carlson PM, Bradley D, Warner RR, Caselle JE. 2017. Predation risk influences feeding rates but competition structures space use for a common Pacific parrotfish. Oecologia 184, 139–149. ( 10.1007/s00442-017-3857-9) [DOI] [PubMed] [Google Scholar]
- 23.Papastamatiou YP, Lowe CG, Caselle JE, Friedlander AM. 2009. Scale-dependent effects of habitat on movements and path structure of reef sharks at a predator-dominated atoll. Ecology 90, 996–1008. ( 10.1890/08-0491.1) [DOI] [PubMed] [Google Scholar]
- 24.Bradley D, Conklin E, Papastamatiou YP, McCauley DJ, Pollock K, Pollock A, Kendall BE, Gaines SD, Caselle JE. 2017. Resetting predator baselines in coral reef ecosystems. Sci. Rep. 7, 43131 ( 10.1038/srep43131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Field IC, Meekan MG, Speed CW, White W, Bradshaw CJA. 2011. Quantifying movement patterns for shark conservation at remote coral atolls in the Indian Ocean. Coral Reefs 30, 61–71. ( 10.1007/s00338-010-0699-x) [DOI] [Google Scholar]
- 26.Lea JSE, Humphries NE, von Brandis RG, Clarke CR, Sims DW. 2016. Acoustic telemetry and network analysis reveal the space use of multiple reef predators and enhance marine protected area design. Proc. R. Soc. B 283, 20160717 ( 10.1098/rspb.2016.0717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papastamatiou YP, Bodey TW, Friedlander AM, Lowe CG, Bradley D, Weng K, Priestly V, Caselle JE. In press Spatial separation without territoriality in shark communities. Oikos. ( 10.1111/oik.04289) [DOI] [Google Scholar]
- 28.Vanni MJ. 2002. Nutrient cycling by animals in freshwater ecosystems. Annu. Rev. Ecol. Syst. 33, 341–370. ( 10.1146/annurev.ecolsys.33.010802.150519) [DOI] [Google Scholar]
- 29.McCauley DJ, DeSalles PA, Young HS, Gardner JPA, Micheli F. 2016. Use of high-resolution acoustic cameras to study reef shark behavioral ecology. J. Exp. Mar. Bio. Ecol. 482, 128–133. ( 10.1016/j.jembe.2016.04.012) [DOI] [Google Scholar]
- 30.Jacoby DMP, Brooks EJ, Croft DP, Sims DW. 2012. Developing a deeper understanding of animal movements and spatial dynamics through novel application of network analyses. Methods Ecol. Evol. 3, 574–583. ( 10.1111/j.2041-210X.2012.00187.x) [DOI] [Google Scholar]
- 31.Wetherbee BM, Gruber SH. 1993. Absorption efficiency of the lemon shark Negaprion brevirostris at varying rates of energy intake. Copeia 1993, 416–425. ( 10.2307/1447140) [DOI] [Google Scholar]
- 32.Wetherbee BM, Crow GL, Lowe CG. 1997. Distribution, reproduction and diet of the gray reef shark Carcharhinus amblyrhynchos in Hawaii. Mar. Ecol. Prog. Ser. 151, 181–189. ( 10.3354/meps151181) [DOI] [Google Scholar]
- 33.Wetherbee B, Cortés E, Bizzarro J. 2012. Food consumption and feeding habits. In Biology of sharks and their relatives (eds Carrier JC, Musick JA, Heithaus MR), pp. 239–264. Boca Raton, FL: CRC Press. [Google Scholar]
- 34.Nelson JA, Stallings CD, Landing WM, Chanton J. 2013. Biomass transfer subsidizes nitrogen to offshore food webs. Ecosystems 16, 1130–1138. ( 10.1007/s10021-013-9672-1) [DOI] [Google Scholar]
- 35.Bradley D, Conklin E, Papastamatiou YP, McCauley DJ, Pollock K, Kendall BE, Gaines SD, Caselle JE. 2017. Growth and life history variability of the grey reef shark (Carcharhinus amblyrhynchos) across its range. PLoS ONE 12, e0172370 ( 10.1371/journal.pone.0172370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 37.QGIS Development Team. 2016. QGIS geographic information system. Open Source Geospatial Foundation Project. See http://www.qgis.org/ (accessed April 2016).
- 38.NOAA National Ocean Service, National Centers for Coastal Ocean Science. 2016. Project details. Benthic Habitat Mapping of Palmyra Atoll. See https://coastalscience.noaa.gov/projects/detail?key=70 (accessed 1 May 2016).
- 39.Heupel MR, Simpfendorfer CA. 2014. Importance of environmental and biological drivers in the presence and space use of a reef-associated shark. Mar. Ecol. Prog. Ser. 496, 47–57. ( 10.3354/meps10529) [DOI] [Google Scholar]
- 40.Vianna GMS, Meekan MG, Meeuwig JJ, Speed CW. 2013. Environmental influences on patterns of vertical movement and site fidelity of grey reef sharks (Carcharhinus amblyrhynchos) at aggregation sites. PLoS ONE 8, e60331 ( 10.1371/journal.pone.0060331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hearn CJ, Atkinson MJ, Falter JL. 2001. A physical derivation of nutrient-uptake rates in coral reefs: effects of roughness and waves. Coral Reefs 20, 347–356. ( 10.1007/s00338-001-0185-6) [DOI] [Google Scholar]
- 42.Meyer JL, Schultz ET, Helfman GS. 1983. Fish schools: an asset to corals. Science 220, 1047–1049. ( 10.1126/science.220.4601.1047) [DOI] [PubMed] [Google Scholar]
- 43.Allgeier JE, Yeager LA, Layman CA. 2013. Consumers regulate nutrient limitation regimes and primary production in seagrass ecosystems. Ecology 94, 521–529. ( 10.1890/12-1122.1) [DOI] [PubMed] [Google Scholar]
- 44.Allgeier JE, Valdivia A, Cox C, Layman CA. 2016. Fishing down nutrients on coral reefs. Nat. Commun. 7, 12461 ( 10.1038/ncomms12461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allgeier JE, Burkepile DE, Layman CA. 2017. Animal pee in the sea: consumer-mediated nutrient dynamics in the world's changing oceans. Glob. Chang. Biol. 23, 2166–2178. ( 10.1111/gcb.13625) [DOI] [PubMed] [Google Scholar]
- 46.Sandin SA, et al. 2008. Baselines and degradation of coral reefs in the Northern Line Islands. PLoS ONE 3, e1548 ( 10.1371/journal.pone.0001548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gove JM, et al. 2016. Near-island biological hotspots in barren ocean basins. Nat. Commun. 7, 1–8. ( 10.1038/ncomms10581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papastamatiou YP, Meyer CG, Carvalho F, Dale JJ, Hutchinson MR, Holland KN. 2013. Telemetry and random-walk models reveal complex patterns of partial migration in a large marine predator. Ecology 94, 2595–2606. ( 10.1890/12-2014.1) [DOI] [PubMed] [Google Scholar]
- 49.Heithaus MR, Frid A, Wirsing AJ, Worm B. 2008. Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 23, 202–210. ( 10.1016/j.tree.2008.01.003) [DOI] [PubMed] [Google Scholar]
- 50.Barnett A, Abrantes K, Seymour J, Fitzpatrick R. 2012. Residency and spatial use by reef sharks of an isolated seamount and its implications for conservation. PLoS ONE 7, e36574 ( 10.1371/journal.pone.0036574) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw acoustic telemetry data can be found at https://github.com/JJWilliams24/Palmyra_Atoll.