Abstract
Oceans of the future are predicted to be more acidic and noisier, particularly along the productive coastal fringe. This study examined the independent and combined effects of short-term exposure to elevated CO2 and boat noise on the predator–prey interactions of a pair of common coral reef fishes (Pomacentrus wardi and its predator, Pseudochromis fuscus). Successful capture of prey by predators was the same regardless of whether the pairs had been exposed to ambient control conditions, the addition of either playback of boat noise, elevated CO2 (925 µatm) or both stressors simultaneously. The kinematics of the interaction were the same for all stressor combinations and differed from the controls. The effects of CO2 or boat noise were the same, suggesting that their effects were substitutive in this situation. Prey reduced their perception of threat under both stressors individually and when combined, and this coincided with reduced predator attack distances and attack speeds. The lack of an additive or multiplicative effect when both stressors co-occurred was notable given the different mechanisms involved in sensory disruptions and highlights the importance of determining the combined effects of key drivers to aid in predicting community dynamics under future environmental scenarios.
Keywords: anthropogenic noise, ocean acidification, escape response, predator–prey, coral reef fish
1. Introduction
Future oceans are predicted to be more acidic and noisier [1–3]. Dissolved CO2 levels in the ocean are rising in line with atmospheric CO2 [4], and CO2 levels are projected to exceed 900 ppm from the current 390 ppm by 2100 [5]. Rising atmospheric CO2 over the last century has already led to a reduction in ocean pH (ocean acidification) [5]. Research has shown that elevated CO2 can affect the acid–base balance of marine organisms [6,7] and alter the processing of sensory information for organisms as diverse as crabs, molluscs and fishes [8–10]. This has marked effects on the behaviour of both fishes and invertebrates, which can affect survival [11–13]. Moreover, recent evidence suggests that some fishes may not be able to acclimate to the behavioural effects of elevated CO2 over multiple generations [14,15].
High levels of CO2 also alter the fundamental properties of seawater so that by the end of the century there will be reduced absorption of sound energy and thus increased propagation of acoustic energy over greater distances [16]. Absorption of low-frequency, biologically important sound is predicted to be reduced by 60% in high-latitude areas by 2100. When this physical amplification of sound through the alteration of the transmission medium is combined with the increase in anthropogenic sound predicted through the increased usage of coastal waterways [1], noise becomes one of the most rapidly increasing and largely unmanaged forms of pollution that will impact the oceans of tomorrow. Marine organisms of the near future will live in coastal areas that are both noisier and more acidic, but the effect of these two factors on community dynamics and the extent to which they interact are unknown.
Marine organisms hear and produce sound at frequencies that directly overlap with those emitted by the operation of a variety of motorboats, ships, seismic surveys and pile-driving operations [1,17]. Anthropogenic noise can compete with naturally produced sound, leading to masking of vocal communication (i.e. failure to recognize the occurrence of one type of sound as a result of the interfering presence of noise [18]) and inappropriate decisions (e.g. when faced with a simulated predator [19]). Fishes and invertebrates produce sounds during reproductive behaviour, territorial defence and predator avoidance [20,21]. Fishes also use this biological sound for orientation [21,22], and to inform important decisions, such as where to settle at the end of the larval phase [23–25]. Masking, distraction and the avoidance of some sounds have all been shown in laboratory studies and in some cases are predicted to occur under natural conditions, based on auditory capabilities and noise levels (e.g. [26,27]). Boat noise has recently been shown to affect predator–prey dynamics [19,28], but the mechanisms underlying these effects are poorly understood.
Recent research suggests that the physiology and behaviour of fishes is sensitive to elevated CO2 and anthropogenic noise when examined in isolation. For instance, both noise and elevated CO2 can affect foraging behaviour, space use, activity levels, mating success, metabolism and even offspring survival [19,29–31]. While there can be some consistency in the nature of the reaction to isolated stressors, the type of response to coincident multiple stressors is seldom clear. For example, if multiple stressors affect similar mechanistic pathways, an additive effect may be found [32]. By contrast, antagonistic or synergistic effects may occur if the stressors affect different pathways, depending on the magnitude and direction of the response to each stressor [33].
Coral reefs are an excellent study system in which to explore the interactive effects of elevated CO2 and anthropogenic noise. Coral reef fishes have evolved in oceans with relatively stable chemistry [34] and they appear to live close to their upper tolerance limits because small changes in CO2 can lead to major behavioural affects [12]. Moreover, tropical regions have some of the fastest-growing human populations and these communities rely on the coastal areas to supply much of their protein. This pressure and the increasing globalization of economies have meant that the exposure of inshore tropical coastal areas to noise from shipping and boats has dramatically increased in recent decades [35,36].
The aim of this study was to explore the interactive effects of elevated CO2 and boat noise on the dynamics (kinematics) that underlie predator–prey interactions in fishes. A common fish predator and juvenile fish prey were exposed to control (385 µatm) or elevated CO2 (925 µatm) and playback of ambient reef noise or recordings that included motorboats, and placed together to examine in detail how the two factors affected the fishes' interaction. Newly settled fish were chosen as prey because the life-history shift between pelagic larvae and settled juvenile represents an important bottleneck where mortality is intense and selective [37,38]. This means that anything that changes the interaction between predators and their prey can have marked consequences for the distribution of traits that enter the juvenile population. Our prediction based on previous research was that predators may be less affected by both elevated CO2 [39,40] and noise from small motorboats [19] than the damselfish prey, and this will have implications for prey mortality trajectories.
2. Material and methods
(a). Study species
Juveniles of the damselfish, Pomacentrus wardi (Pomacentridae), were used as the prey species, while the dottyback, Pseudochromis fuscus (Pseudochromidae), was used as the predator. Pseudochromis fuscus is common, widely distributed throughout the Indo-Pacific and is an important predator of newly settled coral reef fishes [41].
Settlement-stage larvae of P. wardi (13.6 ± 1.3 mm mean standard length (SL) ± s.d.) were collected overnight using light traps moored in open water around Lizard Island (14′40° S, 145′28° E), in the northern Great Barrier Reef, Australia. Fish were sorted to species and transferred to 35 l aquaria supplied with a continuous flow of either control (present-day CO2; 385 µatm) or elevated-CO2 seawater (925 µatm; see electronic supplementary material, file) for 6 to 8 days, with the inflow pipe underwater to reduce noise. Four or more days in elevated CO2 water has been found to be sufficient to elicit the full extent of behavioural effects of high CO2 [29,42]. Pomacentrus wardi were fed 3 times daily to satiation with newly hatched Artemia sp.
Adult Ps. fuscus were collected with a hand net and a dilute solution of clove oil and ethanol from around the shallow fringing reef off Lizard Island. Immediately after collection, fish were transported back to the Lizard Island Research Station where they were housed separately in mesh baskets within 30 l aquaria to avoid aggressive interactions. Fish were randomly assigned to either of the two CO2 treatments for 6–8 days and were fed 2 juvenile reef fish morning and night, and then not fed for the last 24 h prior to the interaction trial to standardize for satiation.
(b). Sound recordings and playback
Ambient reef sounds were recorded mid-water from the shallow (6 m) back reef of Lizard Island, 20 m from the nearest reef edge. The noise from four 5 m long dinghies with 30 hp 2-stroke Suzuki outboard motors (DT30) was also recorded over the same area as the ambient reef sound, with the boats travelling 20–100 m from the recording devices. Acoustic pressure was measured using a calibrated omnidirectional hydrophone (HiTech HTI-96-MIN with inbuilt preamplifier, with a manufacturer-calibrated sensitivity of 164.3 dB re 1 V µPa−1; frequency range 0.02–30 kHz; calibrated by the manufacturer; High Tech Inc., Gulfport, MS) and a digital recorder (PCM-M10, 48 kHz sampling rate; Sony Corporation, Tokyo, Japan). Particle acceleration was measured using a calibrated triaxial accelerometer (M20 L; sensitivity following a curve over the frequency range 0–3 kHz; calibrated by the manufacturer; Geospectrum Technologies, Dartmouth, Canada) and a digital 4-track recorder (Boss BR-800, 44.1 kHz sampling rate; Roland Corporation, Los Angeles, CA). Recording levels used with each set-up were calibrated using pure sine wave signals from a function generator with a measured voltage recorded in line on an oscilloscope.
Ambient reef sound and boat noise tracks were looped (using Audacity v. 2.0.2, http://audacity.sourceforge.net) to form 20 min tracks and these were played in random order through underwater speakers as treatment sound sources. The sound systems used for playback of ambient and boat noise recordings consisted of a battery (12v 7.2 Ah sealed lead-acid), WAV/MP3 player (GoGEAR Vibe, frequency response 0.04–20 kHz; Philips, The Netherlands), amplifier (M033N, 18 W, frequency response 0.04–20 kHz; Kemo Electronic GmbH, Germany) and speaker (University Sound UW-30; maximal output 156 dB re 1 mPa at 1 m, frequency response 0.1–10 kHz; Lubell Labs, Columbus, OH). These playback recordings were played within 1000 l plastic cattle troughs (Reln; water depth 70 cm) 650 mm from the polypropylene interaction arena (electronic supplementary material, figure S1).
The same hydrophone that recorded the initial ambient reef and boat noise tracks in the field was used to record the sound in the middle of the interaction arena of the ambient reef and boat noise played through the speakers within the 1000 l tank. The frequency of the ambient reef sound, boat noise tracks and their playback recordings were then compared to determine the extent to which playback represented boat noise within a shallow reef (figure 1).
Figure 1.
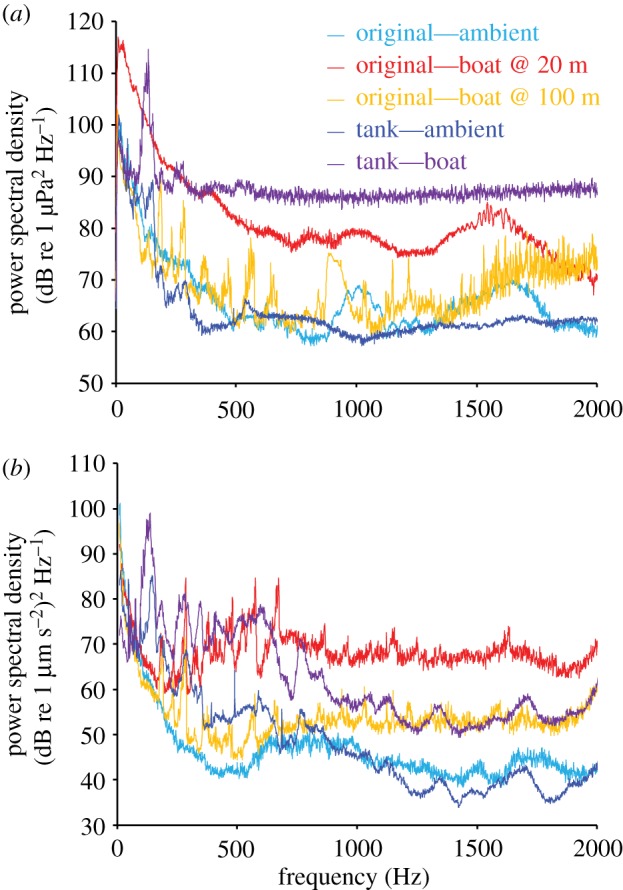
Analysis of acoustic conditions. Spectral content of field recordings of ambient and motorboat noise (at 20 and 100 m), and measurements of playback within a 1000 l mesocosm, measured in both (a) acoustic pressure and (b) particle acceleration. Mean power spectral density of all conditions are shown; recordings averaged over 1 min. The hydrophone and accelerometer were placed in the middle of the interaction arena for recordings of playback characteristics. Sounds were analysed using the PaPAM acoustics analysis package (see [43]) using Matlab v. 2014a, with fft length = sampling frequency (48 kHz), Hamming evaluation window and 50% window overlap.
(c). Interaction trials
Predator–prey interactions were measured using the standard protocol established by Allan et al. [40,44]. Briefly, this involved placing a predator and prey fish into an experimental white polypropylene tank (Nally IH051, 645 × 413 × 276 mm, 10 cm water height; 2.5 mm wall thickness), with a predator being placed in the main elliptical arena (1452 cm2) and prey in a 50 mm diameter grey pipe. The whole arena was covered with a white plastic (Corflute) board to remove any visual disturbances. Fish were given a 15 min acclimation period prior to the commencement of the trial. The start of the trial involved carefully lifting up the pipe enclosing the prey by means of a nylon string, which secured the pipe to the ceiling of the tank. The interaction between predator and prey was then filmed at high speed (480 fps) for 10 min or until the prey had been consumed (Casio EX-ZR1000). To standardize the position of the predator, trials only commenced when the predator was at least 10 cm away from the prey's acclimation pipe. Water within the arena was the same temperature as the holding tanks and because of the difficulties in producing enough CO2-enriched seawater, control CO2 seawater was used in the test arena. Previous research has shown that fishes exposed to elevated CO2 water maintain their altered behavioural effects for least 48 h once transferred to seawater containing present-day CO2 levels [29,42]. Moreover, the fast start of juvenile damselfish has been shown to be affected by conditioning with elevated CO2, regardless of whether they were tested in water that had control or elevated levels of CO2 [42].
Kinematic variables were measured based on the centre of mass (COM) of the fish when stretched straight (based on [45]). COM was assumed to be at 35% of the body length from the tip of the snout as it is the case for generalist fish [46]. Stage 1 and 2 where defined is based upon directional changes of the anterior part of the body of the fish, based on Domenici & Blake [46]. Predator attacks were measured only when a predator showed a fast-directed burst towards the prey (greater than 3 body lengths s−1). All variables, with the exception of number of prey caught, were measured using only the first attack that occurred within a trial. This was done to control for any anaerobic stress either the predator or prey may have experienced due to prolonged attacks. Both predators and prey were used once to avoid habituation to the experimental procedure. Prey suffering was minimal as prey were consumed immediately following a successful strike.
The following performance variables were measured:
Prey
(1) Prey reaction distance (m): the distance between the prey COM and the tip of the predator's snout at the onset of the escape response to a predator attack.
(2) Apparent looming threshold (ALT) for prey avoidance responses to a predatory strike, a measure of the magnitude of the prey's response to the perceived threat of predation. The higher the perceived threat, the higher is the ALT (in radians s−1) measured at the onset of the escape response and measured as the rate of change of the angle (α) subtended by the predator's frontal profile as seen by the prey. Previous work has shown that fish tend to react to an approaching stimulus (a predator) when a given threshold of dα/dt (i.e. ALT) is reached. ALT is calculated as (4US)/(4D2 + S2), based on Dill [47] and Webb [48], where U = predator speed, calculated as the speed of the predator in the frame prior to the prey's response; S = maximum frontal profile calculated as (max. depth + max. width)/2, where max. depth was estimated to be positioned at one-quarter of the body length of the predator (personal observation) and max. width at 0.25 l of the predator; and D is the distance from the prey's eye to the point of the predator's maximum profile calculated as RD + 0.25Lpred, where RD is the prey's reaction distance.
(3) Prey escape distance (m): the straight-line distance between the prey COM at the onset of the escape response and at the end of the escape response (i.e. when the prey came to a halt).
(4) Mean prey escape speed (m s−1): was measured as the distance covered within a fixed time (24 ms). This fixed duration was based on the average duration (22.8 ms) of the first two flips of the tail (the first two axial bends, i.e. stages 1 and 2 defined based on Domenici & Blake [46], which is the period considered crucial for avoiding ambush predator attacks [45]).
Predator
(5) Capture success: percentage of trials in which the predator ingested the prey within the 10 min filming period, out of the total number of trials for each treatment.
(6) Predation rate: capture success divided by the number of attacks per unit time.
(7) Attack rate: number of attacks per unit time, measured for each interaction.
(8) Predator attack distance (m): the straight-line distance between the predator COM at the time the attack commenced and the end of the attack (end is defined as when the predator came to a halt).
(9) Maximum predator attack speed (m s−1): the top speed achieved at any point in time during the attack, based on the predator COM.
(d). Statistical analyses
To determine whether predator attack rate was affected by CO2 pretreatment or boat noise a two-factor ANOVA was undertaken. Data were rank-transformed to meet the assumption of normality. To test whether capture rate was independent of sound or CO2 exposure, capture success was compared among treatment combinations using 4 × 2 contingency table analysis. To test whether the kinematics of the predator–prey interaction were affected by preconditioning with elevated CO2, boat noise or their interaction, a multivariate analysis of variance (MANOVA) was undertaken. MANOVA has the advantage over univariate tests because it tests for the equality of all independent variables among treatments while taking into account the interactions between variables. Canonical discriminant analysis (CDA) was then used to summarize, identify and display the nature of the significant differences found by MANOVA. CDA identifies a number of trends in the dataset (canonical variates) that maximally discriminate among the treatment group centroids. Trends in the original variables (prey reaction distance, prey escape distance, prey escape speed, ALT, predator attack distance and predator attack speed) were represented as vectors given by correlations of these variables with the canonical variates. These vectors were plotted on the first two canonical axes, together with centroids of the CO2 by sound treatment combinations. The strength or importance of each of the original variables in discriminating among groups was displayed graphically as the length of these vectors. Paired tests of Mahalanobis distances enabled the determination of whether the position of the centroids differed from one another. To further aid interpretation of the trends in kinematics, two-factor ANOVAs were conducted on the individual dependent variables. Prior to all analysis the assumptions of normality and homogeneity were examined using residual analysis. Prey reaction distance and predator attack distance required square-root transformation, while prey escape distance, prey escape speed, ALT and predator attack speed required log10 transformation. Statistics were undertaken using Statistica (v. 13.2).
3. Results
The pressure and particle acceleration conditions during the ambient noise treatment were matched and compared with the original field recordings (figure 1a,b). Acoustic pressure with motorboat noise at frequencies greater than 500 Hz was slightly greater in the experimental tank compared with that measured in field conditions (figure 1a). By contrast, particle acceleration was lower in the tank at frequencies greater than 500 Hz during playback of motorboat noise compared with field recordings (figure 1b). There was a clear difference between boat and ambient conditions in the field recordings and also during playback of these recordings in tanks. While playback using underwater speakers combined with tank-based acoustics can cause deviations from the original field acoustic conditions, levels of pressure and particle acceleration in the experimental arena were balanced as well as possible to match field conditions, and were both higher during playback of motorboat noise than during playback of ambient noise, providing a treatment with additional noise and a control.
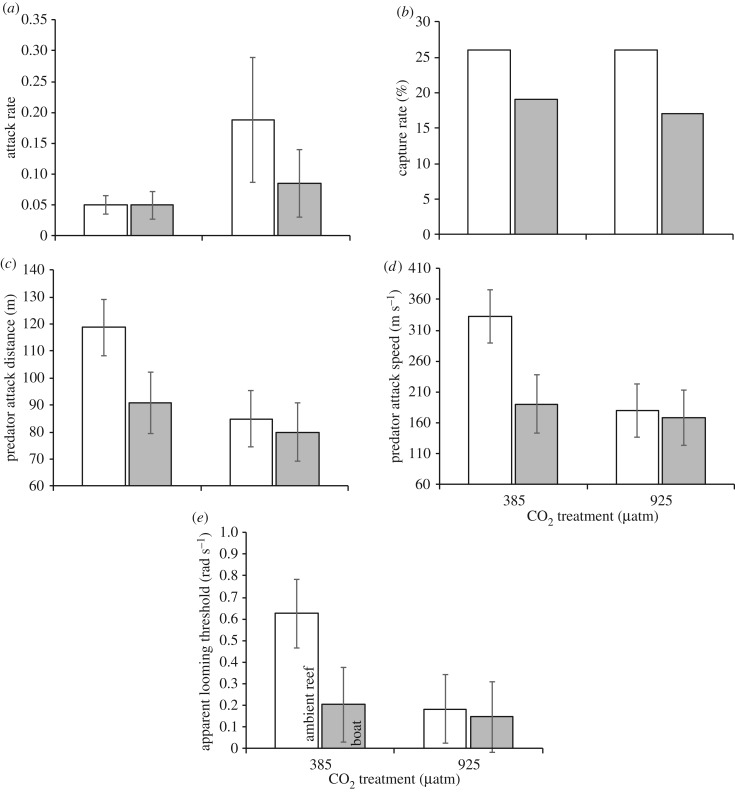
The attack rate of dottybacks was not affected by CO2 pretreatment, sound treatment or their interaction (p > 0.2; figure 2a). The capture rate also did not differ among treatment combinations (χ2 = 0.80, p = 0.85; figure 2b).
Figure 2.
Comparison of capture success and kinematics (means ± s.e.) of the dottyback predator (Pseudochromis fuscus) in its interaction with a juvenile damselfish prey (Pomacentrus wardi). (a) Attack rate (attacks per 10 min), (b) capture rate (% per 10 min), (c) predator attack distance, (d) predator attack speed and (e) apparent looming threshold. (a–e) Left to right: n = 19, 16, 19, 18.
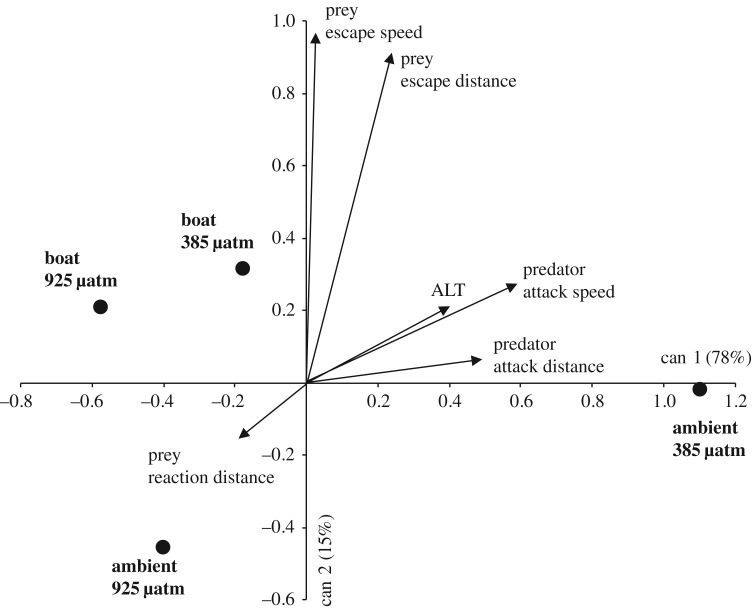
There was a significant effect of CO2 pretreatment on the overall kinematics of the predator–prey interaction (MANOVA, Pillai's trace = 0.21, F6,63 = 2.83, p = 0.017), but no effect of boat noise (p = 0.059) or their interaction (p = 0.30). A CDA showed that treatment combinations were principally discriminated by the efficiency of predators (i.e. predator attack distance and speed), and the ability of the prey to detect the threat (i.e. ALT). The first canonical variate accounted for 78% of the discrimination among treatments and separated the 385 µatm CO2-ambient sound treatment from the other three treatment combinations (figure 3). Tests between the treatment centroids on the Mahalanobis distances found that the 385 µatm CO2-ambient sound treatment differed from the other three treatments (p < 0.03), which did not differ from one another (p > 0.47). Interpreting the CDA in relation to the strength and direction of trends in the original variables suggested that fish in the 385 µatm CO2-ambient sound treatment had higher predator attack distances, attack speeds, ALT, but lower prey reaction distances than the other three treatment combinations. Separate two-factor ANOVAs reinforced this interpretation, with non-significant tests for all variables except predator attack distances, attack speeds and ALT that had significant effects of CO2 pretreatment (figure 2c–e; electronic supplementary material, table S2).
Figure 3.
Comparison of how the kinematics of the interaction between juvenile Ward's damselfish (Pomacentrus wardi; prey) and a predator dottyback (Pseudochromis fuscus) differ when preconditioned with two CO2 levels (385, or 925 µatm) and exposed to one of two noise regimes (ambient reef sound, or boat noise). The plot is the output of a canonical discriminant analysis showing the position of the group centroids (i.e. treatments) with respect to the kinematic variables that characterized the predator–prey interaction.
4. Discussion
Both elevated CO2 and boat noise in isolation has been previously shown to negatively affect the performance of juvenile fishes under the threat of a real or simulated predator [19,29–31]. However, this is the first study to examine the potential interactive effects of elevated CO2 and boat noise conditions on predation for any system. Our study indicates that elevated anthropogenic noise, as represented by the playback of boat noise, had a similar effect on the kinematics of the predator–prey response to elevated CO2 conditions when in isolation. There was no evidence of an interaction between stressors in the way they affected the predator–prey interaction studied. Our finding that all the stressor treatments grouped together with respect to their effects on interaction dynamics suggests that elevated CO2 and noise influence the predator–prey interaction in a similar way. This finding is important as one of the challenges to predicting future community dynamics is the unpredictability of synergistic effects that can manifest when stressors co-occur.
Previous experiments have found that elevated CO2 on its own is sufficient to alter the dynamics between predators and prey [40]. For our study, capture rates were statistically similar under ambient reef sound conditions when fish had been pre-exposed to current or elevated CO2. Despite a similarity in capture success, the kinematics underlying the interaction were fundamentally different. Apparent looming threshold, which is a measure of the magnitude of threat perceived by prey, was lower under elevated CO2, which meant predators were able to get closer to their prey before an escape was initiated. Moreover, predator success was the same across CO2 treatments despite their attack speed after the CO2 treatment being half the speed of the predators preconditioned with ambient CO2. This finding contrasts with the only two detailed studies of kinematics of predator–prey reactions under elevated CO2, both of which have used similar interaction arenas to the present study. Similar to the current study, Allan et al. [40] found that the treatment of both predator (Ps. fuscus) and prey (juvenile P. amboinensis) with elevated CO2 had no effect on predator success. However, unlike the current study, Allan et al. found that none of the kinematic variables recorded in the current study were affected by CO2 treatment, with the exception of prey escape distance, which was 40% shorter in CO2-treated prey. These differences between studies may be due to species-specific differences in prey tolerance to CO2 affecting the predator–prey dynamic, and marked differences in the tolerance of four damselfish species of the genus Pomacentrus to elevated CO2 have previously been demonstrated [49]. Interestingly, Allan et al. [44] used the same species as the current study in an examination of the interactive effects of elevated temperature and CO2. Elevated CO2 led to an increase in predator success but not attack rate, in contrast to the current study. However, the mechanism underlying the predator success was also similar, with most prey kinematic variables not being affected by elevated CO2, suggesting that P. wardi are relatively tolerant to the behavioural effects of high CO2.
Playback of noise from small motorboats also had a marked effect on the predator–prey interaction. Under ambient CO2 conditions, the addition of boat noise did not increase predator capture success, but boat noise did reduce both predator attack distance and speed. These predator effects may represent an alteration in the dottyback foraging strategy as a consequence of a reduction in the ability of the P. wardi to respond to the threat, as indicated by a reduction in ALT under boat noise. A previous series of laboratory and field experiments using dottyback and P. amboinensis found that dottybacks are less affected by boat noise than their damselfish prey [19]. Boat noise elevated the respiration of the juvenile damselfish but not the dottyback, suggesting that the prey were stressed by boat noise, while the predator was largely unaffected. This stress effect appeared to reduce the latency to respond to a looming stimulus in the presence of real boat noise, and led to a marked increase in capture success in both laboratory and field-based experiments. Evidence to date therefore suggests that, while the kinematics affected by boat noise under ambient CO2 were mostly predator-related variables, these may simply represent an active behavioural adjustment by the predator to the altered reaction of the damselfish prey to threat.
Capture rates by predators were the same between control conditions (i.e. ambient sound and CO2) and future multi-stressor conditions (i.e. higher noise and CO2). However, kinematic analysis suggests that while the capture rates were the same, the dynamics of the predator–prey interaction that led to successful capture were different. Despite the predators in the ambient treatment having the fastest attack speeds and longest attack distances, this did not improve their capture success, probably due to the extra-vigilant prey, as indicated by the high apparent looming threshold of the prey. In contrast, predators from treatments that elevated CO2 or noise levels underperformed, as did the prey with respect to their ability to perceive threat (i.e. ALT). Results of this and other studies that have looked at the combined effects of stressors on predator–prey dynamics emphasize that the outcome of the interaction depends strongly not only on the way each species is affected by the stressor, but also their motivation to respond (e.g. [44]).
The way boat noise affected predator success was altered by exposure to elevated CO2, suggesting an interaction between stressors on the predator–prey dynamic, though this was not statistically significant due to high variability among replicate trials and consequently low effect sizes in univariate tests. This trend for an interaction between stressors is clear in the multivariate and univariate analyses, with the effects of boat noise differing in the presence of elevated CO2. Interestingly, when boat noise and elevated CO2 occurred on the same individuals, the effects on capture success and kinematics were the same as the effects of boat noise and elevated CO2 independent of one another, suggesting a lack of additive or multiplicative effects when the stressors co-occurred. This is perhaps surprising given mechanisms suggested for the independent effects are quite different for each stressor. Research suggests that the effects of elevated CO2 on behaviour are manifest in fishes and some invertebrates through an imbalance in plasma chloride and bicarbonate ion concentrations as a result of acid–base regulation, causing the reversal of ionic fluxes through GABA(A) receptors, leading to altered neuronal function [7]. By contrast, boat noise has been suggested to operate through sensory disruption [50], sensory cell damage [51] or acoustic masking [18]. The combined stressors may have been sufficient to distract the predator and reduce their motivation to feed as suggested by a trend towards a reduced mean attack rate. This is the first study to suggest that there may be an interaction between elevated CO2 and anthropogenic noise on predator–prey dynamics, and these findings suggest that further study is warranted.
This study, like many others, used stable CO2 levels. However, in nature CO2 levels can vary, at times markedly over tidal or seasonal cycles [52,53]. Jarrold et al. [54] experimentally examined the effects of elevated CO2 on the behaviour of the spiny chromis, Acanthochromis polyacanthus, but allowed CO2 levels to vary diurnally by 300 or 500 µatm around the average levels. They found that diel fluctuations offset the negative impacts of high CO2 on behaviour, but these behavioural abnormalities were manifest once levels reached mean CO2 levels of above 900 µatm, similar to the levels used in the current study. Though the current research design represents a simplified version of future environmental conditions, our findings are a useful first step in determining how future CO2 and noise conditions may affect predator–prey interactions on reefs of the future.
There is a mounting acknowledgement from researchers, managers and policymakers that anthropogenic stressors, such as elevated CO2 and marine noise, are not reversible but are inevitable [55–57]. Management is now turning towards the prediction of a future in which degraded natural resources must sustain existing and new forms of exploitation. Our unique examination of the independent and interactive effects of future CO2 and noise on a key community process is an initial important step towards understanding how community dynamics will change under future environmental conditions. Undertaking experiments that explore the interaction of community stressors on a broader range of species and under a greater range of environmental conditions will be crucial for the realistic management of future marine resources.
Supplementary Material
Acknowledgements
We thank all the staff at the Lizard Island Research Station, and all the students and volunteers who helped with the light traps and sorting of fish.
Ethics
All work carried out herein was in accordance with the James Cook University Animal Ethics guidelines (JCU Animal Ethics approval A2080).
Data accessibility
Data are accessible from the Tropical Data Hub (http://dx.doi.org/10.4225/28/5a1cd71b4ef8a).
Authors' contributions
M.I.M. and B.J.M.A. designed the study and carried out the experiments. B.J.M.A. analysed the videos. M.I.M. performed the analysis. S.-A.W. operated the CO2 dosing system. S.D.S. undertook, analysed and plotted the sound measurements. M.I.M. wrote the first draft of the manuscript. M.I.M., B.J.M.A. and S.D.S. contributed to editing the manuscript.
Competing interests
No conflict of interest is noted.
Funding
Funding was provided by an Australian Research Council Centre of Excellence for Coral Reef Studies (EI140100117), an ARC discovery (M.I.M.) and a NERC Knowledge Exchange Fellowship (for S.D.S.; NE/J500616/2).
References
- 1.Slabbekoorn H, Bouton N, Van Opzeeland I, Coers A, Ten Cate C, Popper AN. 2010. A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 25, 419–427. ( 10.1016/j.tree.2010.04.005) [DOI] [PubMed] [Google Scholar]
- 2.Normandeau Associates, Inc. 2012. Effects of noise on fish, fisheries, and invertebrates in the US Atlantic and Arctic from energy industry sound-generating activities: a literature synthesis for the US Dept. of the Interior, Bureau of Ocean Energy Management. See http://www.data.boem.gov/homepg/data_center/other/espis/espismaster.asp?appid=1.
- 3.IPCC Climate Change 2015. 2014. synthesis report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Core Writing Team, Pachauri RK, Meyer LA). Geneva, Switzerland: IPCC. [Google Scholar]
- 4.Doney SC, Fabry VJ, Feely RA, Kleypas JA. 2009. Ocean acidification: the other CO2 problem. Ann. Rev. Mar. Sci. 1, 169–192. ( 10.1146/annurev.marine.010908.163834) [DOI] [PubMed] [Google Scholar]
- 5.Collins M, et al. 2013. Long-term climate change: projections, commitments and irreversibility. In Climate change 2013: the physical science basis: contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, pp. 1029–1136. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Pörtner HO, Langenbuch M, Reipschlager AJ. 2004. Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. Oceanogr. 60, 705–718. ( 10.1007/s10872-004-5763-0) [DOI] [Google Scholar]
- 7.Heuer RM, Welch MJ, Rummer JL, Munday PL, Grosell M. 2016. Altered brain ion gradients following compensation for elevated CO2 are linked to behavioural alterations in a coral reef fish. Sci. Rep. 6, 33216 ( 10.1038/srep33216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briffa M, de la Haye KL, Munday PL. 2012. High CO2 and marine animal behaviour: potential mechanisms and ecological consequences. Mar. Poll. Bull. 64, 1519–1528. ( 10.1016/j.marpolbul.2012.05.032) [DOI] [PubMed] [Google Scholar]
- 9.Munday PL, Cheal AJ, Dixson DL, Rummer JL, Fabricius KE. 2014. Behavioural impairment in reef fishes caused by ocean acidification at CO2 seeps. Nat. Clim. Change 4, 487–492. ( 10.1038/nclimate2195) [DOI] [Google Scholar]
- 10.Watson S-A, Lefevre S, McCormick MI, Domenici P, Nilsson GE, Munday PL. 2014. Marine mollusc predator-escape behaviour altered by near-future carbon dioxide levels. Proc. R. Soc. B 281, 20132377 ( 10.1098/rspb.2013.2377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clements JC, Hunt HL. 2015. Marine animal behaviour in a high CO2 ocean. Mar. Ecol. Prog. Ser. 536, 259–279. ( 10.3354/meps11426) [DOI] [Google Scholar]
- 12.Nagelkerken I, Munday PL. 2016. Animal behaviour shapes the ecological effects of ocean acidification and warming: moving from individual to community-level responses. Glob. Change Biol. 22, 974–989. ( 10.1111/gcb.13167) [DOI] [PubMed] [Google Scholar]
- 13.Watson S-A, Fields JB, Munday PL. 2017. Ocean acidification alters predator behaviour and reduces predation rate. Biol. Let. 13, 20160797 ( 10.1098/rsbl.2016.0797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allan BJM, Miller GM, McCormick MI, Domenici P, Munday PL. 2014. Parental effects improve escape performance of juvenile reef fish in a high CO2 world. Proc. R. Soc. B 281, 1777 ( 10.1098/rspb.2013.2179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welch MJ, Watson S, Welsh J, McCormick MI, Munday PL. 2014. Effects of elevated CO2 undiminished by transgenerational acclimation. Nat. Clim. Change 4, 1086–1089. ( 10.1038/nclimate2400) [DOI] [Google Scholar]
- 16.Ilyina T, Zeebe RE, Brewer PG. 2010. Future ocean increasingly transparent to low-frequency sound owing to carbon dioxide emissions. Nat. Geosci. 3, 18–22. ( 10.1038/ngeo719) [DOI] [Google Scholar]
- 17.Hildebrand JA. 2009. Anthropogenic and natural sources of ambient noise in the ocean. Mar. Ecol. Prog. Ser. 395, 5–20. ( 10.3354/meps08353) [DOI] [Google Scholar]
- 18.Pine MK, Jeffs AG, Wang D, Radford CA. 2016. The potential for vessel noise to mask biologically important sounds within ecologically significant embayments. Ocean Coast. Manage. 127, 63–73. ( 10.1016/j.ocecoaman.2016.04.007) [DOI] [Google Scholar]
- 19.Simpson SD, Radford AN, McCormick MI, Ferrari MCO, Chivers DP, Holles S, Meekan MG. 2016. Anthropogenic noise increases fish mortality by predation. Nat. Comm. 7, 10544 ( 10.1038/ncomms10544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myrberg AA, Fuiman LA. 2002. The sensory world of coral reef fishes. In Coral reef fishes: dynamics and diversity in a complex ecosystem (ed. Sale PF.), pp. 123–148. New York, NY: Academic Press. [Google Scholar]
- 21.Leis JM, Siebeck UE, Dixson D. 2011. How Nemo finds home: the neuroecology of dispersal and of population connectivity in larvae of marine fishes. Integr. Comp. Biol. 51, 826–843. ( 10.1093/icb/icr004) [DOI] [PubMed] [Google Scholar]
- 22.Simpson S, Meekan M, McCauley R, Jeffs A. 2004. Attraction of settlement-stage coral reef fishes to reef noise. Mar. Ecol. Prog. Ser. 276, 263–268. ( 10.3354/meps276263) [DOI] [Google Scholar]
- 23.Simpson SD, Meekan MG, Montgomery J, McCauley R, Jeffs A. 2005. Homeward sound. Science 308, 221 ( 10.1126/science.1107406) [DOI] [PubMed] [Google Scholar]
- 24.Radford CA, Stanley JA, Simpson SD, Jeffs AG. 2011. Juvenile coral reef fish use sound to locate habitats. Coral Reefs 30, 295–305. ( 10.1007/s00338-010-0710-6) [DOI] [Google Scholar]
- 25.Holles S, Simpson SD, Radford AN, Berten L, Lecchini D. 2013. Boat noise disrupts orientation behaviour in a coral reef fish. Mar. Ecol. Prog. Ser. 485, 295–300. ( 10.3354/meps10346) [DOI] [Google Scholar]
- 26.Clark CW, Ellison WT, Southall BL, Hatch L, Van Parijs SM, Frankel A, Ponirakis D. 2009. Acoustic masking in marine ecosystems: intuitions, analysis, and implication. Mar. Ecol. Prog. Ser. 395, 201−222. ( 10.3354/meps08402) [DOI] [Google Scholar]
- 27.Purser J, Radford AN. 2011. Acoustic noise induces attention shifts and reduces foraging performance in three-spined sticklebacks (Gasterosteus aculeatus). PLoS ONE 6, e17478 ( 10.1371/journal.pone.0017478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voellmy IK, Purser J, Simpson SD, Radford AN. 2014. Increased noise levels have different impacts on the anti-predator behaviour of two sympatric fish species. PLoS ONE 9, e102946 ( 10.1371/journal.pone.0102946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munday PL, Dixson DL, McCormick MI, Meekan MG, Ferrari MCO, Chivers DP. 2010. Replenishment of fish populations is threatened by ocean acidification. Proc. Natl Acad. Sci. USA 107, 12 930–12 934. ( 10.1073/pnas.1004519107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simpson SD, Purser J, Radford AN. 2015. Anthropogenic noise compromises antipredator behaviour in European eels. Glob. Change Biol. 21, 586–593. ( 10.1111/gcb.12685) [DOI] [PubMed] [Google Scholar]
- 31.Nedelec SL, Radford AN, Pearl L, Nedelec B, McCormick MI, Meekan MG, Simpson SD. 2017. Motorboat noise impacts parental behaviour and offspring survival in a reef fish. Proc. R. Soc. B 284, 20170143 ( 10.1098/rspb.2017.0143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crain CM, Kroeker K, Halpern BS. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315. ( 10.1111/j.1461-0248.2008.01253.x) [DOI] [PubMed] [Google Scholar]
- 33.Kneitel JM, Chase JM. 2004. Trade-offs in community ecology: linking spatial scales and species coexistence. Ecol. Lett. 7, 69–80. ( 10.1046/j.1461-0248.2003.00551.x) [DOI] [Google Scholar]
- 34.Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. ( 10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 35.GBRMPA. 2014. Great barrier reef outlook report 2014. Townsville, Australia: Great Barrier Reef Marine Park Authority. [Google Scholar]
- 36.UNCTAD. 2016. Review of maritime transport 2015. Geneva, Switzerland: UNCTAD. [Google Scholar]
- 37.Almany GR, Webster MS. 2006. The predation gauntlet: early post-settlement mortality in coral reef fishes. Coral Reefs 25, 19–22. ( 10.1007/s00338-005-0044-y) [DOI] [Google Scholar]
- 38.Irschick DJ, Meyers JJ, Husak JF, Le Galliard J-F. 2008. How does selection operate on whole-organism functional performance capacities? A review and synthesis. Evol. Ecol. Res. 10, 177–196. [Google Scholar]
- 39.Cripps IL, Munday PL, McCormick MI. 2011. Ocean acidification affects prey detection by a predatory reef fish. PLoS ONE 6, e22736 ( 10.1371/journal.pone.0022736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allan BJM, Domenici P, McCormick MI, Munday PL. 2013. Elevated CO2 affects predator–prey interactions through altered performance. PLoS ONE 8, e58520 ( 10.1371/journal.pone.0058520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feeney WE, Lönnstedt OM, Bosiger YJ, Martin J, Jones GP, Rowe RJ, McCormick MI. 2012. High rate of prey consumption in a small predatory fish on coral reefs. Coral Reefs 31, 909–918. ( 10.1007/s00338-012-0894-z) [DOI] [Google Scholar]
- 42.Munday PL, Welch MJ, Allan B, Watson SA, McMahon S, McCormick MI. 2016. Effects of elevated CO2 on predator avoidance behaviour by reef fishes is not altered by experimental test water. PeerJ 4, e2501 ( 10.7717/peerj.2501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nedelec SL, Campbell J, Radford AN, Simpson SD, Merchant ND. 2016. Particle motion: the missing link in underwater acoustic ecology. Methods Ecol. Evol. 7, 836–842. ( 10.1111/2041-210x.12544) [DOI] [Google Scholar]
- 44.Allan BJM, Domenici P, Watson S, Munday PL, McCormick MI. 2017. Warming has a greater effect than elevated CO2 on predator–prey interactions in coral reef fish. Proc. R. Soc. B 284, 20170784 ( 10.1098/rspb.2017.0784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webb PW. 1976. The effect of size on the fast-start performance of rainbow trout Salmo cairdneri, and a consideration of piscivorous predator–prey interactions. J. Exp. Biol. 65, 157–177. [DOI] [PubMed] [Google Scholar]
- 46.Domenici P, Blake RW. 1997. The kinematics and performance of fish fast-start swimming. J. Exp. Biol. 200, 1165–1178. [DOI] [PubMed] [Google Scholar]
- 47.Dill LM. 1974. The escape response of the zebra danio (Brachydanio rerio) II. The effect of experience. Anim. Behav. 22, 723–730. ( 10.1016/S0003-3472(74)80023-0) [DOI] [Google Scholar]
- 48.Webb PW. 1982. Avoidance responses of fathead minnow to strikes by four teleost predators. J. Comp. Physiol. 147, 371–378. ( 10.1007/BF00609671) [DOI] [Google Scholar]
- 49.Ferrari MCO, Dixson DL, Munday PL, McCormick MI, Meekan MG, Sih A, Chivers DP. 2011. Intrageneric variation in tolerance of coral reef fishes to ocean acidification: implications for climate change projections on marine communities. Glob. Change Biol. 17, 2980–2986. ( 10.1111/j.1365-2486.2011.02439.x) [DOI] [Google Scholar]
- 50.Holmes LJ, McWilliam J, Ferrari MCO, McCormick MI. 2017. Juvenile damselfish are affected but desensitize to small motor boat noise. J. Exp. Mar. Biol. Ecol. 494, 63–68. ( 10.1016/j.jembe.2017.05.009) [DOI] [Google Scholar]
- 51.Smith ME. 2016. Relationship between hair cell loss and hearing loss in fishes. In Effects of noise on aquatic life II (eds Popper AN, Hawkins A), pp. 1067–1074. Berlin, Germany: Springer. [DOI] [PubMed] [Google Scholar]
- 52.Hofmann GE, et al. 2011. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6, e28983 ( 10.1371/journal.pone.0028983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duarte CM, et al. 2013. Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuar. Coasts. 36, 221–236. ( 10.1007/s12237-013-9594-3) [DOI] [Google Scholar]
- 54.Jarrold M, Humphrey C, McCormick MI, Munday PL. 2017. Diel CO2 cycles alleviate behavioural abnormalities in coral reef fish under ocean acidification. Sci. Rep. 7, 10153 ( 10.1038/s41598-017-10378-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogers A, et al. 2015. Anticipative management for coral reef ecosystem services in the 21st century. Glob. Change Biol. 21, 504–514. ( 10.1111/gcb.12725) [DOI] [PubMed] [Google Scholar]
- 56.Harborne AR, Rogers A, Bozec YM, Mumby PJ. 2017. Multiple stressors and the functioning of coral reefs. Ann. Rev. Mar. Sci. 9, 445–468. ( 10.1146/annurev-marine-010816-060551) [DOI] [PubMed] [Google Scholar]
- 57.Mumby PJ, et al. 2017. Avoiding a crisis of motivation for ocean management under global environmental change. Glob. Change Biol. 23, 4453–5020. ( 10.1111/gcb.13698) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are accessible from the Tropical Data Hub (http://dx.doi.org/10.4225/28/5a1cd71b4ef8a).