Abstract
Artificial light at night has shown a dramatic increase over the last decades and continues to increase. Light at night can have strong effects on the behaviour and physiology of species, which includes changes in the daily timing of activity; a clear example is the advance in dawn song onset in songbirds by low levels of light at night. Although such effects are often referred to as changes in circadian timing, i.e. changes to the internal clock, two alternative mechanisms are possible. First, light at night can change the timing of clock controlled activity, without any change to the clock itself; e.g. by a change in the phase relation between the circadian clock and expression of activity. Second, changes in daily activity can be a direct response to light (‘masking’), without any involvement of the circadian system. Here, we studied whether the advance in onset of activity by dim light at night in great tits (Parus major) is indeed attributable to a phase shift of the internal clock. We entrained birds to a normal light/dark (LD) cycle with bright light during daytime and darkness at night, and to a comparable (LDim) schedule with dim light at night. The dim light at night strongly advanced the onset of activity of the birds. After at least six days in LD or LDim, we kept birds in constant darkness (DD) by leaving off all lights so birds would revert to their endogenous, circadian system controlled timing of activity. We found that the timing of onset in DD was not dependent on whether the birds were kept at LD or LDim before the measurement. Thus, the advance of activity under light at night is caused by a direct effect of light rather than a phase shift of the internal clock. This demonstrates that birds are capable of changing their daily activity to low levels of light at night directly, without the need to alter their internal clock.
Keywords: entrainment, artificial light at night, light pollution, circadian period, circadian phase
1. Introduction
Artificial light at night is omnipresent in the civilized world and continues to increase by economic growth and urbanization [1,2]. Consequently, ecosystems are increasingly exposed to unnatural levels of light at night, on a worldwide scale [3,4]. Artificial light has effects on many species groups, and among these, birds are well represented. Such effects include direct attraction to light, an effect which can induce severe mortality [5,6]. Other effects of light at night involve changes of temporal organization of behaviour. For example, exposure to light at night may cause birds to breed earlier in the season [7], an effect which has been observed in the laboratory as well [8]. On a shorter temporal scale, light at night affects the daily organization of bird behaviour and physiology. Effects of nocturnal illumination may be quite direct as light enables diurnal birds to forage during the night [9–11]. In songbirds, a well-known effect of light at night is the advancement of the daily onset of activity and dawn song. Recordings in the field show that the magnitude of this advance depends on light intensity [12–14] and the species specific onset of dawn song in unlit conditions. The combined effect ranges from a few minutes to over half an hour [15,16], and even modest changes in the temporal organization of birds can have important fitness consequences [16]. In the laboratory, diurnal bird species confine their activity to the light phase of the light/dark (LD) cycle [17–19]. Like in the field, birds advance their onset of activity when exposed to light at night in the laboratory [17,19]. In great tits (Parus major) this advance is dose-dependent, and can measure up to several hours when captive birds are exposed to five lux at night [17].
Differences in the daily timing of activity as a result of environmental changes have been known for a long time [20] and are reported for several species [21–25]. There are three potential ways in how environmental variables can affect daily activity patterns.
The first is by a change in the way the internal clock and the external photoperiod interact to shape these temporal activity patterns. This is defined as the phase of entrainment; i.e. the phase relation between the master circadian clock and the external LD cycle.
A second possibility is that the timing of activity has a different phase relation with the central pacemaker; i.e. where the central circadian pacemaker is unaffected and activity is triggered at a different time of day. Such changes in phase-relation between activity and the central circadian pacemaker are for example reported for nocturnal mice (Mus musculus domesticus) that change to daytime activity when subjected to restricted feeding schedules and low ambient temperature [26,27]. Evidence of changes in phase-relation as a result of low light at night is thus far limited to hamsters (Mesocricetus auratus) [28,29] and mice [30] in the laboratory.
A third possibility is that changes in daily timing of activity are caused by a direct response, and do not involve a shift of the clock nor a change in phase relation with the central circadian pacemaker. Such a response is called masking, and may encompass a direct, positive or negative, response to the presence of light, resulting in an increase or decrease in activity which may conceal the phase of the circadian clock [31,32].
Here, we investigate whether the advancement of the onset of activity in the great tit (P. major) by dim light levels at night is caused by change in the phase of entrainment (the first option described above). We do this by advancing the birds' onset of activity by exposure to the same dim light levels as applied in [17], and subsequently measure the circadian rhythm in constant darkness. The release of animals in an environment in absence of time cues is the classical way of uncovering the phase of entrainment. The initial timing of activity in constant conditions reflects the phase of entrainment in the preceding conditions [32–34]. In case the phase of entrainment is unaffected by dim light at night, e.g. comparable to the phase of entrainment in normal LD conditions without dim light at night, the dim light induced advance in onset is not caused by a phase shift of the internal circadian clock.
2. Methods
(a). Animals and data collection
We included in total 29 male great tits (P. major), collected at 10 days post-hatch from a long-term studied population in the Hoge Veluwe National Park in the Netherlands during the breeding season of 2014. Nestlings were subsequently hand-reared at the Netherlands Institute of Ecology (NIOO-KNAW); the males were included in a breeding programme in spring 2015. At the end of September 2015, we placed the birds individually in light-tight cages distributed over three walls in two rooms. Birds were kept in the same cage for the entire experiment. Each cage was equipped with two wooden perches, one fitted with a micro switch connected to a computer [35] to register perch-hopping activity (software developed by T&M Automation, Leidschendam, The Netherlands). We first entrained birds to L : D 12.3 : 11.7 (lights on: 6.21; lights off: 18.38) with 1000 Lux fluorescent light (18 W Havells Sylvania Activa 172, East Sussex, UK) at perch level. After 6 days, we left the lights off and kept the birds for 14 days in constant darkness. Birds were then re-entrained to the same LD schedule for six days and then subjected to an LDim schedule. This was done by illuminating the ‘dark’ phase with dim light, with an intensity of either 0.15, 0.5, 1.5 or 5.0 Lux (warmwhite LED light, Philips, Eindhoven, The Netherlands, see the electronic supplementary material, figure S1 for spectrum) at the perch level in the cages, with LDim treatments randomly assigned over individual cages. Temperature was kept constant at 14°C over the entire course of the experiment and did not vary over the day. To prevent brief moments of darkness in between the bright light phase and the dim lit ‘dark’ phase, dim lights were switched on 30 min before the end of the light phase, and switched off 30 min after the end of the ‘dark’ phase (on at 18.08 and off at 6.51). After 13 days in the LDim cycle, we left both the day- and dim lights off, starting at the end of the last bright light phase. All birds were subsequently kept in DD for 14 days (see also figure 1). White noise was continuously present to cover perch hopping sounds and vocalizations of birds, and each cage was equipped with a green night light (Alecto ANV-17, 0.5 Lux at perch level as used in [36], see also the electronic supplementary material) when birds were in kept constant darkness in order to enable birds to find food. Bird feeding times were randomized within the daytime bright light phase, and randomly distributed between the times of 8.00 and 14.00 when birds were in DD. Birds were supplied with a surplus of food to ensure permanent ad libitum access.
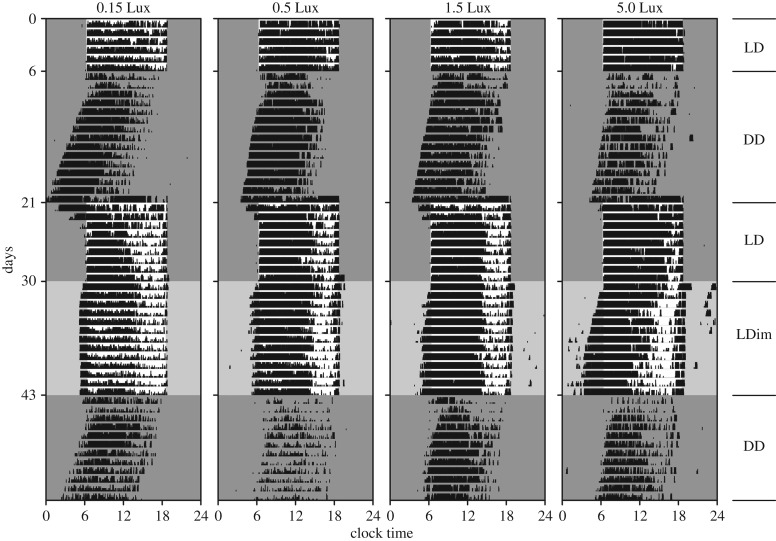
Figure 1.
Example actograms of four great tits exposed to different levels of dim light at night between days 31–43. Birds were entrained to LD 12.3 : 11.7 (L = 1000 Lux; days 1–6), free running in DD (days 7–21), re-entrained to LD 12.3 : 11.7 (L = 1000 Lux; days 21–30), entrained to LDim 12.3 : 11.7 (L = 1000 Lux; dim intensity above actogram; days 31–43), and free running in DD (days 44–55). Dark grey indicates darkness; light grey indicates dim light; and white bright light.
(b). Data analysis
Data were analysed with Chronoshop 1.1 (written by K.S.; freely available, see the electronic supplementary material). First, we measured the phase angle difference between the onset of activity and the time of lights on, both in DD and LDim conditions according to the routines implemented in [37], procedure examples are provided in the electronic supplementary material, figure S2. We then assessed the phase of entrainment by backwards extrapolating onsets of activity in the activity pattern in constant darkness to the change from LD to DD or LDim to DD cycles following the Aschoff type II protocol [38] (see the electronic supplementary material, figures S3 and S4). Subsequently the effect of dim light at night on the advance in onset of activity was tested using a linear mixed effect model with phase relative to lights on as dependent, and dim light treatment as explanatory variable and cage position as random term. Cage position identifies the wall (1–3) at which the cage was placed. With a Mantel test [39] we tested for possible influences by neighbouring birds on the advance of activity in LDim light conditions. We then tested for the effect of dim light levels at night on the (onset) phase of entrainment directly after the switch from LDim to DD. We used the individual initial phase in DD directly after the switch from LD to DD as a control. We did this in a linear model fitting the initial phases in DD (both after LD and LDim) to the interaction between the presence of dim light (yes or no) and treatment (dim light at night intensity, four levels), with bird identity and cage position as random terms. Models were compared to null models (without explanatory variable or interaction term) using analysis of residual variance. We also analysed whether dim light at night had an effect on the length of the circadian period (τ) measured in DD, in a model with the difference between τ after LD and τ after LDim as a dependent variable, and treatment as an independent variable. We further tested for a relation between τ and the timing of onset of activity during entrainment in LD and LDim, and the timing of onset of activity (phase of entrainment) in DD directly after LD and LDim. In all models testing circadian period, we added cage position as a random term. For all statistical tests we used R v. 3.3.1 [40] with a significance level of 5%.
3. Results
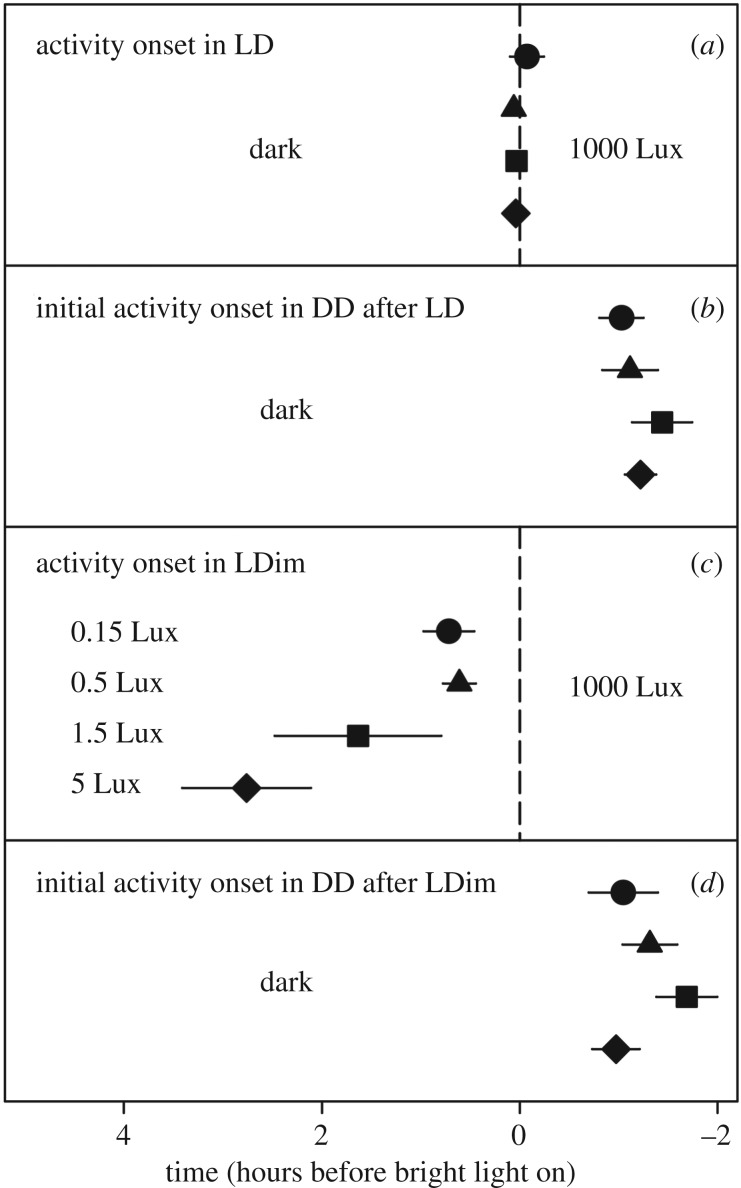
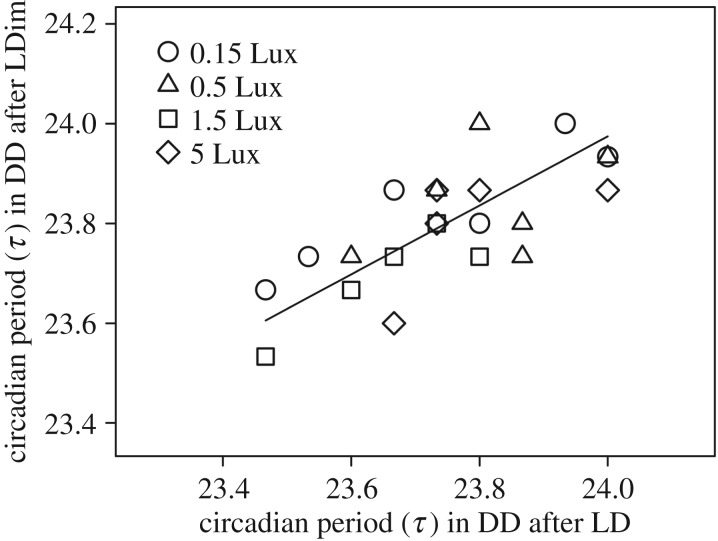
All birds entrained well to the LD schedule; see figure 1 for example actograms (actograms of all birds are provided in the electronic supplementary material). We had to exclude three birds because of low activity in DD after the LD schedule, and three birds because we could not obtain a good activity recording. This resulted in a sample size of six birds per treatment, except for five birds for the 1.5 Lux group. In LD, the difference between average onset of activity and lights on was for all four groups less than 5 min. After release in DD after the LD cycle, the birds delayed their initial onset by 69 ± 7.3 min (relative to previous light-on time; average ±1 s.e.m.). The dim light at night significantly advanced the onset of the birds (p < 0.005, table 1), with 43 ± 15 min, 37 ± 10 min, 98 ± 51 min and 166 ± 39 min for the 0.15, the 0.5, the 1.5 and the 5.0 Lux dim light treated groups, respectively (figure 2). We did not detect any effects of neighbouring birds on the onset of activity in the Mantel tests (p = 0.28 or higher, table 1). After release in DD following the LDim cycle, birds immediately delayed their onset. There was no effect of the interaction between the presence of dim light at night (yes or no) and treatment, indicating no effect of dim light at night on the phase of entrainment (p = 0.64). The initial timing of onset in DD after LDim was on average 72 ± 8.4 min after the moment the bright daylights went on during the preceding LDim schedule (figure 2). We did not find any difference between the circadian period (τ) after LD and after LDim conditions (p = 0.62; figure 3), and individual τ did neither explain the onset of activity in LD (p = 0.37) nor in LDim (p = 0.32). However, individual τ did explain the (onset) phase of entrainment directly after the switch from LD to DD (p = 0.02) and from LDim to DD (p = 0.03), with a shorter τ related to an earlier onset of activity.
Table 1.
Results of the linear model comparisons. (a) Onset of activity and LDim light intensity; (b) within individual comparison of the phase of entrainment after LD (without dim light at night) and LDim (with dim light at night). This is done by testing for an effect of the interaction of LDim presence and the LDim light intensity on the initial phase of onset in DD (either following LD or LDim). (c) Effect of dim light at night on circadian period length in following DD phase; (d,e) effect of circadian period on the phase of onset in normal LD and LDim, respectively; (f,g) effect of circadian period on the initial phase of onset in in DD after LD and LDim, respectively; (h) Mantel test output on the influence of neighbouring birds.
| d.f. | AIC | loglik | d.f. | χ2 | p | |
|---|---|---|---|---|---|---|
| (a) effect of dim light intensity on the onset of activity | ||||||
| 1|cage position | 3 | 84.2 | 87.6 | |||
| LDim intensity + 1|cage position | 6 | 77.4 | 84.2 | 3 | 12.84 | <0.005 |
| (b) effect of the interaction between the presence of dim light at night and its intensity on the initial phase of onset in DD | ||||||
| LDim(y/n) + LDim intensity + 1|cage position + 1|birdID | 8 | 88.3 | −36.2 | |||
| LDim(y/n) × LDim intensity + 1|cage position + 1|birdID | 11 | 92.6 | −35.3 | 3 | 1.69 | 0.64 |
| (c) effect of the preceding LDim intensity on the circadian period (τ) in DD | ||||||
| 1|cage position | 3 | 124.4 | −59.2 | |||
| LDim intensity + 1|cage position | 4 | 125.3 | −58.7 | 1 | 1.08 | 0.30 |
| (d) effect of τ on the phase of onset of activity in LD | ||||||
| 1|cage position | 3 | −49.4 | 27.7 | |||
| τ + 1|cage position | 4 | −48.2 | 28.1 | 1 | 0.80 | 0.37 |
| (e) effect of τ on the phase of onset of activity in LDim | ||||||
| 1|cage position | 3 | 84.2 | −39.1 | |||
| τ + 1|cage position | 4 | 85.2 | −38.6 | 1 | 0.99 | 0.32 |
| (f) effect of τ on the initial phase of onset of activity in DD (directly after LD) | ||||||
| 1|cage position | 3 | 39.1 | −16.5 | |||
| τ + 1|cage position | 4 | 35.8 | −13.9 | 1 | 5.33 | 0.02 |
| (g) effect of τ on the initial phase of onset of activity in DD (directly after LDim) | ||||||
| 1|cage position | 3 | 85.2 | −39.6 | |||
| τ + 1|cage position | 4 | 82.5 | −37.2 | 1 | 4.78 | 0.03 |
| Rsq | observed | expected | var | p | ||
|---|---|---|---|---|---|---|
| (h) influence neighbouring birds with different LDim light levels on the onset of activity (Mantel test, 99 permutations) | ||||||
| neighbouring birds room 1, wall 1 | 0.19 | 0.68 | 0.02 | 0.07 | 0.28 | |
| neighbouring birds room 1, wall 2 | 0.14 | 0.49 | 0.02 | 0.06 | 0.30 | |
| neighbouring birds room 2, wall 3 | 0.00 | −0.14 | 0.02 | 0.04 | 0.50 | |
Figure 2.
Average timing of daily activity onset. The four groups received equal treatment except in the LDim phase (c) of the experiment, in which with one group was exposed to 0.15 Lux dim light at night (circles); 0.5 Lux (triangles); 1.5 Lux (squares) and 5 Lux (diamonds). (a) Average time of activity onset in LD 12.3 : 11.7; (b) average time of activity onset immediately after switch to DD after preceding LD; (c) average time of activity onset during LDim 12.3 : 11.7; (d) average time of activity onset immediately after the switch to DD after LDim. The dashed line indicates the time of bright light on in (a) and (c).
Figure 3.
Individual circadian period (τ) in constant darkness (DD) after a light/dark (LD) cycle without dim light at night against the circadian period in DD after an LD cycle with dim light at night (LDim). Symbols indicate the dim light at night intensity, which had no effect on τ (p = 0.62).
4. Discussion
The immediate return of the onset in constant darkness (DD) to the same time, irrespective of prior LD or LDim conditions, clearly excludes a change in the main circadian pacemaker phase (i.e. the phase of entrainment). Therefore, the advance in the onset of activity by dim light at night must be attributable to either masking or an altered phase relation between the expression of activity (whether or not governed by a possible peripheral oscillator) and the main circadian pacemaker.
This direct response of the birds to low light at night implies that birds are flexible in their response to ambient light levels at night, independent of the circadian system. This observation is in line with the immediate disappearance of the advancement of dawn song in the dark nights in an outdoor set-up with intermittent lighting [15]. Changes in the phase of entrainment of the circadian system may be even less likely in the field as the difference between the low levels of artificial light and the powerful natural LD cycle are much greater than the difference between the dim and bright light levels we used in the laboratory. However, to conclusively exclude such changes in the field, free-ranging birds have to be brought directly into a laboratory setting with constant conditions, or alternatively the phase of core-clock gene products in the master circadian clock has to be assessed.
We did not observe differences between τ after entrainment to either completely dark nights (LD) and nights with dim light (LDim). If any, these changes are probably subtle, and may have disappeared within the first cycles in DD. The absence of a general effect of the circadian period on the timing of onset of activity during LD and LDim may have been obscured by the strong effect of the bright daylight lamps. This is plausible as the individual τ did significantly explain the phase of onset in DD directly after both the end of the LD, and the end of the LDim conditions. Effects of the circadian period on the daily timing of activity in entrained conditions are however known for humans e.g. [41,42] and have been shown for birds in the laboratory [43] (but see [36]), and more recently also for free-ranging birds in the field [44].
Interestingly, the dim light at night does not induce activity throughout the night but only during the last part of the night, effectively advancing activity onset, comparable to the birds in the study by de Jong et al. [17]. This means that there is no compulsive response to the nightly dim light presence, and suggests circadian control over the expression of dim light induced nocturnal activity. A dependency of masking on circadian phase has indeed been shown before, in for example hamsters [45]. Although artificial light at night may well affect the phase of entrainment of the circadian system under certain circumstances and for different species, the results we present here show that this mechanism is not behind the advance of activity onset in birds exposed to dim light at night . Our observations imply that we should be cautious with the assumption that changes in daily timing reflect changes of the circadian system by artificial light at night.
Supplementary Material
Acknowledgements
We thank Marylou Aaldering, Coretta Jongeling, Franca Kropman, Anouk de Plaa and Ruben de Wit for taking good care of our experimental birds, and Marlies Vollebregt for measuring the spectrum of the green night lights.
Ethics
This study was performed under the approval by the Animal Experimentation Committee (DEC), Amsterdam, The Netherlands, protocol NIOO 14.10 addendum 2.
Data accessibility
The data collected during this study are available from the Dataverse Digital Depository, and can be accessed via the link: https://dataverse.nl/dataset.xhtml?persistentId=hdl:10411/XMONBC; The Chronoshop software can be accessed via the link: https://dataverse.nl/dataset.xhtml?persistentId=hdl:10411/YHJEFV.
Authors' contributions
All authors contributed to the design and set-up of the study. Data were collected by I.V. and D.M., and analysed by K.S. K.S. wrote the paper, and all authors commented on the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research is supported by the Dutch Technology Foundation STW, which is part of the Netherlands Organization for Scientific Research (NWO), and which is partly funded by the Ministry of Economic Affairs. The project is supported by Philips and the Nederlandse Aardolie Maatschappij (NAM).
References
- 1.Falchi F, Cinzano P, Duriscoe D, Kyba CCM, Elvidge CD, Baugh K, Portnov BA, Rybnikova NA, Furgoni R. 2016. The new world atlas of artificial night sky brightness. Sci. Adv. 2, e1600377 ( 10.1126/sciadv.1600377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyba CCM, et al. 2015. Worldwide variations in artificial skyglow. Sci. Rep. 5, 1–6. ( 10.1038/srep08409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennie J, Duffy JP, Davies TW, Correa-Cano ME, Gaston KJ. 2015. Global trends in exposure to light pollution in natural terrestrial ecosystems. Remote Sens. 7, 2715–2730. ( 10.3390/rs70302715) [DOI] [Google Scholar]
- 4.Gaston KJ, Visser ME, Hölker F. 2015. The biological impacts of artificial light at night: the research challenge. Phil. Trans. R. Soc. B 370, 20140133 ( 10.1098/rstb.2014.0133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Corre M, Ollivier A, Ribes S, Jouventin P. 2002. Light-induced mortality of petrels: a 4-year study from Réunion Island (Indian Ocean). Biol. Conserv. 105, 93–102. ( 10.1016/S0006-3207(01)00207-5) [DOI] [Google Scholar]
- 6.Rodriguez A, Rodriguez B. 2009. Attraction of petrels to artificial lights in the Canary Islands: effects of the moon phase and age class. Ibis 151, 299–310. ( 10.1111/j.1474-919X.2009.00925.x) [DOI] [Google Scholar]
- 7.de Jong M, Ouyang JQ, Silva AD, Grunsven RHA, Kempenaers B, Visser ME, Spoelstra K. 2015. Effects of nocturnal illumination on life-history decisions and fitness in two wild songbird species. Phil. Trans. R. Soc. B 370, 20140128 ( 10.1098/rstb.2014.0128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominoni D, Quetting M, Partecke J. 2013. Artificial light at night advances avian reproductive physiology. Proc. R. Soc. B 280, 20123017 ( 10.1098/rspb.2012.3017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dwyer RG, Bearhop S, Campbell HA, Bryant DM. 2013. Shedding light on light: benefits of anthropogenic illumination to a nocturnally foraging shorebird. J. Anim. Ecol. 82, 478–485. ( 10.1111/1365-2656.12012) [DOI] [PubMed] [Google Scholar]
- 10.Lebbin DJ, Harvey MG, Lenz TC, Andersen MJ, Ellis JM. 2007. Nocturnal migrants foraging at night by artificial light. Wilson J. Ornithol. 119, 506–508. ( 10.1676/06-139.1) [DOI] [Google Scholar]
- 11.Santos CD, Miranda AC, Granadeiro JP, Lourenço PM, Saraiva S, Palmeirim JM. 2010. Effects of artificial illumination on the nocturnal foraging of waders. Acta Oecolog. 36, 166–172. ( 10.1016/j.actao.2009.11.008) [DOI] [Google Scholar]
- 12.Da Silva A, Jong M, Grunsven RHA, Visser ME, Kempenaers B, Spoelstra K. 2017. Experimental illumination of a forest: no effects of lights of different colours on the onset of the dawn chorus in songbirds. R. Soc. open sci. 4, 160638 ( 10.1098/rsos.160638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominoni DM, Carmona-Wagner EO, Hofmann M, Kranstauber B, Partecke J. 2014. Individual-based measurements of light intensity provide new insights into the effects of artificial light at night on daily rhythms of urban-dwelling songbirds. J. Anim. Ecol. 83, 681–692. ( 10.1111/1365-2656.12150) [DOI] [PubMed] [Google Scholar]
- 14.Miller MW. 2006. Apparent effects of light pollution on singing behavior of American robins. Condor 108, 130–139. ( 10.1650/0010-5422(2006)108%5B0130:AEOLPO%5D2.0.CO;2) [DOI] [Google Scholar]
- 15.Da Silva A, Valcu M, Kempenaers B. 2016. Behavioural plasticity in the onset of dawn song under intermittent experimental night lighting. Anim. Behav. 117, 155–165. ( 10.1016/j.anbehav.2016.05.001) [DOI] [Google Scholar]
- 16.Kempenaers B, Borgström P, Loës P, Schlicht E, Valcu M. 2010. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 20, 1735–1739. ( 10.1016/j.cub.2010.08.028) [DOI] [PubMed] [Google Scholar]
- 17.de Jong M, Jeninga L, Ouyang JQ, van Oers K, Spoelstra K, Visser ME. 2016. Dose-dependent responses of avian daily rhythms to artificial light at night. Physiol. Behav. 155, 172–179. ( 10.1016/j.physbeh.2015.12.012) [DOI] [PubMed] [Google Scholar]
- 18.Kumar V, Gwinner E, Van't Hof TJ. 2000. Circadian rhythms of melatonin in European starlings exposed to different lighting conditions: relationship with locomotor and feeding rhythms. J. Comp. Physiol. A 186, 205–215. ( 10.1007/s003590050020) [DOI] [PubMed] [Google Scholar]
- 19.Singh J, Rani S, Kumar V. 2012. Functional similarity in relation to the external environment between circadian behavioral and melatonin rhythms in the subtropical Indian weaver bird. Horm. Behav. 61, 527–534. ( 10.1016/j.yhbeh.2012.01.015) [DOI] [PubMed] [Google Scholar]
- 20.Daan S. 1981. Adaptive daily strategies in behavior. In Handbook of behavioral neurobiology; biological rhythms (ed. Aschoff J.), pp. 275–298. New York, NY: Plenum Press. [Google Scholar]
- 21.Castillo-Ruiz A, Paul MJ, Schwartz WJ. 2012. In search of a temporal niche: social interactions. Prog. Brain Res. 199, 267–280. ( 10.1016/B978-0-444-59427-3.00016-2) [DOI] [PubMed] [Google Scholar]
- 22.Cohen R, Smale L, Kronfeld-Schor N. 2010. Masking and temporal niche switches in spiny mice. J. Biol. Rhythms 25, 47–52. ( 10.1177/0748730409351672) [DOI] [PubMed] [Google Scholar]
- 23.Hut RA, Kronfeld-Schor N, van der Vinne V, De la Iglesia H. 2012. In search of a temporal niche. Prog. Brain Res. 199, 281–304. ( 10.1016/B978-0-444-59427-3.00017-4) [DOI] [PubMed] [Google Scholar]
- 24.Mrosovsky N, Hattar S. 2005. Diurnal mice (Mus musculus) and other examples of temporal niche switching. J. Comp. Physiol. A 191, 1011–1024. ( 10.1007/s00359-005-0017-1) [DOI] [PubMed] [Google Scholar]
- 25.Ziv Y, Abramsky Z, Kotler BP, Subach A. 1993. Interference competition and temporal and habitat partitioning in two gerbil species. Oikos 66, 237–246. ( 10.2307/3544810) [DOI] [Google Scholar]
- 26.Mistlberger RE. 1994. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci. Biobehav. Rev. 18, 171–195. ( 10.1016/0149-7634(94)90023-X) [DOI] [PubMed] [Google Scholar]
- 27.van der Vinne V, Riede SJ, Gorter JA, Eijer WG, Sellix MT, Menaker M, Daan S, Pilorz V, Hut RA. 2014. Cold and hunger induce diurnality in a nocturnal mammal. Proc. Natl Acad. Sci. USA 111, 15 256–15 260. ( 10.1073/pnas.1413135111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans JA, Elliott JA, Gorman MR. 2005. Circadian entrainment and phase resetting differ markedly under dimly illuminated versus completely dark nights. Behav. Brain Res. 162, 116–126. ( 10.1016/j.bbr.2005.03.014) [DOI] [PubMed] [Google Scholar]
- 29.Gorman MR, Evans JA, Elliott JA. 2006. Potent circadian effects of dim illumination at night in hamsters. Chronobiol. Int. 23, 245–250. ( 10.1080/07420520500521905) [DOI] [PubMed] [Google Scholar]
- 30.Fonken LK, Aubrecht TG, Meléndez-Fernández OH, Weil ZM, Nelson RJ. 2013. Dim light at night disrupts molecular circadian rhythms and increases body weight. J. Biol. Rhythms 28, 262–271. ( 10.1177/0748730413493862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aschoff J. 1988. Masking of circadian rhythms by zeitgebers as opposed to entrainment. Trends Chronobiol. Adv. Biosci. 73, 149–161. [Google Scholar]
- 32.Mrosovsky N. 1999. Masking: history, definitions, and measurement. Chronobiol. Int. 16, 415–429. ( 10.3109/07420529908998717) [DOI] [PubMed] [Google Scholar]
- 33.Aschoff J. 1960. Exogenous and endogenous components in circadian rhythms. Cold Spr. Harb. Symp. Quant. Biol. 25, 11–28. [DOI] [PubMed] [Google Scholar]
- 34.Roenneberg T, Daan S, Merrow M. 2003. The art of entrainment. J. Biol. Rhythms 18, 183–194. ( 10.1177/0748730403018003001) [DOI] [PubMed] [Google Scholar]
- 35.Gänshirt G, Daan S, Gerkema MP. 1984. Arrhythmic perch hopping and rhythmic feeding of starlings in constant light: separate circadian oscillators? J. Comp. Physiol. A 154, 669–674. ( 10.1007/BF01350220) [DOI] [Google Scholar]
- 36.Helm B, Visser ME. 2010. Heritable circadian period length in a wild bird population. Proc. R. Soc. B 277, 3335–3342. ( 10.1098/rspb.2010.0871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spoelstra K, Albrecht U, van der Horst GTJ, Brauer V, Daan S. 2004. Phase responses to light pulses in mice lacking functional per or cry genes. J. Biol. Rhythms 19, 518–529. ( 10.1177/0748730404268122) [DOI] [PubMed] [Google Scholar]
- 38.Aschoff J. 1965. Response curves in circadian periodicity. In Circadian clocks—proceedings of the Feldafing summer school (ed. Aschoff J.), pp. 95–111. Amsterdam, the Netherlands: North-Holland Publishing. [Google Scholar]
- 39.Mantel N. 1967. The detection of disease clustering and a generalized regression approach. Cancer Res. 27, 209–220. [PubMed] [Google Scholar]
- 40.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org. [Google Scholar]
- 41.Duffy JF, Rimmer DW, Czeisler CA. 2001. Association of intrinsic circadian period with morningness–eveningness, usual wake time, and circadian phase. Behav. Neurosci. 115, 895 ( 10.1037/0735-7044.115.4.895) [DOI] [PubMed] [Google Scholar]
- 42.Vanselow K, et al. 2006. Differential effects of PER2 phosphorylation: molecular basis for the human familial advanced sleep phase syndrome (FASPS). Genes Dev. 20, 2660–2672. ( 10.1101/gad.397006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aschoff J, Wever R. 1966. Circadian period and phase-angle difference in chaffinches (Fringilla coelebs L.). Comp. Biochem. Physiol. 18, 397–404. ( 10.1016/0010-406X(66)90197-6) [DOI] [PubMed] [Google Scholar]
- 44.Dominoni DM, Helm B, Lehmann M, Dowse HB, Partecke J. 2013. Clocks for the city: circadian differences between forest and city songbirds. Proc. R. Soc. B 280, 20130593 ( 10.1098/rspb.2013.0593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aschoff J, von Goetz C. 1988. Masking of circadian activity rhythms in hamsters by darkness. J. Comp. Physiol. A 162, 559–562. ( 10.1007/BF00612521) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data collected during this study are available from the Dataverse Digital Depository, and can be accessed via the link: https://dataverse.nl/dataset.xhtml?persistentId=hdl:10411/XMONBC; The Chronoshop software can be accessed via the link: https://dataverse.nl/dataset.xhtml?persistentId=hdl:10411/YHJEFV.