Abstract
Species traits are thought to predict feeding specialization and the vulnerability of a species to extinctions of interaction partners, but the context in which a species evolved and currently inhabits may also matter. Notably, the predictive power of traits may require that traits evolved to fit interaction partners. Furthermore, local abiotic and biotic conditions may be important. On islands, for instance, specialized and vulnerable species are predicted to be found mainly in mountains, whereas species in lowlands should be generalized and less vulnerable. We evaluated these predictions for hummingbirds and their nectar-food plants on Antillean islands. Our results suggest that the rates of hummingbird trait divergence were higher among ancestral mainland forms before the colonization of the Antilles. In correspondence with the limited trait evolution that occurred within the Antilles, local abiotic and biotic conditions—not species traits—correlate with hummingbird resource specialization and the vulnerability of hummingbirds to extinctions of their floral resources. Specifically, hummingbirds were more specialized and vulnerable in conditions with high topographical complexity, high rainfall, low temperatures and high floral resource richness, which characterize the Antillean Mountains. These findings show that resource specialization and species vulnerability to extinctions of interaction partners are highly context-dependent.
Keywords: island biology, mountains, mutualistic networks, endemics, specialization, taxon cycles
1. Introduction
For more than 150 years, ecologists have realized that species are entangled in networks of interactions with locally co-occurring species [1,2]. Species interaction networks can be used to investigate whether species traits influence resource specialization and species vulnerability to extinctions of their interaction partners [3–5], but how these crucial aspects of species ecology are predicted by local abiotic and biotic conditions is less well known [6–8]. Moreover, although traits may evolve to exploit specific resources and minimize competition [9,10], it is poorly understood whether the importance of traits in determining resource specialization and species vulnerability is related to rates of trait evolution. In other words, the context in which a species evolved, and is currently distributed, is often neglected as a determinant of resource specialization and vulnerability to the extinction of interaction partners.
With respect to resource specialization, island organisms are often found to be more generalized than their mainland counterparts, and sometimes engage in interactions rarely observed on the mainland, e.g. pollination by lizards [11–13]. Such generalized behaviour may be owing to the limited number of species and reduced interspecific competition on islands, resulting in ecological release and the evolution of generalized feeding niches [11]. Colonization history may also matter, as mainland generalists from the lowlands should more easily colonize and establish on islands than mainland specialists and mountainous species [14,15]. However, not all island species have generalized feeding niches, particularly if they undergo sequential phases of range expansion and contraction as predicted by taxon cycle theory. Notably, according to taxon cycle theory, newly colonized species that are in a period of range expansion often have fairly generalized niches and establish in marginal lowland habitats, whereas species at the end of the taxon cycle are endemics specialized to interior mountain abiotic and biotic environments [15–17]. Thus, contrary to the prevailing trend for island species to be generalists, mountain endemics may provide extreme examples of specialization. One such example is the purple-throated carib (Eulampis jugularis), an endemic hummingbird specialized to feed on nectar from the flowers of Heliconia plants in the Lesser Antillean Mountains [9,18]. On islands, there should, therefore, be an association between local conditions and the level of resource specialization: lowland species being generalized and species in mountains being more specialized.
In addition to these geographical trends in resource generalization–specialization, island species are known to be more vulnerable to extinction than mainland species [19,20]. For example, during the last 400 years, approximately 90% of all bird extinctions have occurred on islands [19]. This is thought to be a consequence of island populations being small—many are endemic to one or a few islands—and because island species have evolved largely in isolation. Thus, species on islands are susceptible to natural disasters, habitat destruction and introduced species [20], such as rats or cats, causing extinctions and threatening endemics across numerous islands throughout the world. The negative consequences of disrupting mutualistic associations are a further potentially important influence upon the extinction risk of island taxa, but these are less well known [21]. Bird pollination provides a good example of how disrupting mutualistic associations can influence reproductive output and population density [22]. Recently, species vulnerability to the extinction of their mutualistic partners has been modelled using networks of interactions between animal and plant communities [5,7,23,24], illustrating that plant extinctions are more likely to cause animal coextinctions than vice versa [7]. However, it remains poorly understood how animal vulnerability to plant extinctions is affected by their traits and local abiotic and biotic conditions.
Here, we use mutualistic plant–hummingbird networks in the Antillean archipelago to ask how species traits and local abiotic and biotic conditions relate to resource specialization and hummingbird vulnerability to plant extinctions. Hummingbirds have a long coevolutionary history with their nectar-food plants, which they are energetically highly dependent on. Likewise, although plants pollinated by hummingbirds may also be pollinated by other animals [18], many are, to a large extent, dependent on hummingbirds’ pollination services [25,26]. Notably, large-bodied and long-billed hummingbirds, and those living in wet, cool and topographically heterogeneous environments, seem to establish specialized interactions with their nectar-food plants [9,18,27–29]. High resource specialization may reduce competition between hummingbirds, increase the likelihood of pollen transfer among conspecific plants [3,30] and thus benefit both the hummingbirds and their nectar-food plants. However, high resource specialization may also make them more vulnerable to extinctions of their mutualistic partners [31,32]. It is, therefore, important to understand to what degree resource specialization and vulnerability to plant extinctions are associated with hummingbird traits and local abiotic and biotic conditions. As the evolution of hummingbird traits, notably bill length and body mass, may be associated with specialization on specific floral resources that have matching corolla morphologies and nectar-production rates [9], it is relevant to assess the degree of trait evolution among the Lesser Antillean hummingbirds. As such, we expect hummingbird traits to determine specialization and vulnerability when traits have coevolved in situ to fit their floral partners in the Antilles. Thus, we ask: (i) have the rates of hummingbird body size and bill length evolution been highest early or late in the history of Antillean hummingbirds, i.e. did hummingbird body size and bill length evolve primarily among ancestral mainland forms prior to colonizing the islands or more recently on the Antillean islands? (ii) within the Antilles, is hummingbird resource specialization and vulnerability to plant extinctions associated mainly with (a) morphological traits that are important for partitioning floral resources, i.e. hummingbird bill length and body mass, or (b) the abiotic and biotic conditions of the localities in which they occur? As measures of local abiotic and biotic conditions, we include topographic heterogeneity, precipitation, temperature, and richness of hummingbirds and their nectar-food plants. These abiotic and biotic factors are hypothesized to influence how hummingbirds partition floral resources. Specifically, species-rich communities and wet, cool mountain environments are hypothesized to support more specialized and vulnerable hummingbird species [18,27].
2. Material and methods
(a). Hummingbird trait evolution
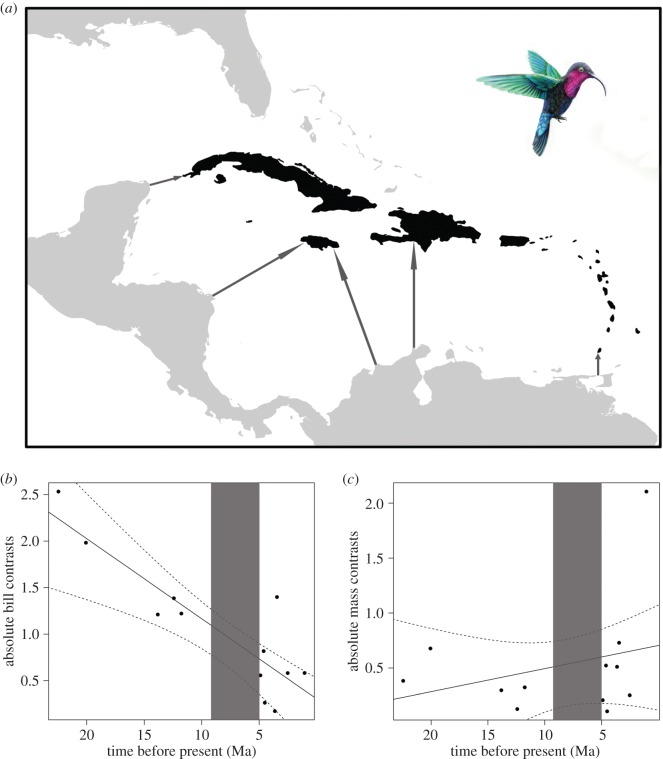
Extant hummingbirds colonized South America approximately 22 Ma and arrived through multiple colonization events to the Antillean archipelago approximately 5–9 Ma (figure 1a) [33,34]. All Antillean hummingbirds are endemic to the archipelago, except the rufous-breasted hermit (Glaucis hirsutus) which is found both on the South American mainland and on Grenada, the most southern island of the Antilles. We used phylogenetic comparative methods to evaluate changes in the rate of morphological evolution among Antillean hummingbird species through time and to subsequently assess whether the body size and bill length of Antillean hummingbirds probably evolved on the mainland prior to colonizing the islands, or on the Antillean islands. To achieve this, we used a recent hummingbird phylogeny [34] which represents a highly resolved time-calibrated analysis of molecular data from 284 hummingbird species (approx. 86% of all hummingbird species). The maximum clade credibility tree from this analysis was pruned to contain 13 of the 14 Antillean hummingbird species. Among the Antillean species, only Mellisuga helenae is missing from the phylogeny [34]. We analysed rates of hummingbird morphological diversification using phylogenetic independent contrasts (PICs; [35]) and Blomberg's K in the R packages APE and phytools [36–38]. The absolute values of the contrasts for body mass and bill length were regressed against node age using linear models, which enables assessment of how evolutionary rates change through time. Subsequently, we used Blomberg's K to test for the phylogenetic signal in both body mass and bill length. PICs and Blomberg's K represent alternative methods of assessing rates of trait evolution and how these compare to the null expectation under a Brownian motion model of evolution [35–38]. The smallest bird in the world, the bee hummingbird (M. helenae), is endemic to Cuba in the Greater Antilles. Although M. helenae is missing from the phylogeny [34], and therefore not included in our analysis, we expect the results presented to be robust to the inclusion of this species given the similarity in body mass and bill length with its congener Mellisuga minima (2.2 versus 2.4 g body mass; 10.8 versus 10.5 mm bill length; electronic supplementary material, table S1).
Figure 1.
(a) Map of the Caribbean Sea, with the Antillean archipelago shown in black, and surrounding islands and the American mainland in light grey. The major colonization routes of Antillean hummingbirds are indicated by dark grey arrows, following Abrahamczyk et al. [33]. We show the rate of morphological evolution of hummingbird (b) bill length and (c) body mass through evolutionary time assessed with phylogenetically independent contrasts. The regression line and ±95% confidence intervals derived from repeating the analyses across 1000 post burn-in phylogenies are shown with the solid and dashed lines, respectively. The dark grey shadings reflect the estimated time of hummingbird colonization to the Antilles some 5–9 Ma, following Abrahamczyk et al. [33]. Note that high rates of divergence in hummingbird bill length primarily occurred early in the evolutionary history, before the colonization of the Antilles, while the evolution of body mass differences was more constant through time. The main exception to this general trend was a large recent divergence in body mass between the members of the genus Eulampis. The drawing illustrates E. jugularis (credit: Pedro Lorenzo). (Online version in colour.)
(b). Hummingbird specialization on nectar-food plants
To estimate hummingbird specialization on nectar-food plants in the Antilles, we assembled a database of our own Antillean hummingbird–plant network studies, which recorded mutualistic interactions between assemblages of hummingbirds and their nectar-food plants. Details on the sampling can be found in [18], but here we give a brief overview. The database includes eight networks covering 12 of the 14 hummingbird species in the Antilles, only missing the Jamaican mango (Anthracothorax mango) and Hispaniolan emerald (Chlorostilbon swainsonii). Within the study plots of each network, we surveyed all flowering plant species for hummingbird visitation. In all networks, the link weight of each legitimate plant–hummingbird interaction was reported as the visitation rates of hummingbirds to flowers. Compared with binary networks, which only record whether an interaction occurred or not, weighted networks better reflect the dependencies between species [39]. Additionally, network-derived specialization indices based on weighted networks are less sensitive to sampling effort than their binary counterparts, making cross-network comparisons more reliable [40–43]. All studies collected hummingbird–plant interactions within the same season (March to July) and approximately the same sampling length (1.5–3.5 months; mean three months). The size of the study plots were 200 m × 5 m (Cuba, Puerto Rico, Grenada; five plots), 400 m × 5 m (Dominica; two plots) and 870 m × 6 m (Jamaica; one plot). In every study, we observed all hummingbird species whose range distribution overlapped the study area. The raw quantitative hummingbird–plant networks can be downloaded from Dryad (http://dx.doi.org/10.5061/dryad.5770gm7) [44]. See the electronic supplementary material, table S1 for details on the local abiotic and biotic conditions of each network: topographic heterogeneity, precipitation, temperature, and richness of hummingbirds and nectar-food plants.
To estimate hummingbird specialization, we used the ‘complementary specialization’ metric, which is a measure of the partitioning of interactions among species in weighted networks [40]. We calculated the species-level version of complementary specialization d′ (Kullback–Leibler divergence), which measures the interaction specialization of a given species by quantifying the deviation of interaction frequencies from a null expectation that assumes that all partners interact proportionally to their availability, using interaction frequency as a surrogate for abundance [40]. Values of d′ are scaled to range from 0 to 1, indicating the extremes of generalization and specialization, respectively. Complementary specialization d′ is conceived to account for differences in species richness among networks [40]. Calculations of hummingbird specialization d′ were conducted in the bipartite package v. 1.20 in R [38,45].
Next, we used linear mixed-effects models to examine how hummingbird specialization d′ associates with local conditions (abiotic: topography, temperature and precipitation; and biotic: richness of hummingbirds and plants, i.e. network size) and hummingbird traits (bill length and body mass). First, however, because of the relatively small sample size (n = 12 hummingbird species) and large number of intercorrelated variables (e.g. for hummingbird bill length and body mass: n = 12 species, r = 0.87, p < 0.05), we reduced the number of predictor variables to two using principal component analysis (PCA). To do this, for each of the two groups of predictors (local conditions and species traits), we used PCA and the broken stick method, identifying one PCA axis each for local conditions (explaining 69% of the total variation; loading: temperature = −0.607, precipitation = 0.832, topography = 0.941, network size = 0.894) and species traits (95%; loading: bill length = 0.975, body mass = 0.975). We note that the data are suitable for PCA analysis, being approximately linear, homoscedastic and normally distributed (−1.0 < skewness < 1.1; [46]). The resulting PCA axes were likewise suitable for linear mixed-effects modelling and were not correlated (r = 0.174; p > 0.05), i.e. there was no association between the local conditions and hummingbird traits. We then ran two linear mixed-effects models, one for each of the PCA axes as fixed effects, to predict hummingbird specialization d′. We used linear mixed-effects models because three hummingbird species were observed in more than one network (electronic supplementary material, table S1); therefore, we included hummingbird species identity as a random factor to account for the non-independence of the observations of the same species occurring in different networks [6,47]. We also ran a full model including both PCA axes (local conditions and hummingbird traits) as predictors of hummingbird specialization d′. Finally, we performed a set of supplementary models, analysing the association between hummingbird specialization d′ and the three components of local conditions: (i) local abiotic conditions, (ii) local floral resource richness, and (iii) local hummingbird richness (the latter two factors representing local biotic conditions). To do this, we first used PCA and the broken stick method for the local abiotic conditions, identifying one PCA axis (explaining 68% of the total variation; loading: temperature = −0.681, precipitation = 0.849, topography = 0.920). This axis was strongly positively correlated with local resource plant richness (r = 0.721; p < 0.05), indicating that in the Antilles there are more hummingbird-visited plant species in topographically complex, high precipitation and low temperature localities than in topographically simple, drier and warmer areas [18,48]. There was no association between the size of the study plots, the length of the sampling period and the floral resource richness within each site (study plot size: n = 8 sites, Pearson's r = −0.141; p = 0.74; sampling period: n = 8 sites, Pearson's r = 0.187; p = 0.66), or with hummingbird specialization (study plots: R2 marginal = 0.01, R2 conditional = 0.01; p = 0.68; sampling period: R2 marginal = 0.12, R2 conditional = 0.12; p = 0.16). Thus, neither the different sizes of the study plots nor differences in the sampling periods influence our results. For all linear mixed-effects models, we report Akaike information criterion corrected for small sample size (AICc), marginal R2 and conditional R2 values to evaluate model performance. To evaluate the performance of each predictor variable, we report coefficient estimates and corresponding p-values, standard errors and 95% confidence intervals. The PCA analyses were conducted in SAM v. 4.0 [49] and the linear mixed models in SPSS v. 22 [50].
(c). Hummingbird vulnerability to plant extinctions
We assessed the vulnerability of hummingbird species to iterative plant extinctions in the networks using the recently developed stochastic coextinction model (SCM), full details of which can be found in [51]; here, we give a brief overview. In the model, Pij = Ri dij is the probability of species i going extinct following the extinction of its partner species j. Ri is a species-level property between 0 and 1 that reflects the intrinsic demographic dependence of i on the pollination mutualism. In this study, where species i is a plant, Ri reflects the plant's dependence on hummingbird pollination. Where species i is an animal, Ri reflects hummingbird dependence on floral nectar. dij is the dependence of i on j, defined as the interaction strength between i and j divided by the total interaction strength between i and all its mutualistic partners. In the SCM, let A and B be the two sets of species in the network, with A representing plant species and B representing hummingbird species. Simulations start with the extinction of a single plant species in A. Next, all hummingbird species in B have a probability of extinction following the equation Pij = Ri dij. For each extinction in B, if any, all species in A have a probability of extinction, and so on. This process continues until there are no extinctions and equilibrium is reached. In this way, the SCM allows for complex coextinction cascades: a plant extinction can lead to a hummingbird extinction, which in turn can lead to a plant extinction, and so forth. A pervasive phenomenon in nature is the ability for pollinators to rewire, i.e. compensate for the loss of a partner plant species by reallocating lost interactions to other species in the community [52]. In this respect, it is also important to note that, in the SCM, a given species may survive even if all of its partners become extinct, reflecting that hummingbirds may be able to switch to other food resources and plants may not only be pollinated by hummingbirds [18]. Therefore, simulating species vulnerability to extinction using mutualistic networks may not reflect real extinctions in nature, but should reflect reduction in fitness. It is thus an appropriate tool to understand which species are most vulnerable to extinction of their mutualistic partners [24].
We ran two groups of simulations. In the first group (random plant R values), R values for plants were sampled from a uniform distribution between 0 and 1, with all plant species being assigned the same R value in each model run [51]. In the second group (expert-assigned plant R values), R values for plants were assigned based on expert opinion using fieldwork knowledge on floral phenotype and known insect visitation, which may contribute to pollination of hummingbird-visited flowers [18]. Each plant species was assigned low (0–0.33), medium (0.33–0.66) or high (0.66–1) R values, or values spanning two or more of these categories. Plants assigned as ‘low’ have flowers pollinated by both hummingbirds and insects; plants scored as ‘medium’ were plants with intermediate dependence on pollination by hummingbirds; ‘high’ categorized plants have ornithophilous syndrome flowers rarely visited by insects (for details, see the electronic supplementary material, table S2). In each run, all ‘low’ species were assigned the same randomly sampled value in the ‘low’ range, and all ‘medium’ species were assigned the same randomly sampled value in the ‘medium’ range, etc. In both group of simulations, as hummingbirds are highly dependent on floral nectar, R values for hummingbirds were assigned as ‘high’ (0.66–1). In each run, all hummingbird species were assigned the same randomly sampled value in the ‘high’ range. For both groups of model runs (random plant R values and expert-assigned plant R values), we carried out three sets of simulations, corresponding to three different plant extinction sequences [5,24]. In the first set of simulations, we iteratively removed the lowest degree plant species, i.e. those plant species with fewest hummingbird pollinators. This represents a ‘realistic extinction scenario’ as specialist species tend to be more vulnerable to extinction [24,31,32]. In the second set of simulations, we iteratively removed the highest degree plant species. This quantifies the ‘attack tolerance’ of the network; a ‘worst-case scenario’ where high degree nodes are lost first [53,54]. Finally, we simulated the loss of plants in a random order: this represents the null expectation between the two systematic removal orders discussed above [24]. All simulations were run 10 000 times.
From these six simulations (two methods of assigning plant R values and three plant extinction sequences), we calculated three measures of hummingbird vulnerability to plant extinctions: (i) the probability of extinction, PE, defined as the proportion of the 10 000 runs in which the species went extinct; (ii) the average proportion of plant species which had to be removed for a given hummingbird species to go extinct across the 10 000 runs (excluding model runs when it survived), representing the speed of extinction, SE; and (iii) a novel index of vulnerability of extinction, VE, where VE = PE (1 − SE). This index captures two components of vulnerability, such that species are considered more vulnerable when they have a higher PE and when, on average, their extinction occurs early in an extinction sequence (when 1 − SE is high). The R-codes for calculating the three measures of vulnerability can be downloaded from Dryad (http://dx.doi.org/10.5061/dryad.5770gm7) [44].
We examined the association between the three measures of hummingbird vulnerability (PE, SE and VE) and local conditions (temperature, precipitation, topography and network size, i.e. species richness of hummingbirds and plants) and hummingbird traits (bill length and body mass) using linear mixed-effects models. In all cases, the calculations were performed for all three extinction scenarios (iteratively removing species with the lowest degree; iteratively removing species with the highest degree, removing species at random) and for both methods of assigning plant R values: random (table 2; electronic supplementary material, tables S4 and S7) and expert-assigned dependency values based on floral phenotype and insect visitation (electronic supplementary material, tables S5 and S6). The linear mixed-effects modelling approach was identical to the one for hummingbird specialization d′, including the use of PCA axes, except in this instance we used hummingbird vulnerability as the response variable. We report the results for both the VE (table 2; electronic supplementary material, tables S5 and S7) and the 1 − SE (electronic supplementary material, tables S4 and S6). As we forced the networks to collapse completely in terms of simulating the removal of all plant species (see above), the PE was very high for nearly all species (0.83 < PE < 0.98), and thus, PE showed no association to either local conditions or to hummingbird traits (results not shown).
Table 2.
Linear mixed models, analysing the association between hummingbird vulnerability of extinction and two types of predictor models: (i) species traits and (ii) local conditions. (We also performed a full model including both predictors. In all models, we used species identity as a random factor, as for three species the level of vunerability VE was estimated in more than one network. Plant dependencies R on mutualism were assigned at random, and hummingbird vulnerabilities to plant extinction, VE, were calculated as VE = PE (1 − SE). This index captures two components of vulnerability, such that hummingbird species are considered more vulnerable to iterative plant extinctions when they have a higher probability of extinction (PE) and when, on average, their extinction occurs early in an extinction sequence (1 − SE). We modelled plant extinctions in three orders of deletion: iteratively removing the lowest degree plant species, iteratively removing the highest degree plant species and at random. Note that hummingbird vulnerability to plant extinction is only associated with the local conditions. See the electronic supplementary material, table S7 for similar calculations analysing if the importance of local conditions is mainly owing to abiotic or biotic conditions. See ‘Material and methods’ for details on the modelling, and the electronic supplementary material, table S4 for similar calculations but with the speed of extinction 1 − SE as the estimate of species vulnerability. Finally, see the electronic supplementary material, tables S5 and S6 for similar calculations but assigning plant species dependencies R based on their floral phenotype and known insect-pollination visitors. **p < 0.001; *p < 0.05; NSp > 0.05.)
| model | AICc | coefficient | s.e. | 95% CI, lower | 95% CI, upper | R2 marginal | R2 conditional |
|---|---|---|---|---|---|---|---|
| order of deletion: removing species with lowest degree | |||||||
| species traits | −10.716 | +0.024NS | +0.027 | −0.035 | +0.083 | 0.01 | 0.79 |
| local conditions | −19.800 | +0.05* | +0.012 | +0.022 | +0.075 | 0.46 | 0.89 |
| full model | −13.987 | 0.44 | 0.90 | ||||
| species traits | +0.008NS | +0.021 | −0.041 | +0.056 | |||
| local conditions | +0.048* | +0.013 | +0.020 | +0.076 | |||
| order of deletion: removing species with highest degree | |||||||
| species traits | −16.367 | −0.008NS | +0.022 | −0.056 | +0.040 | 0.01 | 0.74 |
| local conditions | −16.007 | −0.016NS | +0.014 | −0.041 | +0.020 | 0.04 | 0.70 |
| full model | −10.156 | 0.03 | 0.73 | ||||
| species traits | −0.004NS | +0.022 | −0.055 | +0.046 | |||
| local conditions | −0.010NS | +0.015 | −0.042 | +0.023 | |||
| order of deletion: random removal of species | |||||||
| species traits | −46.526 | +0.009NS | +0.007 | −0.010 | +0.027 | 0.09 | 0.20 |
| local conditions | −57.175 | +0.018** | +0.004 | +0.010 | +0.026 | 0.47 | 0.86 |
| full model | −48.917 | 0.49 | 0.87 | ||||
| species traits | +0.002NS | +0.007 | −0.013 | +0.016 | |||
| local conditions | +0.018** | +0.004 | +0.009 | +0.026 | |||
3. Results
(a). Hummingbird trait evolution
The absolute contrast values of bill length showed a significant negative correlation with distance from the root of the phylogeny (figure 1b; r = −0.87, p < 0.001), indicating higher rates of morphological evolution early in the history of Antillean hummingbirds, which has subsequently slowed towards the present day. Differences in hummingbird bill length appear to have accumulated mainly before the colonization of the Antilles 5–9 Ma (figure 1b). Blomberg's K indicated a greater amount of evolution in bill length than expected under Brownian motion (K = 1.65; p = 0.001); thus, species that share recent evolutionary history are more similar in bill length than expected by chance. Conversely, body mass contrasts were positively but non-significantly correlated with distance from the root of the tree (figure 1c; r = 0.28, p= 0.38). Blomberg's K suggested body mass to be more divergent than expected under Brownian motion; however, this phylogenetic signal was also non-significant upon performing a randomization test (K = 0.41, p = 0.21). These results suggest the lack of a general trend in the evolution of body mass through time among Antillean hummingbirds; however, we note a large recent contrast among congeners of the genus Eulampis, which is endemic to the Antilles (figure 1c).
(b). Hummingbird specialization on nectar-food plants
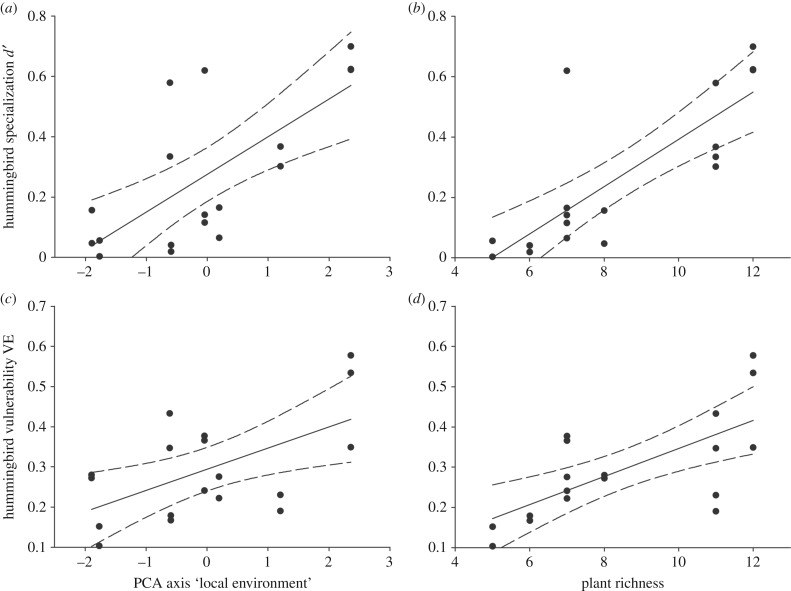
The specialization of hummingbirds on their nectar plants was not significantly associated with hummingbird body mass or bill length (table 1). Instead, hummingbird specialization was strongly associated with the local conditions (table 1), with specialization increasing as a factor of the local abiotic conditions (high topographic complexity, high precipitation and low temperature promoting specialization; electronic supplementary material, table S3; figure 2a) and hummingbird and floral resource richness (electronic supplementary material, table S3; figure 2b).
Table 1.
Linear mixed models, analysing the association between hummingbird resource specialization d′ and two types of predictor models: (i) species traits and (ii) local conditions. (We also performed a full model including both predictors. In all models, we used species identity as a random factor, as for three species the degree of specialization d′ was estimated in more than one network. Note that hummingbird specialization d′ is only associated with the local conditions. See the electronic supplementary material, table S3 for similar calculations analysing if the importance of local conditions is mainly owing to local abiotic or biotic conditions. **p < 0.001; NSp > 0.05.)
| model | AICc | coefficient | s.e. | 95% CI, lower | 95% CI, upper | R2 marginal | R2 conditional |
|---|---|---|---|---|---|---|---|
| species traits | 12.033 | +0.048NS | +0.044 | −0.045 | +0.140 | 0.07 | 0.07 |
| local conditions | −3.211 | +0.12** | +0.022 | +0.074 | +0.168 | 0.64 | 0.73 |
| full model | 1.709 | 0.66 | 0.71 | ||||
| species traits | +0.019NS | +0.029 | −0.046 | +0.084 | |||
| local conditions | +0.118** | +0.023 | +0.069 | +0.167 |
Figure 2.
The association between hummingbird specialization d′ (a,b) and hummingbird vulnerability to plant extinctions VE (c,d) with the two main components of ‘local conditions’, i.e. local abiotic conditions (topography, precipitation and temperature) and local biotic conditions (nectar-food plant richness). High values of the PCA axis ‘local environment’ reflect topographical complex, high precipitation and low temperature localities, i.e. Antillean Mountains [18]. The regression line and ±95% confidence intervals (CIs) are shown. Note that some of the data points represent the same hummingbird species observed in several localities; hence, we conducted linear mixed models with species identity as a random factor (tables 1 and 2).
(c). Hummingbird vulnerability to plant extinctions
The vulnerability of hummingbirds to plant extinctions VE was not associated with the morphological traits analysed: small- and large-bodied/-billed hummingbirds were equally vulnerable (table 2; electronic supplementary material, table S5). Instead, when removing plant species in a ‘realistic extinction scenario’ (iteratively removing lowest degree plant species) and at random, hummingbird vulnerability was strongly associated with the local conditions (table 2): hummingbirds were most vulnerable in conditions characterized by high topographic complexity, high precipitation, low temperature and high floral resource richness (figure 2c,d). The ‘attack tolerance’ of the network (iteratively removing highest degree plant species) showed no association to either hummingbird traits or local conditions. The results were qualitatively similar when vulnerability was modelled using the speed of hummingbird extinctions 1 − SE (electronic supplementary material, tables S4 and S6). All results were also qualitatively consistent, irrespective of whether plant dependencies on hummingbird pollination, R, were assigned randomly or by expert opinion (table 2; electronic supplementary material, tables S4–S6).
4. Discussion
Our results suggest that high rates of divergence in hummingbird bill length primarily occurred before lineages colonized the Antilles approximately 5–9 Ma, while the evolution of body mass differences was more constant through time (figure 1). In correspondence with the limited trait evolution occurring within the Antilles, especially bill length that have often been shown to determine floral niche partitioning [42,55], we found that hummingbird specialization on floral resources and their corresponding vulnerability to plant extinctions were not predicted by their bill length and body mass. Instead, hummingbird resource specialization and vulnerability to plant extinctions were strongly associated with high topographical complexity, more rainfall, cooler temperatures and greater richness of flowers used by hummingbirds (figure 2), conditions which characterize the Antillean Mountains [18,48].
These findings fit well with expectations of taxon cycle theory, which predicts that endemic species found in interior mountains are specialized and vulnerable species, whereas species in the lowlands are more generalized and resilient [15,16]. The repeated structuring of Antillean hummingbird communities into lowland and highland communities—each consisting of one small- and one large-bodied/-billed species—has been suggested to be a consequence of competition, possibly for floral resources [14,56]. Similar patterns have been observed for other pollination systems, such as coexisting bumblebees that differ in proboscis length to minimize competition [57]. For Antillean hummingbirds, there is also recent evidence of competitive exclusion, as the Lesser Antillean species Orthorhyncus cristatus and Eulampis holosericeus have expanded north in recent years displacing the Greater Antillean species Anthracothorax dominicus from many of the Virgin Islands and northeastern Puerto Rico [14,58]. Combined with the apparently limited trait evolution that occurred in the Antilles (figure 1), this suggests that the structuring of hummingbird communities largely reflects an assembly process, possibly driven by competition for the limited nectar-food plant richness observed in the Antilles. The competition for floral resources may have allowed hummingbirds already differentiated in size, i.e. only one small- and one large-bodied/-billed hummingbird species, to enter a given community, thereby resulting in minor evolutionary changes in bill length and body mass among the constituent Antillean hummingbird species. This idea is similar to Janzen's ‘ecological fitting’ hypothesis, whereby species use niches based on the traits they carried with them but which evolved somewhere else [59,60]. The main exception to this general trend was a large divergence in body mass between the members of the genus Eulampis (figure 1c). We suggest that this most likely reflects evolutionary increases in the mass of E. jugularis, a large and highly dimorphic mountain endemic that is highly specialized on Heliconia flowers [9,18]. Conversely, E. holosericeus is a smaller and less dimorphic species feeding on an array of flowers throughout the Lesser Antillean lowlands [18]. This would further underline that the Antillean Mountains have provided optimal conditions for specialized associations between hummingbirds and their nectar-food plants.
Taken together, our results indicate that mountain environments, and the corresponding high richness of flowering plants attracting hummingbird pollinators, have influenced the evolution and maintenance of a highly specialized and vulnerable endemic hummingbird fauna in the Antillean Mountains. These results have implications for the conservation of species engaged in mutualistic associations. Notably, as climate change and anthropogenic activity disrupt mutualistic associations, and cause pollinator and plant extinctions [7,61,62], the montane biota of the Antilles is more susceptible to extinction of mutualistic partners than the biota in the lowlands. The role of mountain environments in sustaining a highly specialized and vulnerable endemic fauna may be a general phenomenon also occurring in other taxa and in a mainland context [29,63]. This underlines that resource specialization and vulnerability is highly context-dependent, and that the local abiotic and biotic conditions should be more integrated into studies predicting resource specialization and vulnerability to extinctions of interaction partners.
Supplementary Material
Acknowledgements
We thank Helle Sørensen for the statistical advice and Bjørn Hermansen for constructing the map in figure 1.
Data accessibility
The raw quantitative hummingbird–plant networks, morphological and phylogentic data can be downloaded at Dryad (http://dx.doi.org/10.5061/dryad.5770gm7) [44]. For each hummingbird–plant network, details on the local abiotic and biotic conditions, i.e. temperature, precipitation, topography and richness of hummingbirds and their nectar-food plants, are presented in the electronic supplementary material, table S1. In the electronic supplementary material, table S1, for each hummingbird species, we also give its body mass, bill length and network-derived estimates of resource specialization d′ and vulnerability VE. Assigned R values for plants based on expert opinion, using knowledge on floral phenotype and known insect visitation [18], are available in the electronic supplementary material, table S2.
Authors' contributions
B.D. designed the study, conducted the mixed models analysis and wrote the manuscript. J.D.K. conducted the phylogenetic analysis. B.I.S. conducted the plant extinction analysis. A.M.M.G. conducted the network analysis. A.C.B., A.M.M.G. and A.T. contributed plant–hummingbird networks. All authors contributed to interpretation of the data and manuscript writing.
Competing interests
We declare we have no competing interests.
Funding
B.D., J.D.K., A.C.B., A.M.M.G. and C.R. thank the Danish National Research Foundation for its support of the Center for Macroecology, Evolution and Climate (grant no. DNRF96). P.K.M. thanks the São Paulo Research Foundation (FAPESP) for the postdoctoral grant (grant no. 2015/21457-4). B.I.S. was supported by the Natural Environment Research Council as part of the Cambridge Earth System Science NERC DTP (NE/L002507/1).
References
- 1.Darwin C. 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London, UK: John Murray. [PMC free article] [PubMed] [Google Scholar]
- 2.Montoya JM, Pimm SL, Solé RV. 2006. Ecological networks and their fragility. Nature 442, 259–264. ( 10.1038/nature04927) [DOI] [PubMed] [Google Scholar]
- 3.Maglianesi MA, Blüthgen N, Böhning-Gaese K, Schleuning M. 2014. Morphological traits determine specialization and resource use in plant–hummingbird networks in the neotropics. Ecology 95, 3325–3334. ( 10.1890/13-2261.1) [DOI] [Google Scholar]
- 4.Dehling DM, Jordano P, Schaefer HM, Böhning-Gaese K, Schleuning M. 2016. Morphology predicts species' functional roles and their degree of specialization in plant–frugivore interactions. Proc. R. Soc. B 283, 20152444 ( 10.1098/rspb.2015.2444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Memmott J, Waser NM, Price MV. 2004. Tolerance of pollination networks to species extinctions. Proc. R. Soc. B 271, 2605–2611. ( 10.1098/rspb.2004.2909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maruyama PK, et al. 2016. The integration of alien plants in mutualistic plant–hummingbird networks across the Americas: the importance of species traits and insularity. Divers. Distrib. 22, 672–681. ( 10.1111/ddi.12434) [DOI] [Google Scholar]
- 7.Schleuning M, et al. 2016. Ecological networks are more sensitive to plant than to animal extinction under climate change. Nat. Comm. 7, 13965 ( 10.1038/ncomms13965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tinoco BA, Graham CH, Aguilar JM, Schleuning M. 2017. Effects of hummingbird morphology on specialization in pollination networks vary with resource availability. Oikos 126, 52–60. ( 10.1111/oik.02998) [DOI] [Google Scholar]
- 9.Temeles EJ, Kress J. 2003. Adaptation in a plant-hummingbird association. Science 300, 630–633. ( 10.1126/science.1080003) [DOI] [PubMed] [Google Scholar]
- 10.Grant PR, Grant BR. 2006. Evolution of character displacement in Darwin's finches. Science 313, 224–226. ( 10.1126/science.1128374) [DOI] [PubMed] [Google Scholar]
- 11.Olesen JM, Eskildsen LI, Venkatasamy S. 2002. Invasion of pollination networks on oceanic islands: importance of invader complexes and endemic super generalists. Divers. Distrib. 8, 181–192. ( 10.1046/j.1472-4642.2002.00148.x) [DOI] [Google Scholar]
- 12.Olesen JM, Valido A. 2003. Lizards as pollinators and seed dispersers: an island phenomenon. Trends Ecol. Evol. 18, 177–181. ( 10.1016/S0169-5347(03)00004-1) [DOI] [Google Scholar]
- 13.Dalsgaard B, Baquero AC, Rahbek C, Olesen JM, Wiley JW. 2016. Speciose opportunistic nectar-feeding avifauna in Cuba and its association to hummingbird island biogeography. J. Ornithol. 157, 627–634. ( 10.1007/s10336-016-1326-6) [DOI] [Google Scholar]
- 14.Lack D. 1973. The number of species of hummingbirds in the West Indies. Evolution 27, 326–337. ( 10.2307/2406972) [DOI] [PubMed] [Google Scholar]
- 15.Ricklefs RE, Cox GW. 1972. Taxon cycles in the West Indian avifauna. Am. Nat. 106, 195–215. ( 10.1086/282762) [DOI] [Google Scholar]
- 16.Wilson EO. 1961. The nature of the taxon cycle in the Melanesian ant fauna. Am. Nat. 95, 169–193. ( 10.1086/282174) [DOI] [Google Scholar]
- 17.Ricklefs RE, Bermingham E. 2002. The concept of the taxon cycle in biogeography. Glob. Ecol. Biogeogr. 11, 353–361. ( 10.1046/j.1466-822x.2002.00300.x) [DOI] [Google Scholar]
- 18.Dalsgaard B, Martín González AM, Olesen JM, Ollerton J, Timmermann A, Andersen LH, Tossas AG. 2009. Plant-hummingbird interactions in the West Indies: floral specialisation gradients associated with environment and hummingbird size Oecologia 159, 757–766. ( 10.2307/40309943) [DOI] [PubMed] [Google Scholar]
- 19.Johnson TH, Stattersfield AJ. 1990. A global review of island endemic birds. Ibis 132, 167–180. ( 10.1111/j.1474-919X.1990.tb01036.x) [DOI] [Google Scholar]
- 20.Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ. 2004. Avian extinction and mammalian introductions on oceanic islands. Science 305, 1955–1958. ( 10.1126/science.1101617) [DOI] [PubMed] [Google Scholar]
- 21.Kaiser-Bunbury CN, Traveset A, Hansen DM. 2010. Conservation and restoration of plant–animal mutualisms on oceanic islands. Perspect. Plant Ecol. Evol. Syst. 12, 131–143. ( 10.1016/j.ppees.2009.10.002) [DOI] [Google Scholar]
- 22.Anderson SH, Kelly D, Ladley JJ, Molloy S, Terry J. 2011. Cascading effects of bird functional extinction reduce pollination and plant density. Science 331, 1068–1071. ( 10.1126/science.1199092) [DOI] [PubMed] [Google Scholar]
- 23.Kaiser-Bunbury CN, Muff S, Memmott J, Müller CB, Caflisch A. 2010. The robustness of pollination networks to the loss of species and interactions: a quantitative approach incorporating pollinator behavior. Ecol. Lett. 13, 442–452. ( 10.1111/j.1461-0248.2009.01437.x) [DOI] [PubMed] [Google Scholar]
- 24.Dáttilo W, et al. 2016. Unravelling Darwin's entangled bank: architecture and robustness of mutualistic networks with multiple interaction types. Proc. R. Soc. B 283, 20161564 ( 10.1098/rspb.2016.1564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feinsinger P, Colwell RK. 1978. Community organization among neotropical nectar-feeding birds. Am. Zool. 18, 779–796. ( 10.1093/icb/18.4.779) [DOI] [Google Scholar]
- 26.Stiles FG. 1978. Ecological and evolutionary implications of bird pollination. Am. Zool. 18, 715–727. ( 10.1093/icb/18.4.715) [DOI] [Google Scholar]
- 27.Dalsgaard B, et al. 2011. Specialization in plant-hummingbird networks is associated with species richness, contemporary precipitation and quaternary climate-change velocity. PLoS ONE 6, e25891 ( 10.1371/journal.pone.0025891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martín González AM, et al. 2015. The macroecology of phylogenetically structured hummingbird-plant networks. Glob. Ecol. Biogeogr. 24, 1212–1224. ( 10.1111/geb.12355) [DOI] [Google Scholar]
- 29.Sonne J, et al. 2016. High proportion of smaller ranged hummingbird species coincides with ecological specialization across the Americas. Proc. R. Soc. B 283, 20152512 ( 10.1098/rspb.2015.2512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brosi BJ, Briggs HM. 2013. Single pollinator species losses reduce floral fidelity and plant reproductive function. Proc. Natl Acad. Sci. USA 110, 13 044–13 048. ( 10.1073/pnas.1307438110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olesen JM, Jain SK. 1994. Fragmented plant populations and their lost interactions. In Conservation genetics (eds Loeschcke V, Tomiuk J, Jain SK), pp. 417–426. Basel, Switzerland: Birkhäuser. [Google Scholar]
- 32.Bond WJ. 1995. Assessing the risk of plant extinction due to pollinator and disperser failure. In Extinction rates (eds Lawton JH, May RM.), pp. 131–146. Oxford, UK: Oxford University Press. [Google Scholar]
- 33.Abrahamczyk S, Souto-Vilarós D, McGuire JA, Renner SS. 2015. Diversity and clade ages of West Indian hummingbirds and the largest plant clades dependent on them: a 5–9 Myr young mutualistic system. Biol. J. Linn. Soc. 114, 848–859. ( 10.1111/bij.12476) [DOI] [Google Scholar]
- 34.McGuire JA, Witt CC, Remsen JV Jr, Corl A, Rabosky DL, Altshuler DL, Dudley R. 2014. Molecular phylogenetics and the diversification of hummingbirds. Curr. Biol. 24, 910–916. ( 10.1016/j.cub.2014.03.016) [DOI] [PubMed] [Google Scholar]
- 35.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15. ( 10.1086/284325) [DOI] [Google Scholar]
- 36.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 37.Revell LJ. 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–3223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 38.R Development Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 39.Vázquez DP, Morris WF, Jordano P. 2005. Interaction frequency as a surrogate for the total effect of animal mutualists on plants. Ecol. Lett. 8, 1088–1094. ( 10.1111/j.1461-0248.2005.00810.x) [DOI] [Google Scholar]
- 40.Blüthgen N, Menzel F, Blüthgen N. 2006. Measuring specialization in species interaction networks. BMC Ecol. 6, 9 ( 10.1186/1472-6785-6-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fründ J, McCann KS, Williams NM. 2015. Sampling bias is a challenge for quantifying specialization and network structure: lessons from a quantitative niche model. Oikos 125, 502–513. ( 10.1111/oik.02256) [DOI] [Google Scholar]
- 42.Vizentin-Bugoni J, Maruyama PK, Debastiani VJ, Duarte LS, Dalsgaard B, Sazima M. 2016. Influences of sampling effort on detected patterns and structuring processes of a Neotropical plant-hummingbird network. J. Anim. Ecol. 85, 262–272. ( 10.1111/1365-2656.12459) [DOI] [PubMed] [Google Scholar]
- 43.Dalsgaard B, et al. 2017. Opposed latitudinal patterns of network-derived and dietary specialization in avian plant-frugivore interaction systems. Ecography 40, 1395–1401. ( 10.1111/ecog.02604) [DOI] [Google Scholar]
- 44.Dalsgaard B, et al. 2018. Data from: Trait evolution, resource specialisation and vulnerability to plant extinctions among Antillean hummingbirds Dryad Digital Repository. ( 10.5061/dryad.5770gm7) [DOI] [PMC free article] [PubMed]
- 45.Dormann CF, Gruber B, Fründ J. 2008. Introducing the bipartite package: analysing ecological networks. R News 8, 8–11. [Google Scholar]
- 46.McCune B, Mefford MJ. 2006. PC-ORD. Multivariate analysis of ecological data. Version 5.10. Gleneden Beach, OR: MjM Software. [Google Scholar]
- 47.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 48.Martín González AM, Dalsgaard B, Ollerton J, Timmermann A, Olesen JM, Andersen L, Tossas AG. 2009. The effect of climate on pollination networks in the West Indies. J. Trop. Ecol. 25, 493–506. ( 10.1017/S0266467409990034) [DOI] [Google Scholar]
- 49.Rangel TF, Diniz-Filho JAF, Bini LM. 2010. SAM: a comprehensive application for spatial analysis in macroecology. Ecography 33, 46–50. ( 10.1111/j.1600-0587.2009.06299.x) [DOI] [Google Scholar]
- 50.IBM Corp. Released. 2013. IBM SPSS statistics for windows, version 22.0. Armonk, NY: IBM Corp. [Google Scholar]
- 51.Vieira MC, Almeida-Neto M. 2015. A simple stochastic model for complex coextinctions in mutualistic networks: robustness decreases with connectance. Ecol. Lett. 18, 144–152. ( 10.1111/ele.12394) [DOI] [PubMed] [Google Scholar]
- 52.CaraDonna PJ, Petry WK, Brennan RM, Ginnningham JL, Bronstein JL, Waser NM, Sanders NJ. 2017. Interaction rewiring and the rapid turnover of plant-pollinator networks. Ecol. Lett. 20, 385–394. ( 10.1111/ele.12740) [DOI] [PubMed] [Google Scholar]
- 53.Solé RV, Montoya JM. 2001. Complexity and fragility in ecological networks. Proc. R. Soc. Lond. B 268, 2039–2045. ( 10.1098/rspb.2001.1767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunne JA, Williams RJ, Martinez ND. 2002. Network structure and biodiversity loss in food webs: robustness increases with connectance. Ecol. Lett. 5, 558–567. ( 10.1046/j.1461-0248.2002.00354.x) [DOI] [Google Scholar]
- 55.Weinstein BG, Graham CH. 2017. Persistent bill and corolla matching despite shifting temporal resources in tropical hummingbird-plant interactions. Ecol. Lett. 20, 326–335. ( 10.1111/ele.12730) [DOI] [PubMed] [Google Scholar]
- 56.Brown JH, Bowers MA. 1985. Community organization in hummingbirds: relationship between morphology and ecology. Auk 102, 251–269. ( 10.2307/4086767) [DOI] [Google Scholar]
- 57.Pyke G. 1982. Local geographic distributions of bumblebees near Crested Butte, Colorado: competition and community structure. Ecology 63, 555–573. ( 10.2307/1938970) [DOI] [PubMed] [Google Scholar]
- 58.BirdLife International. 2008. Important bird areas in the Caribbean: key sites for conservation. Cambridge, UK: Birdlife International (BirdLife Conservation Series No. 15). [Google Scholar]
- 59.Janzen DH. 1985. On ecological fitting. Oikos 45, 308–310. ( 10.2307/3565565) [DOI] [Google Scholar]
- 60.Agosta SJ, Klemens JA. 2008. Ecological fitting by phenotypically flexible genes: implications for species associations, community assembly and evolution. Ecol. Lett. 11, 1123–1134. ( 10.1111/j.1461-0248.2008.01237.x) [DOI] [PubMed] [Google Scholar]
- 61.Ollerton J, Erenler H, Edwards M, Crockett R. 2014. Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 346, 1360–1362. ( 10.1126/science.1257259) [DOI] [PubMed] [Google Scholar]
- 62.Sebastián-González E, Dalsgaard B, Sandel B, Guimarães PR. 2015. Macroecological trends in nestedness and modularity of seed-dispersal networks: human impact matters. Glob. Ecol. Biogeogr. 24, 293–303. ( 10.1111/geb.12270) [DOI] [Google Scholar]
- 63.Graves G. 1985. Elevational correlates of speciation and intraspecific geographic variation in plumage in Andean forest birds. Auk 102, 556–579. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Dalsgaard B, et al. 2018. Data from: Trait evolution, resource specialisation and vulnerability to plant extinctions among Antillean hummingbirds Dryad Digital Repository. ( 10.5061/dryad.5770gm7) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The raw quantitative hummingbird–plant networks, morphological and phylogentic data can be downloaded at Dryad (http://dx.doi.org/10.5061/dryad.5770gm7) [44]. For each hummingbird–plant network, details on the local abiotic and biotic conditions, i.e. temperature, precipitation, topography and richness of hummingbirds and their nectar-food plants, are presented in the electronic supplementary material, table S1. In the electronic supplementary material, table S1, for each hummingbird species, we also give its body mass, bill length and network-derived estimates of resource specialization d′ and vulnerability VE. Assigned R values for plants based on expert opinion, using knowledge on floral phenotype and known insect visitation [18], are available in the electronic supplementary material, table S2.