Abstract
Competition plays a crucial role in determining adaptation of species, yet we know little as to how adaptation is affected by the strength of competition. On the one hand, strong competition typically results in population size reductions, which can hamper adaptation owing to a shortage of beneficial mutations; on the other hand, specificity of adaptation to competitors may offset the negative evolutionary consequences of such population size effects. Here, we investigate how competition strength affects population fitness in the bacterium Pseudomonas fluorescens. Our results demonstrate that strong competition constrains adaptation of focal populations, which can be partially explained by population size reductions. However, fitness assays also reveal specific adaptation of focal populations to particular competitors varying in competitive ability. Additionally, this specific adaptation can offset the negative effects of competitor-mediated population size reductions under strong competition. Our study, therefore, highlights the importance of opposing effects of strong competition on species adaptation, which may lead to different outcomes of colonization under intense and relaxed competitive environments in the context of population dispersal.
Keywords: adaptation, competition strength, population size reduction, Pseudomonas fluorescens
1. Introduction
The extent to which populations adapt is dependent on ecological and evolutionary interactions with other species or populations [1–9]. One such ubiquitous interaction is competition. Between-population competition is predicted to constrain adaptation by reducing population sizes [10,11], and this adaptive constraint should increase with the strength of competition. Competition can also have more nuanced evolutionary consequences, owing to possible trade-offs between biotic and abiotic adaptations [1,2,10,12] and specificity in adaptation to particular competitors [13,14]. For example, character displacement, and the underlying niche differentiation, is a competitor-specific adaptation [14–18]. All things being equal, the negative effects of competitors on adaptation are likely to increase with competition strength. However, costs of evolving in the presence of strong competitors may be offset during colonization of new habitats. Most organisms commonly encounter biotically stressful environments when colonizing new habitats [13,19]; hence, a history of evolving with strong competitors may confer an advantage following colonization as a consequence, for example, of pre-adaptation for exploitation of low-productivity resources or reduced resource requirement for basic life activities. We, therefore, hypothesize that in competition-intense environments, specific pre-adaptation to strong competitors may offset the negative evolutionary consequence of competition-imposed population size reductions.
To experimentally determine how competitor's superiority affects adaptation, we used rapidly evolving in vitro populations of the bacterium Pseudomonas fluorescens SBW25 [20]. Pseudomonas fluorescens populations have been used extensively as a model system in evolutionary ecology, because of their propensity to rapidly adapt to both abiotic conditions [21], including genetically diversifying into morphologically distinct spatial niche specialists in spatially heterogeneous environments [15], and biotic conditions (e.g. bacteriophage [22], intra- and interspecific competitors [23]). We first allowed focal populations to pre-adapt to environments with no, inferior, equivalent or superior competitors, and then measured each focal population's performance against all competitor types, using a full factorial design. To avoid our results being affected by potentially nuanced adaptation to different species, all competitors (including the inferior, equivalent and superior competitors) were derived from the same ancestor of P. fluorescens SBW25. We aimed to answer three specific questions: (i) do focal populations specifically adapt to competitors varying in their competitive ability? (ii) is population size a good predictor of adaptive potential as focal populations experience different strength of competition? and (iii) can the specific adaptation offset the negative evolutionary consequences of competitor-imposed population size reductions?
2. Material and methods
(a). Strains and culture conditions
The bacterium P. fluorescens SBW25 [20] and an isogenic strain modified by integrating a lacZ gene and a gentamicin resistance cassette [24] were used to set up competitor and focal populations in our experiment, respectively. The presence of a lacZ gene results in blue colonies when culture is plated on agar plates supplemented with X-gal, allowing for easy distinction between focal and competitor colony forming units, and the gentamicin resistance allows for easy isolation of focal populations. Populations were incubated statically in 30 ml glass universal vials containing 6 ml of standard King's medium B (KB) at 28°C.
(b). Selection experiment
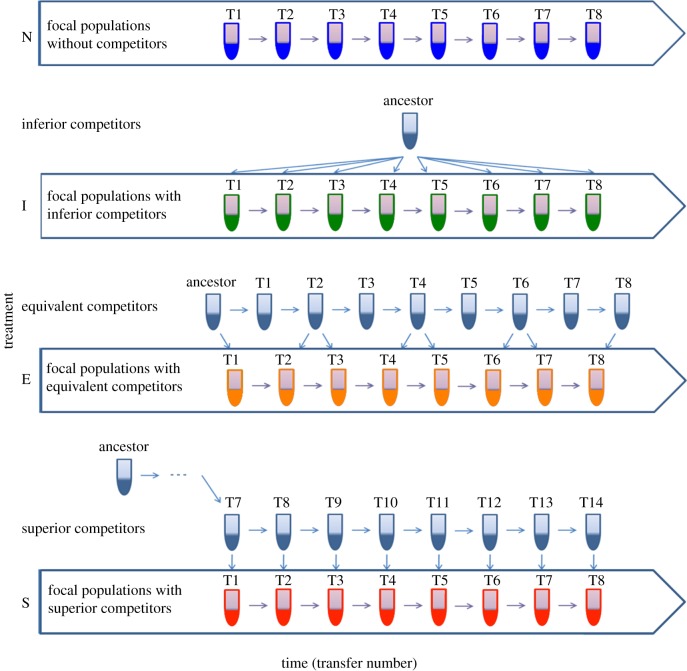
We imposed four different selection regimes on focal populations by evolving these populations with no (N), inferior (I), equivalent (E) and superior (S) competitors (figure 1). Each microcosm was established by inoculating approximately 108 cells of the lacZ marked SBW25 strain in the presence or absence of approximately 108 cells of the appropriate unmarked competitor (see below). Each microcosm was incubated statically at 28°C for 48 h, after which the densities of the focal and competitor populations were measured by plating serial diluted cultures onto KB agar plates supplemented with X-gal. Focal populations were isolated by plating 100 µl of 10−5-diluted cultures onto KB agar plates supplemented with X-gal and gentamicin (15 µl ml−1); the latter was used to select against the competitor. Following incubation, all blue colonies (approx. 1000) were scraped, mixed, inoculated into fresh medium and grown overnight in KB microcosms. One per cent (60 µl) of focal population cultures was then re-inoculated into fresh microcosms together with the same volume of the treatment-specific competitor cultures (figure 1). This cycle was continued for eight transfers, and samples of isolated focal populations were stored at −80°C every two transfers. There were 12 replicates in each treatment, yielding 48 microcosms in total.
Figure 1.
A graphic illustration of the design of selection experiment, where focal populations evolved for eight transfers with no (N), inferior (I), equivalent (E) or superior (S) competitors. Each treatment consisted of 12 replicate microcosms. (Online version in colour.)
The ancestor of P. fluorescens SBW25 was used as inferior competitors in treatment I. Specifically, the competitor was grown from the ancestral freezer stock for each transfer. For treatment E, we used the ancestor as equivalent competitors for the first transfer, and for subsequent transfers we used competitors that had evolved for approximately the same amount of time as the focal populations (figure 1). These competitor populations were evolved by transferring 1% of culture into fresh microcosms every 48 h, with samples frozen at −80°C in 20% (v: v) glycerol : culture solution. Preliminary experiments revealed that equivalent competitors typically had marginally higher fitness than focal populations from the same transfer, because of their greater population size. To offset this effect we used only competitors from even-numbered transfers, resulting in the equivalent competitors coming from either the same (n) transfer as the focal population, or transfer n − 1. For treatment S we used competitors that had an evolutionary head-start over the focal populations, having evolved for six transfers (approximately 40 generations) (figure 1). Preliminary work also showed that this six-transfer head-start resulted in superior competitors having a higher fitness than the focal population. Note that the aim of continually reintroducing competitors at each transfer was to ensure the maintenance of our treatment effects throughout the experiment, as well as to prevent potential extinction of the focal strain in the presence of the superior competitor.
(c). Measuring adaptation of focal populations
To obtain a proxy of adaptive ability, we measured the fitness of all focal populations from the final transfer relative to that of three reference populations: inferior, equivalent and superior competitors of the final transfer of the selection experiment. In order to measure adaptation of focal populations to competition strength, we pooled all 12 treatment-specific replicates of the competitor populations at equal ratios to obtain a mixed culture as a reference population. This pooling was done because when determining fitness with respect to competitors from different treatments, there is no pairing between competitors and focal populations.
The focal and reference populations were grown overnight in shaken microcosms separately, after which 1% of both cultures were mixed, inoculated into fresh cultures and incubated statically for 48 h, with initial and final densities of both focal and reference populations measured on X-gal supplemented KB agar plates. The relative fitness (W) was estimated as the ratio of the estimated Malthusian growth rate (m) of the focal and reference populations: W = mfocal/mcompetitor, m = ln (Ni/Nf), where Ni is the initial density and Nf is the final density [25].
(d). Statistical analyses
Population densities were log10-transformed in all subsequent statistical analyses. To test how variation in competitive ability affects focal population sizes, we used a one-way analysis of variance (ANOVA) on mean population size of each focal population (averaged across time), with selection regime (i.e. N, I, E, S) as an explanatory variable. The relative fitness of focal populations was analysed using a two-way ANOVA, with selection regime and reference population (competitors I, E, S) as explanatory variables. To investigate the relationship between population size and adaptation of focal populations, we carried out an analysis of covariance (ANCOVA) with relative fitness of focal populations as response variable; selection regime and reference population as explanatory variables, and mean focal population size (averaged across time) as a covariate. Model simplification was performed by removing non-significant terms sequentially from higher to lower-order interaction effects. Tukey's HSD multiple comparisons were also carried out for significant treatment effects. All statistical tests were carried out in the R environment [26].
3. Results
(a). The strength of competition endured by focal populations
As expected, focal populations with differential evolutionary backgrounds varied in their population size as a function of competitor type (one-way ANOVA: F3,44 = 49.420, p < 0.001). Specifically, the presence of inferior competitors did not cause a significant reduction in population sizes of focal populations, while the equivalent and superior competitors did so, with the latter showing the largest effect (figure 2).
Figure 2.
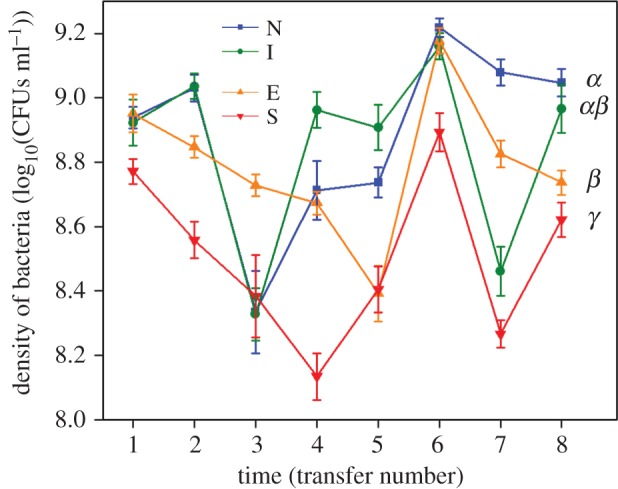
Mean bacterial cell densities ± s.e.m., where focal populations evolved with no competitors (N: blue squares), inferior competitors (I: green circles), equivalent competitors (E: orange triangles), or superior competitors (S: red inverted triangles). Different letters denote significant between-treatment differences in mean focal population densities over time (Tukey multiple comparison, padj < 0.05). (Online version in colour.)
(b). Adaptation of focal populations
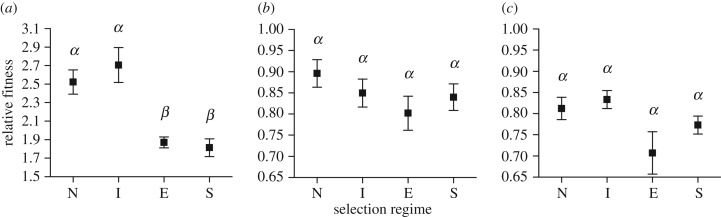
The distinct competitive abilities of competitors resulted in different magnitudes of adaptation of focal populations (two-way ANOVA: selection regime, F3,132 = 15.057, p < 0.001; electronic supplementary material, table S1; figure 3). Crucially, the degree to which competitors hampered adaptation depended on which reference populations were employed for the fitness assays (two-way ANOVA: selection regime × reference populations, F6,132 = 9.456, p < 0.001; electronic supplementary material, table S1). This significant interaction effect suggested that there was specificity of adaptation between the focal populations and competitors. Specifically, focal populations that had evolved in the presence of the equivalent or superior competitors overall showed lower fitness relative to focal populations in the other two treatments (electronic supplementary material, table S2) because of their reduced population sizes (see section (c) in Results); but they acquired additional adaptations specific to the equivalent and superior competitors, resulting in non-significant difference in fitness between the focal populations from different treatments when fitness was measured in competition with the equivalent and superior competitors (figure 3).
Figure 3.
The relative fitness of focal populations when competing with the following reference populations: (a) inferior competitors, (b) equivalent competitors, and (c) superior competitors. The x-axis titles indicate selection regimes (i.e. focal populations evolving with no (N), inferior (I), equivalent (E), and superior (S) competitors). Note the difference in y-axis value ranges. Within each panel, different letters denote significant differences in the relative fitness of focal populations between treatments (Tukey multiple comparison, padj < 0.05).
(c). The relationships between population size and adaptation
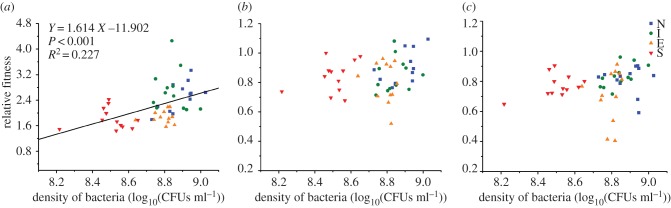
We determined whether these differences in adaptation could be explained by the population sizes of focal populations, and found that relative fitness of focal populations was positively correlated with population size (ANCOVA: population size, F1,43 = 21.951, p < 0.001; electronic supplementary material, table S3; figure 4). However, the reference populations used to measure relative fitness affected the relationships between focal population fitness and their population sizes (ANCOVA: population size × reference population, F2,129 = 13.687, p < 0.001; electronic supplementary material, table S3; figure 5). There was a positive relationship between population size and relative fitness only when inferior competitors were used as reference populations (figure 5).
Figure 4.

The relationship between the extent of adaptation (the relative fitness averaged across the three reference populations) and population size (averaged over time) of focal populations. The symbols represent different treatments, where focal populations evolved with no competitors (N: blue squares), with inferior competitors (I: green circles), with equivalent competitors (E: orange triangles), or with superior competitors (S: red inverted triangles). The regression line is based on data pooled across the four treatments. (Online version in colour.)
Figure 5.
The relationship between the extent of adaptation (the relative fitness measured by the inferior competitors (a), equivalent competitors (b), and superior competitors (c) as reference populations) and population size (averaged over time) of focal populations. The symbols represent different treatments, where focal populations evolved with no competitors (N: blue squares), with inferior competitors (I: green circles), with equivalent competitors (E: orange triangles), or with superior competitors (S: red inverted triangles). Significant relationships are depicted by regression lines. Note the difference in y-axis value ranges. (Online version in colour.)
After accounting for population size effects, selection regime remained significant in determining focal population fitness (ANCOVA: selection regime, F3,129 = 8.293, p < 0.001; electronic supplementary material, table S3). However, only the relative fitness of focal populations that had evolved in competition with equivalent competitors was significantly lower than that of all others (Tukey multiple comparison; E versus N, I and S; all padj < 0.05; electronic supplementary material, table S4).
4. Discussion
Populations frequently compete with other species or populations for resources, which can greatly affect their potential of adaptation [1,8,11,27]. Consistent with theory, we showed that a competitor-mediated reduction in population size was an important determinant of adaptation [10,11]. However, we also found evidence for specificity of adaptation with respect to the strength of competition, resulting in no net effect of competition strength on adaptation when focal populations competed against superior or equally fit competitors during the fitness assays.
Specificity of adaptation between P. fluorescens SBW25 populations has been reported before. SBW25 rapidly evolves into morphologically distinct niche specialists, and adaptation of these niche specialists has been shown to be specific to the time points from which genotypes were isolated [23]. Moreover, the evolutionary diversification of SBW25 is greatly influenced by what genotypes have pre-occupied the habitat [28,29]. The specificity of adaptation observed here may be linked to adaptation to evolved niche specialists, but equally there are many general reasons to expect specific adaptation to the strength of competition in general, including the use of less productive resources and life-history changes to minimize competition [30]. Such competition-specific adaptation may be of greater magnitude if competing against more ecologically distinct competitors than used here (e.g. different species), as this could allow further opportunities for character displacement [11].
Despite the specificity of adaptation, increasing competition strength reduced mean levels of adaptation when measured against multiple competitors, presumably because stronger competitors reduced population sizes and hence the supply of beneficial mutations [10,11]. Once such population size reductions were controlled for, adaptation was significantly lower in populations that had evolved against equivalent competitors compared to either superior or inferior competitors. We do not know why this is, but speculate that the equivalent competitors are probably ecologically most similar to the focal populations given the repeatability of morphological and genomic evolution in this system [31,32]. This may have minimized opportunities to evolve to reduce competition [11], ultimately impeding the fixation of beneficial mutations in focal populations through clonal interference [33].
Our results have important implications for predicting the performance of focal populations in the context of migration. In competition-intense scenarios, the prospects of successful colonization are similar for focal species, independent of the strength of previous competition. However, when competition is relaxed, for instance when focal species colonize vacant habitats after disturbances such as fire, volcanic eruption, and antibiotic treatment [34–37], the strength of competition a focal species previously experienced does matter, with focal species from weak competition backgrounds being more likely to colonize successfully than those from strong competition backgrounds.
In summary, our results suggest that the intense competition may generally have a negative effect on the adaptation of focal species, partly owing to competitor-imposed population size reductions. These results are consistent with previous theoretical studies [10,11], suggesting the mechanism to be quite general. One caveat is that if recombination occurs between competitors—either via sexual reproduction or horizontal gene transfer—superior competitors could enhance mean rates of adaptation. We also found that the strength of competition can drive specific adaptation of focal species, and weaken the relationship between fitness gains and population size. Thus, the evolutionary consequences of competition on a focal species' adaptation should be carefully considered in the context of migration.
Supplementary Material
Supplementary Material
Data accessibility
Datasets supporting this article are uploaded to the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.t0rf6 [38].
Authors' contributions
All authors conceived and designed the study. X.F.Z conducted the experiment, analysed the data and drafted the manuscript, under the guidance of the other authors.
Competing interests
We declare we have no competing interests.
Funding
The work was funded by NERC, BBSRC and the AXA Research Fund. A.B. is supported by the Royal Society. Q.G.Z. is supported by the National Natural Science Foundation of China (31670376 and 31725006).
References
- 1.Collins S. 2010. Competition limits adaptation and productivity in a photosynthetic alga at elevated CO2. Proc. R. Soc. B 278, 247–255. ( 10.1098/rspb.2010.1173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence D, Fiegna F, Behrends V, Bundy JG, Phillimore AB, Bell T, Barraclough TG. 2012. Species interactions alter evolutionary responses to a novel environment. PLoS Biol. 10, e1001330 ( 10.1371/journal.pbio.1001330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer JR, Kassen R. 2007. The effects of competition and predation on diversification in a model adaptive radiation. Nature 446, 432–435. ( 10.1038/nature05599) [DOI] [PubMed] [Google Scholar]
- 4.Northfield TD, Ives AR. 2013. Coevolution and the effects of climate change on interacting species. PLoS Biol. 11, e1001685 ( 10.1371/journal.pbio.1001685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson JN. 2013. Relentless evolution. Chicago, IL: University Chicago Press. [Google Scholar]
- 6.Tomiolo S, van der Putten WH, Tielboerger K. 2015. Separating the role of biotic interactions and climate in determining adaptive response of plants to climate change. Ecology 96, 1298–1308. ( 10.1890/14-1445.1) [DOI] [PubMed] [Google Scholar]
- 7.Zhang QG, Buckling A. 2011. Antagonistic coevolution limits population persistence of a virus in a thermally deteriorating environment. Ecol. Lett. 14, 282–288. ( 10.1111/j.1461-0248.2010.01586.x) [DOI] [PubMed] [Google Scholar]
- 8.Gifford DR, Toll-Riera M, Kojadinovic M, MacLean RC. 2015. Here's to the losers: evolvable residents accelerate the evolution of high-fitness invaders. Am. Nat. 186, 41–49. ( 10.1086/681598) [DOI] [PubMed] [Google Scholar]
- 9.Barraclough TG. 2015. How do species interactions affect evolutionary dynamics across whole communities? Annu. Rev. Ecol. Evol. Syst. 46, 25–48. ( 10.1146/annurev-ecolsys-112414-054030) [DOI] [Google Scholar]
- 10.Johansson J. 2008. Evolutionary responses to environmental changes: how does competition affect adaptation? Evolution 62, 421–435. ( 10.1111/j.1558-5646.2007.00301.x) [DOI] [PubMed] [Google Scholar]
- 11.Osmond MM, De Mazancourt C. 2012. How competition affects evolutionary rescue. Phil. Trans. R. Soc. B 368, 20120085 ( 10.1098/rstb.2012.0085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Mazancourt C, Johnson E, Barraclough TG. 2008. Biodiversity inhibits species' evolutionary responses to changing environments. Ecol. Lett. 11, 380–388. ( 10.1111/j.1461-0248.2008.01152.x) [DOI] [PubMed] [Google Scholar]
- 13.Grady KC, Wood TE, Kolb TE, Hersch-Green E, Shuster SM, Gehring CA, Hart SC, Allan GJ, Whitham TG. 2017. Local biotic adaptation of trees and shrubs to plant neighbors. Oikos 126, 583–593. ( 10.1111/oik.03240) [DOI] [Google Scholar]
- 14.Grant PR, Grant BR. 2006. Evolution of character displacement in Darwin's finches. Science 313, 224–226. ( 10.1126/science.1128374) [DOI] [PubMed] [Google Scholar]
- 15.Rainey PB, Travisano M. 1998. Adaptive radiation in a heterogeneous environment. Nature 394, 69–72. ( 10.1038/27900) [DOI] [PubMed] [Google Scholar]
- 16.Craig MR, Dickson A, Bell G. 2005. Resource competition and adaptive radiation in a microbial microcosm. Ecol. Lett. 8, 38–46. ( 10.1111/j.1461-0248.2004.00689.x) [DOI] [Google Scholar]
- 17.Schluter D, McPhail JD. 1992. Ecological character displacement and speciation in sticklebacks. Am. Nat. 140, 85–108. ( 10.1086/285404) [DOI] [PubMed] [Google Scholar]
- 18.Kooyers NJ, James B, Blackman BK. 2017. Competition drives trait evolution and character displacement between Mimulus species along an environmental gradient. Evolution 71, 1205–1221. ( 10.1111/evo.13200) [DOI] [PubMed] [Google Scholar]
- 19.Leopold DR, Tanentzap AJ, Lee WG, Heenan PB, Fukami T. 2015. Evolutionary priority effects in New Zealand alpine plants across environmental gradients. J. Biogeogr. 42, 729–737. ( 10.1111/jbi.12441) [DOI] [Google Scholar]
- 20.Rainey PB, Bailey MJ. 1996. Physical and genetic map of the Pseudomonas fluorescens SBW25 chromosome. Mol. Microbiol. 19, 521–533. ( 10.1046/j.1365-2958.1996.391926.x) [DOI] [PubMed] [Google Scholar]
- 21.Barrett RDH, MacLean RC, Bell G. 2005. Experimental evolution of Pseudomonas fluorescens in simple and complex environments. Am. Nat. 166, 470–480. ( 10.1086/444440) [DOI] [PubMed] [Google Scholar]
- 22.Buckling A, Rainey PB. 2002. Antagonistic coevolution between a bacterium and a bacteriophage. Proc. R. Soc. Lond. B 269, 931–936. ( 10.1098/rspb.2001.1945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang QG, Buckling A, Ellis RJ, Godfray HCJ. 2009. Coevolution between cooperators and cheats in a microbial system. Evolution 63, 2248–2256. ( 10.1111/j.1558-5646.2009.00708.x) [DOI] [PubMed] [Google Scholar]
- 24.Pal C, Macia MD, Oliver A, Schachar I, Buckling A. 2007. Coevolution with viruses drives the evolution of bacterial mutation rates. Nature 450, 1079–1081. ( 10.1038/nature06350) [DOI] [PubMed] [Google Scholar]
- 25.Lenski RE, Rose MR, Simpson SC, Tadler SC. 1991. Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. Am. Nat. 138, 1315–1341. ( 10.1086/285289) [DOI] [Google Scholar]
- 26.R Development Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 27.Bailey SF, Dettman JR, Rainey PB, Kassen R. 2013. Competition both drives and impedes diversification in a model adaptive radiation. Proc. R. Soc. B 280, 20131253 ( 10.1098/rspb.2013.1253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brockhurst MA, Colegrave N, Hodgson DJ, Buckling A. 2007. Niche occupation limits adaptive radiation in experimental microcosms. PLoS ONE 2, e193 ( 10.1371/journal.pone.0000193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukami T, Beaumont HJE, Zhang X-X, Rainey PB. 2007. Immigration history controls diversification in experimental adaptive radiation. Nature 446, 436–439. ( 10.1038/nature05629) [DOI] [PubMed] [Google Scholar]
- 30.Schluter D. 2000. Ecological character displacement in adaptive radiation. Am. Nat. 156, S4–S16. ( 10.1086/303412) [DOI] [Google Scholar]
- 31.Buckling A, Rainey PB. 2002. The role of parasites in sympatric and allopatric host diversification. Nature 420, 496–499. ( 10.1038/nature01164) [DOI] [PubMed] [Google Scholar]
- 32.Scanlan PD, Hall AR, Blackshields G, Friman VP, Davis MR, Goldberg JB, Buckling A. 2015. Coevolution with bacteriophages drives genome-wide host evolution and constrains the acquisition of abiotic-beneficial mutations. Mol. Biol. Evol. 32, 1425–1435. ( 10.1093/molbev/msv032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerrish PJ, Lenski RE. 1998. The fate of competing beneficial mutations in an asexual population. Genetica 102-3, 127–144. ( 10.1023/a:1017067816551) [DOI] [PubMed] [Google Scholar]
- 34.Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ.M, Relman DA. 2012. The application of ecological theory toward an understanding of the human microbiome. Science 336, 1255–1262. ( 10.1126/science.1224203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gongalsky KB, Persson T. 2013. Recovery of soil macrofauna after wildfires in boreal forests. Soil Biol. Biochem. 57, 182–191. ( 10.1016/j.soilbio.2012.07.005) [DOI] [Google Scholar]
- 36.Sutherland EF, Dickman CR. 1999. Mechanisms of recovery after fire by rodents in the Australian environment: a review. Wildl. Res. 26, 405–419. ( 10.1071/wr97045) [DOI] [Google Scholar]
- 37.Del Moral R, Grishin SY. 1999. Volcanic disturbances and ecosystem recovery. In Ecosystems of disturbed ground (ed. Walker LR.), pp. 137–160. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 38.Zhao X-F, Buckling A, Zhang Q-G, Hesse E. 2018. Data from: Specific adaptation to strong competitors can offset the negative effects of population size reductions Dryad Digital Repository. ( 10.5061/dryad.t0rf6) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Zhao X-F, Buckling A, Zhang Q-G, Hesse E. 2018. Data from: Specific adaptation to strong competitors can offset the negative effects of population size reductions Dryad Digital Repository. ( 10.5061/dryad.t0rf6) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Datasets supporting this article are uploaded to the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.t0rf6 [38].