Abstract
Laparoscopic and endoscopic cooperative surgery (LECS) is an accepted method of laparoscopic wedge resection, which is minimally invasive, for gastrointestinal stromal tumors (GISTs). We established a type of LECS achieving a full-thickness resection, non-exposed endoscopic wall-inversion surgery (NEWS), in an effort to prevent exposure of the peritoneal cavity to gastric intraluminal contents. We employed this surgical technique in 28 gastric GIST patients. We failed to complete NEWS in the initial two patients and in one patient with a large tumor (40 mm × 35 mm), but otherwise carried out the procedure successfully. Although a learning effect is speculated to occur, based on a decreasing trend in the operation time, the median operation time was 184 minutes showing that NEWS is still a time-consuming method. No significant differences were recognized in tumor size or location, except near the esophagogastric junction (EGJ), nor in the cross-sectional circumference. NEWS is feasible and appears to be a good option, especially for small GISTs with mucosal ulceration rendering full-thickness enucleation by opening of the gastric wall unfeasible.
Keywords: Gastrointestinal stromal tumor (GIST), laparoscopic and endoscopic cooperative surgery (LECS), non-exposed technique
Introduction
Gastrointestinal stromal tumor (GIST) is the most common mesenchymal neoplasm of the alimentary tract, and the stomach is the most frequently affected site, accounting for roughly of 60% of all patients with a GIST (1,2). Since gastric GIST rarely metastasizes to perigastric lymph nodes, gastric local resection without lymphadenectomy is accepted as a standard treatment. Laparoscopic local resection was thus introduced as a minimally-invasive approach and has achieved an acceptable outcome.
Tumor rupture is associated with a very high risk of recurrence (2), mainly within the peritoneal cavity (3). Therefore, preservation of the pseudocapsule and avoidance of tumor spillage resulting from rupture are the basic principles adhered to when resecting a GIST. Accordingly, laparotomy is basically employed for large GISTs to prevent unexpected tumor rupture during surgery, and a minimally-invasive approach is recommended only for smaller tumors (4). Furthermore, tumor ulceration is also considered to potentially be associated with tumor cell spillage. Local resection with intentional gastric perforation should be avoided in this situation because it results in a communication between the peritoneal cavity and the gastric intraluminal space.
With the aim of preventing exposure of the peritoneal cavity to gastric intraluminal contents, we established and reported a novel technique achieving full-thickness resection without the risk of gastric perforation; non-exposed endoscopic wall-inversion surgery (NEWS) (5-7). This is a form of laparoscopic and endoscopic cooperative surgery (LECS). The concept of this technique was initially described based on results obtained with ex vivo experimentation (5), and the first application to clinical practice was reported in 2014 (7). We herein describe the technical details and also the short-term results obtained with this procedure.
Procedure of NEWS
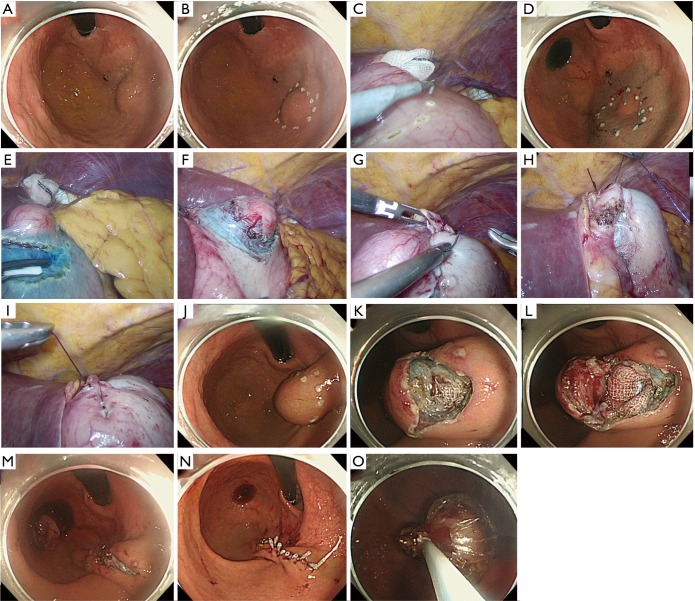
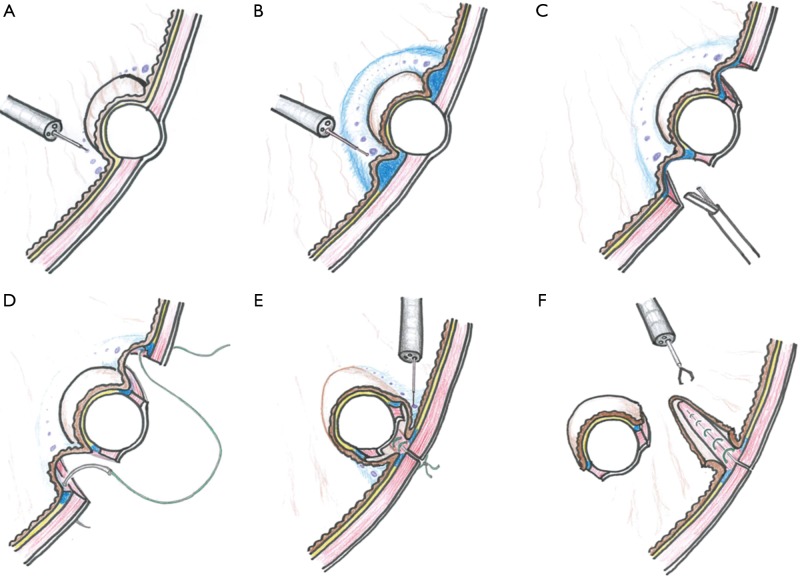
Technical procedures are detailed in the images presented as Figure 1 and illustrated in Figure 2. A 12-mm camera port is inserted via the umbilicus and pneumoperitoneum is then established. Three 5-mm trocars are placed in the left upper, left lower, and right upper quadrants, and one 12-mm trocar in the right lower quadrant. The tumor location is confirmed employing an endoscope with a carbon dioxide supplier (Figure 1A). Markings are made by electrocautery on the mucosa around the lesion under endoscopic vision (Figures 1B and 2A) as well as laparoscopically on the serosa just opposite the mucosal markings, guided by pressing the gastric wall using the endoscopic device, or the fiber-optic probe of a diode laser system (Figure 1C). A 0.4% sodium hyaluronate solution with a small amount of indigo carmine dye is endoscopically injected into the submucosal layer circumferentially around the mucosal markings with a standard injection needle, of the type used during endoscopic submucosal dissection (Figure 1D and 2B). The seromuscular layer is then incised circumferentially around the serosal markings (Figures 1E,F and 2C). The seromuscular layer is linearly sutured using 3-0 absorbable thread (Figures 1G and 2D). The lesion is naturally inverted into the stomach (Figure 2E), and a gauze spacer is inserted between the seromuscular suture plane and the seromuscular surface of the inverted tissue which facilitates the subsequent muco-submucosal incision (Figure 1H,I). Endoscopy shows extensive protrusion of the gastric mucosa due to the inverted tissue (Figure 1J). The muco-submucosal layer is circumferentially incised outside the mucosal markings, using an endoscopic submucosal dissection technique, until the gauze spacer is found (Figure 1K,L). After removal of the gauze spacer, the muco-submucosal incision is completed (Figures 1M and 2F). The resulting artificial linear ulcer is closed using endoscopic clips to promote mucosal healing (Figure 1N). The specimen is extracted orally using an endoscopic retrieval device (Figure 1O).
Figure 1.
Technical detail of non-exposed endoscopic wall-inversion surgery for gastric gastrointestinal stromal tumor. (A) Identification of the tumor location; (B) markings on the mucosa around the lesion; (C) markings on the serosa; (D) injection into the submucosal layer; (E,F) circumferential seromuscular layer cutting; (G-I) seromuscular suturing with inversion of the lesion with a gauze spacer; (J) extensive protrusion of the gastric mucosa due to the inverted tissue; (K-M) incision of the muco-submucosal layer; (N) endoscopic clips placement to close an artificial linear ulcer; (O) oral extraction using an endoscopic retrieval device.
Figure 2.
Illustrations to explain the procedures. (A) Markings on the mucosa around the lesion; (B) injection into the submucosal layer; (C) circumferential seromuscular layer cutting; (D) seromuscular suturing; (E) incision of the muco-submucosal layer after inversion of the lesion; (F) loss of continuity between the lesion and gastric wall.
Results
We employed NEWS in 28 patients with a GIST between January 2012 and August 2017. The clinicopathological characteristics and operative data of our series are presented in Table 1. In the first case, the procedure had to be converted to classical LECS because the tumor was of the totally intraluminal growth type and the tumor margin was poorly recognized on the laparoscopic view. Mucosal injury with a small perforation occurred during the laparoscopic seromuscular cutting phase in case 2. We therefore made two modifications to our technique; employment of the optical fiber system to identify the tumor border clearly from the serosal side and doubling the amount of hyaluronate solution to be injected into the submucosal layer before the laparoscopic seromuscular cutting phase. After these modifications, the full NEWS process was successfully carried out in 25 patients. In case 25, the resected tissue could not be retrieved through the esophagus due to the short axis diameter of the resected GIST being 35 mm, and it was extracted via the gastric window and a small laparotomy incision.
Table 1. Characteristics of 28 consecutive patients with gastric GIST.
| Case No. | Age (years) | Sex | Location | Cross-sectional circumference | Maximum diameter of resected specimen, mm | Tumor size (long axis), mm | Tumor size (short axis), mm | Mucosal ulceration | Mitotic index (Mitoses/hpf) | Malignant risk | Retrieval route | Operation time, min | Perforation | Complication (Clavien-Dindo classification) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | M | U | Post | 45 | 19 | 16 | No | >10/50 hpf | High | Transabdominal | 292 | Yes | None |
| 2 | 61 | M | U | Post | 33 | 26 | 26 | No | ≤5/50 hpf | Low | Transoral | 357 | Yes | None |
| 3 | 71 | F | U | Ant | 38 | 25 | 23 | No | ≤5/50 hpf | Low | Transoral | 265 | No | None |
| 4 | 79 | F | U | Ant | 35 | 25 | 20 | No | ≤5/50 hpf | Low | Transoral | 190 | No | None |
| 5 | 49 | M | U | Ant | 28 | 17 | 17 | No | ≤5/50 hpf | Very low | Transoral | 140 | No | None |
| 6 | 58 | F | M | Post | 48 | 30 | 23 | No | ≤5/50 hpf | Low | Transoral | 191 | No | None |
| 7 | 61 | M | M | Ant | 30 | 25 | 20 | No | ≤5/50 hpf | Low | Transoral | 162 | No | None |
| 8 | 71 | M | U | Ant | 50 | 45 | 25 | Yes | ≤5/50 hpf | Low | Transoral | 263 | No | None |
| 9 | 64 | F | M | Post | 36 | 18 | 18 | No | ≤5/50 hpf | Very low | Transoral | 183 | No | None |
| 10 | 76 | M | M | Post | 24 | 24 | 20 | No | ≤5/50 hpf | Low | Transoral | 175 | No | None |
| 11 | 65 | M | L | Post | 30 | 10 | 9 | No | ≤5/50 hpf | Very low | Transoral | 217 | No | None |
| 12 | 76 | F | U | Post | 25 | 18 | 18 | Yes | ≤5/50 hpf | Very low | Transoral | 214 | No | None |
| 13 | 77 | M | M | Post | 40 | 40 | 20 | No | ≤5/50 hpf | Low | Transoral | 194 | No | None |
| 14 | 70 | F | U | Post | 20 | 17 | 14 | No | 6-10/50 hpf | Low | Transoral | 191 | No | None |
| 15 | 52 | M | U | Post | 30 | 22 | 17 | No | ≤5/50 hpf | Low | Transoral | 159 | No | None |
| 16 | 67 | M | U | Post | 20 | 20 | 18 | No | ≤5/50 hpf | Very low | Transoral | 148 | No | None |
| 17 | 68 | F | U | Less | 38 | 30 | 20 | No | ≤5/50 hpf | Low | Transoral | 156 | No | Grade II |
| 18 | 67 | M | U | Less | 28 | 22 | 19 | No | 6–10/50 hpf | Intermediate | Transoral | 200 | No | None |
| 19 | 50 | F | EGJ | Gre | 37 | 34 | 23 | No | 6–10/50 hpf | Intermediate | Transoral | 327 | No | None |
| 20 | 65 | M | M | Less | 22 | 12 | 9 | No | ≤5/50 hpf | Very low | Transoral | 98 | No | None |
| 21 | 85 | M | U | Ant | 45 | 30 | 26 | No | ≤5/50 hpf | Low | Transoral | 185 | No | None |
| 22 | 54 | M | U | Post | 27 | 25 | 20 | Yes | ≤5/50 hpf | Low | Transoral | 189 | No | None |
| 23 | 55 | M | U | Ant | 35 | 24 | 22 | No | ≤5/50 hpf | Low | Transoral | 176 | No | None |
| 24 | 76 | M | U | Ant | 38 | 15 | 14 | No | ≤5/50 hpf | Very low | Transoral | 132 | No | None |
| 25 | 82 | M | M | Ant | 40 | 40 | 35 | No | ≤5/50 hpf | Low | Transabdominal | 161 | Yes | None |
| 26 | 72 | M | U | Ant | 30 | 17 | 14 | No | ≤5/50 hpf | Very low | Transoral | 146 | No | None |
| 27 | 53 | F | U | Post | 18 | 15 | 12 | No | ≤5/50 hpf | Very low | Transoral | 122 | No | None |
| 28 | 76 | M | U | Ant | 37 | 30 | 22 | No | ≤5/50 hpf | Low | Transoral | 174 | No | None |
GIST, gastrointestinal stromal tumor; M, male; F, female; U, upper third; M, middle third; L, lower third; EGJ, esophagogastric junction; Post, posterior wall; Ant, anterior wall; hpf, high power fields.
Excluding this patient, case 25, the tumor diameter ranges were 10–45 mm for the long axis and 9–26 mm for the short axis. The only postoperative complication was a fever of unknown origin in one case (Clavien-Dindo grade II), with postoperative courses otherwise being uneventful. Neither conversion of the retrieval route nor unexpected gastric perforation during the procedure was associated with negative postoperative outcomes.
The median operation time was 184 minutes. The operation time gradually decreased during the study period and was within 3 hours for most patients managed during the later part of this study, the exception being one patient with a tumor near the esophagogastric junction (EGJ) (327 min). No significant differences were recognized in tumor size or location, except near the EGJ, nor in the cross-sectional circumference.
Discussion
LECS has now become accepted as a minimally invasive surgical technique for gastric GIST, having gained widespread acceptance since the first report of classical LECS in 2008 (8). Extra-gastric growth type GISTs can easily be identified solely based on a laparoscopic view and laparoscopic wedge resection can be achieved even without support from an endoscopist. However, endoscopy does indeed facilitate identifying the exact tumor location, especially for intraluminal growth type GIST with no significant serosal distortion. Furthermore, it allows the boundary of the GIST to be recognized by endoscopy, while also offering the essential negative margin and minimizing the resected gastric tissues thereafter. However, classical LECS has an innate flaw due to the deliberate gastric perforation that is potentially associated with the risks of bacterial infection and/or tumor cell implantation to the peritoneal surface when gastric juice contains tumor cells dispersed from the primary GIST. Therefore, we hesitate to employ the original LECS procedure with intentional gastric perforation for GISTs with either ulceration or delle formation, or even an artificial ulcer after an extensive biopsy procedure, due to possible tumor cell spillage into the peritoneal cavity.
Employing a non-exposed technique for the digestive tract theoretically reduces the surgical site infection rate and, thereby, postoperative inflammatory responses as well. Although this overcomes the flaw of classical LECS and appears to be an ideal method, NEWS has a major limitation in terms of tumor size due to the tumor retrieval route. The esophageal orifice and EGJ are both among the most inherently narrow areas in the human body. In our series, the maximum tumor size which could be extracted orally was 45 mm in the longest axis and 26 mm in the shortest axis. One tumor, 40 mm × 35 mm in size, could not be retrieved orally and had to be extracted via the abdominal wall. NEWS can be employed basically for small GIST. Based on our experience, the short axis diameter of the tumor is the determinant of NEWS feasibility. With a short axis diameter of less than 30 mm, NEWS is feasible. Therefore, meticulous evaluation of tumor size prior to performing NEWS appears to be essential. Endoscopic ultrasound sonography and computed tomography are recommended for evaluating the exact tumor size.
The procedure is still time-consuming though a learning effect, as indicated by the decreasing trend in operation time, is speculated to be present. Insertion of a gauze spacer accompanied by wall inversion after seromuscular cutting has been employed in recent cases. This maneuver reduces the operation time by facilitating the muco-submucosal incision phase owing to the creation of a wider space between the closed seromuscular plane and the tissue to be resected. Given that seromuscular layer suturing alone is acceptable for alimentary tract anastomosis, endoscopic clipping of the artificial linear ulcer might be optional. Further time reduction might thus be achieved by omitting endoscopic clipping.
Local resection with complete preservation of the vagal nerve system, minimal resected volume of the unaffected stomach wall and the least possible deformation of the stomach is ideal for preserving inherent gastric function to the maximum extent possible. Given that gastric GISTs 5 cm or smaller can potentially be removed through laparoscopic wedge resection (9), a non-exposed LECS technique appears to be the best current option for small GISTs with mucosal ulceration rendering full-thickness enucleation by opening of the gastric wall unfeasible. It is not clear that the same concept can be employed for GISTs in other organs such as the esophagus, duodenum, and colon. We hope the unique concept of this technique might promote a discussion about establishing and offering a new treatment modality for alimentary neoplasms, especially given the risk of peritoneal seeding when techniques with exposure are applied.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006;130:1466-78. [DOI] [PubMed] [Google Scholar]
- 2.Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74. 10.1016/S1470-2045(11)70299-6 [DOI] [PubMed] [Google Scholar]
- 3.Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg 2010;97:1854-9. 10.1002/bjs.7222 [DOI] [PubMed] [Google Scholar]
- 4.ESMO/European Sarcoma Network Working Group Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii21-6. 10.1093/annonc/mdu255 [DOI] [PubMed] [Google Scholar]
- 5.Goto O, Mitsui T, Fujishiro M, et al. New method of endoscopic full-thickness resection: a pilot study of non-exposed endoscopic wall-inversion surgery in an ex vivo porcine model. Gastric Cancer 2011;14:183-7. 10.1007/s10120-011-0014-8 [DOI] [PubMed] [Google Scholar]
- 6.Mitsui T, Goto O, Shimizu N, et al. Novel technique for full-thickness resection of gastric malignancy: feasibility of nonexposed endoscopic wall-inversion surgery (news) in porcine models. Surg Laparosc Endosc Percutan Tech 2013;23:e217-21. 10.1097/SLE.0b013e31828e3f94 [DOI] [PubMed] [Google Scholar]
- 7.Mitsui T, Niimi K, Yamashita H, et al. Non-exposed endoscopic wall-inversion surgery as a novel partial gastrectomy technique. Gastric Cancer 2014;17:594-9. 10.1007/s10120-013-0291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiki N, Yamamoto Y, Fukunaga T, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc 2008;22:1729-35. 10.1007/s00464-007-9696-8 [DOI] [PubMed] [Google Scholar]
- 9.Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8 Suppl 2:S1-41; quiz S42-4. [DOI] [PMC free article] [PubMed]