Abstract
Although imatinib is a standard treatment for metastatic or recurrent gastrointestinal stromal tumors (GISTs), acquired c-kit mutations reportedly cause secondary resistance to imatinib. Sunitinib is a tyrosine kinase inhibitor (TKI) that can be used as second-line therapy in imatinib-resistant or -intolerant GISTs. For sunitinib-resistant or -intolerant GISTs, regorafenib is a standard third-line treatment. Although TKI therapies have revolutionized the treatment of recurrent or metastatic GISTs, they cannot cure GISTs. Therefore, in the era of TKIs, role of cytoreductive surgery for recurrent or metastatic GISTs has been discussed. Retrospective studies of treatment strategies with front-line surgery prior to imatinib have shown that initial cytoreduction confers no benefit in cases of advanced or recurrent GIST, and administering imatinib is the principle treatment. Most retrospective studies report cytoreductive surgery to be feasible in patients with metastatic GIST whose disease is stable or responsive to imatinib. Cytoreductive surgery may be indicated in limited disease progression refractory to imatinib when complete resection is possible, but case selection is critical. Cytoreductive surgery for metastatic GIST treated with sunitinib seems less feasible because of high rates of incomplete resections and complications. The role of cytoreductive surgery for metastatic GISTs would be difficult to establish in a prospective study; individualized treatments need to be carefully designed based on c-kit and platelet-derived growth factor receptor alpha (PDGFRA) mutations and other factors.
Keywords: Metastatic gastrointestinal stromal tumor (metastatic GIST), cytoreductive surgery, imatinib, sunitinib, multidisciplinary treatment
Introduction
Gastrointestinal stromal tumors (GISTs), which originate from the interstitial cells of Cajal (ICC) or their progenitor cells, are the most common mesenchymal neoplasm in the human digestive tract (1,2). Most GISTs have a gain-of-function mutation of the c-kit or platelet-derived growth factor receptor alpha (PDGFRA) genes in ICC, which results in ligand-independent activation of the receptors and consequential tumor progression (3-5). Although surgery is the most effective treatment for resectable primary GISTs without metastasis, post-operative recurrence or metastasis occurs in nearly 30% of patients within 3 years after complete resection in the absence of adjuvant therapy, and those metastatic GISTs are difficult to cure with surgery alone (6-8).
An orally bioactive tyrosine kinase inhibitor (TKI), imatinib mesylate (Glivec®, Gleevec®; Novartis, Basel, Switzerland), has been shown to inhibit KIT and PDGFR in vitro (9), and the safety and efficacy of imatinib treatment in patients with metastatic GIST has been confirmed by the results of phase I/II trials (10,11). Although imatinib is thought to be the most effective agent for treating GISTs, about half of patients with unresectable or metastatic GIST develop secondary resistance within 2 years of beginning imatinib therapy (12). A small-molecule TKI, sunitinib malate (Sutent®; Pfizer, New York, NY, USA), has been shown to selectively inhibit KIT, PDGFRA, PDGFRB, vascular endothelial growth factor receptor 1-3 (VEGFR1-3), FMS-like tyrosine kinase 3 (FLT3), and the receptor encoded by the proto-oncogene RET (13,14); the clinical benefits of sunitinib were shown in a phase III trial of patients with advanced GIST after failure of imatinib (15). However, median time to tumor progression was 6.8 months (95% CI: 4.0–8.0 month) in patients treated with sunitinib and most patients developed resistance or intolerance to sunitinib (15). An orally active TKI regorafenib (Stivarga®; Bayer, Leverkusen, Germany) was shown to inhibit KIT, PDGFRB, VEGFR1-3, TIE-2, fibroblast growth factor receptor 1 (FGFR1), RET, RAF-1, and BRAF (16); its clinical benefit was shown in a phase III trial for advanced GISTs after failure of imatinib and sunitinib (17). Although median progression-free survival (PFS) was significantly improved with regorafenib vs placebo control [4.8 vs. 0.9 month, hazard ratio (HR): 0·27, 95% CI: 0·19–0·39 months; P<0·0001], the anti-tumor effect was limited in these advanced GIST patients who had been repeatedly treated with TKIs; most patients developed resistance or intolerance to regorafenib within a year (17).
Since the safety and efficacy of imatinib treatment has been confirmed in clinical trials, treatment strategies for recurrent or metastatic GISTs have dramatically changed. Although other TKIs, including sunitinib and regorafenib, have also improved recurrent or metastatic GISTs treatment, GISTs cannot be cured with TKIs alone. Therefore, in the era of TKIs, a multidisciplinary approach that includes cytoreductive surgery for recurrent or metastatic GISTs has been discussed. In this review, we summarize the current status of surgery for recurrent or metastatic GISTs.
Front-line surgery prior to imatinib therapy
Baseline tumor size when starting imatinib is an important predictive factor for prognosis of advanced GIST patients treated with imatinib, as it reportedly correlates with imatinib resistance in some retrospective analyses (18,19). As this relationship implied that cytoreductive surgery before imatinib therapy would decrease the rate of imatinib resistance and improve the prognosis of advanced GIST patients, some studies have retrospectively evaluated the usefulness of front-line surgery prior to imatinib. An et al. retrospectively reviewed 249 advanced GIST patients, and compared outcomes of patients whose initial cytoreductive surgery removed ≥75% of their tumor bulk (n=35) with outcomes of the other 214 patients, but found that, although these patients had significantly smaller baseline tumors when starting imatinib, their outcomes were not significantly better (20). Chang et al. conducted a prospective collecting retrospective review of advanced GIST patients (metastatic, unresectable, and recurrent GIST) (21). In this study, 76 patients who underwent cytoreductive surgery were divided into two groups; 54 patients who underwent cytoreductive surgery before treatment with imatinib (early group) and 22 patients who received surgery after imatinib therapy (late group). Although PFS and overall survival (OS) were comparable between the early and late groups, the late group had a higher R0 resection rate (21). Sato et al. retrospectively analyzed 14 cases of synchronous metastatic GIST from the Kinki GIST registry in Japan, and investigated outcomes of combined primary surgery and TKI treatments (22). Patients who underwent R0/R1 and those who underwent R2 resection did not significantly differ in 5-year OS, whereas survival time from diagnosis was correlated with duration of imatinib therapy, which suggests that primary surgery alone may not be beneficial, and continuous TKI therapy may be more appropriate as frontline treatment (22). Kanda et al. conducted a multicenter prospective study to clarify the efficacy and safety of surgery and imatinib for liver oligometastasis of GIST (23). Because the trials were prematurely terminated due to amendment of guidelines for adjuvant imatinib therapy and low patient accrual, this study did not yield any evidence supporting the preference for surgical resection in patients with resectable metastatic liver GIST. Notably, all the six patients enrolled in the surgery trial showed hepatic recurrence with median recurrence-free survival of 145 days (range, 62–1,366 days), suggesting that metastatic liver GIST may not be controllable by surgery alone and require concomitant imatinib therapy (23). Taken together, these retrospective and prospective studies suggest that initial cytoreduction does not have a beneficial effect for recurrent or metastatic GISTs. Therefore, imatinib should be the first treatment of choice in this population (Figure 1).
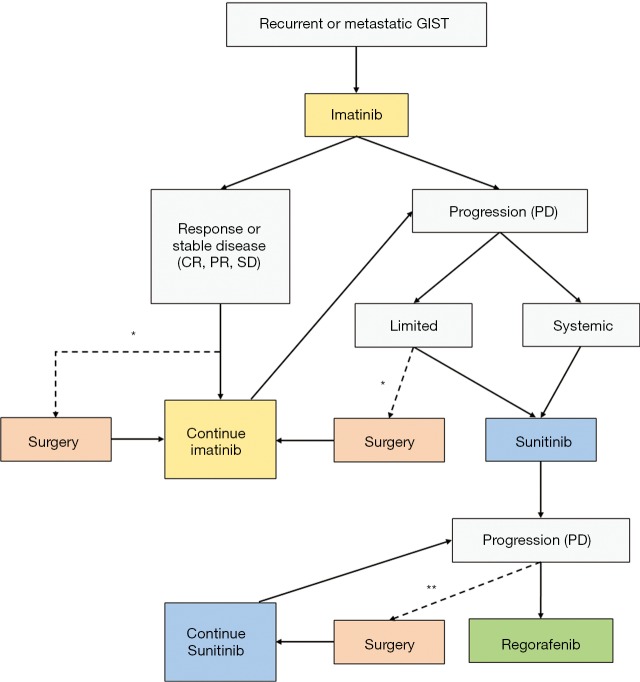
Figure 1.
Proposed algorithm for clinical management of patients with recurrent or metastatic GIST. *, consider surgery if R0 resection can be obtained and imatinib can be restarted early after operation; **, surgery may be indicated for management of symptomatic bleeding or obstruction. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; GIST, gastrointestinal stromal tumor.
Cytoreductive surgery for metastatic GISTs responding to imatinib
Many retrospective studies of the feasibility of cytoreductive surgery during therapy with TKIs in patients with recurrent or metastatic GIST were conducted in American, European, and Asian institutions (24-36). Table 1 summarizes the results of 11 principal retrospective studies on cytoreductive surgery for recurrent or metastatic GISTs treated with TKIs. Of those, 6 studies only analyzed cytoreductive surgery during imatinib therapy and 3 studies included GIST patients treated with imatinib and sunitinib. Although differences in patients’ backgrounds and enrollment periods might have affected outcomes, due to higher availability of sunitinib and regorafenib in later cases, these retrospective studies consistently showed higher complete resection rates and longer PFS and OS for patients who underwent cytoreductive surgery for recurrent or metastatic GISTs that were responding to imatinib compared with those undergoing surgery for imatinib -resistant GISTs. However, the prognosis of patients with recurrent or metastatic GISTs who were treated with imatinib but not cytoreductive surgery also reportedly correlates with response to imatinib (37). Furthermore, these retrospective studies on cytoreductive surgery appear to have selection biases for patients with relatively good status. Therefore, whether cytoreductive surgery has a survival benefit for patients with metastatic GISTs that respond to imatinib is impossible to conclude based only on retrospective studies.
Table 1. Retrospective studies of cytoreductive surgery in patients with recurrent or metastatic GIST on TKI therapy.
| Study | Month/year of surgery | N | TKI at surgery imatinib/sunitinib | Median [range] preoperative TKI duration (months) | Complete resection % by disease status at time of surgery | Median follow-up from time of surgery (months) | Median PFS by disease status at time of surgery (months) | 2-year PFS by disease status at time of surgery (%) | Median OS by disease status at time of surgery (months) | 2-year OS by disease status at time of surgery (%) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | R/S | LR | SR | O | R/S | LR | SR | O | R/S | LR | SR | O | R/S | LR | SR | O | R/S | LR | SR | |||||||||
| Raut, 2006 (24) | Mar 2002–Nov 2004 | 69 | 45/21 | – | – | 78 | 25 | 7 | 14.6 [0.5–36] | – | NR | 7.7 | 2.9 | – | 58 | 18 | 0 | – | NR | 29.8 | 5.6 | – | 90 | 72 | 0 | |||
| DeMatteo, 2007 (25) | Jan 2001–Jul 2005 | 40 | 37/3 | 15 [1–48] | 62.5 | 85 | 46.2 | 28.6 | 15 [6–46] | 15 | NR | 12 | 3 | 39 | 61 | 24 | 0 | 39 | NR | 19 | 11 | 62 | 100 | 36 | 0 | |||
| Gronchi, 2007 (26) | Jan 2001–Jun 2005 | 38 | 38/0 | >12 | 81.5 | 88.9 | 50 | 100 | 29 [6–36] | – | NR | 3 | – | – | 69 | 0 | 0 | – | NR | NR | – | – | 100 | 60 | 0 | |||
| Sym, 2008 (27) | Jun 2001–Jan 2006 | 26 | 26/0 | 18.1 [2.2–53] | 62 | 79 | 33 | 14 | 25.7 [4.3–59] | – | 21.8 | 5.1 | 3.3 | – | 65 | 0 | 0 | – | NR | 22.5 | 23.5 | – | 96 | 68 | 38 | |||
| Yeh, 2010 (28) | Jan 2001–May 2009 | 35 | 35/0 | >14 | 18.4 | 42.9 | 4.8 | 0 | 37 [7.7–75] | – | NR | 8.3 | 2 | – | 59.4 | 35.9 | 0 | – | NR | NR | – | – | 69.6 | 48.4 | – | |||
| Mussi, 2010 (29) | Jul 2002–Oct 2007 | 80 | 80/0 | >15 | – | 88 | 45 | – | >13 [0–76] | – | NR | 8 | – | – | 64.4 | 9.7 | – | – | NR | NR | – | – | 82.9 | 67.6 | – | |||
| Park, 2014 (30) | Jan 2001–Jun 2010 | 42 | 42/0 | 19.1 [7.2–87] | 62 | 62 | – | – | 58.9 [15–129] | 87.7 | 87.7 | – | – | – | – | – | – | NR | NR | – | – | – | – | – | – | |||
| Rubió-Casadevall, 2015 (31) | Jan 2001–Dec 2008 | 27 | 27/0 | 49 | – | 70.4 | 25 | – | 56.6 | 73.4 | 73.4 | – | – | – | – | – | – | 87.6 | 87.6 | – | – | – | – | – | – | |||
| Fairweather, 2017 (32) | Jan 2001–Dec 2014 | 400 | 234/93 | 20 [8–39] | 34 | – | – | – | 33.6 [9.5–45] | 11 | 31/19 | 10 | 5 | – | – | – | – | 81 | NR/110 | 54 | 26 | – | – | – | – | |||
| 234 | 234/0 | 16 [7–36] | – | – | – | – | – | 16 | 36/30 | 11 | 6 | – | – | – | – | 105 | NR/110 | 59 | 24 | – | – | – | – | |||||
| 93 | 0/93 | 25 [12–39] | – | – | – | – | – | 7 | – | – | – | – | – | – | – | 48 | – | – | – | – | – | – | – | |||||
| Raut, 2010 (33) | Feb 2003–Feb 2008 | 50 | 0/50 | 6.7 [1.9–48] | 50 | 40 | 64 | 39 | 15.2 [1.0–54] | 5.8 | 11 | 6.1 | 4.1 | – | – | – | – | 16.4 | NR | 18.5 | 8.9 | – | – | – | – | |||
| Yeh, 2017 (34) | Aug 2001–Dec 2014 | 26 | 0/26 | 6.2 [1–41] | – | – | – | – | 15.2 [1.0–54] | 5.2 | – | 5.2 | – | – | – | – | – | 20 | – | 20 | – | – | – | – | – | |||
TKI, tyrosine kinase inhibitor; GIST, gastrointestinal stromal tumor; O, overall; R/S, response/stable; LR, limited resistance; SR, systemic resistance.
Only few randomized clinical trials (RCTs) on cytoreductive surgery for recurrent or metastatic GIST on imatinib treatment have been performed, with small numbers of patients. Xia et al. randomly assigned 41 patients with GIST and liver metastases to an operation group (neoadjuvant therapy + resection + adjuvant therapy with imatinib) or a nonoperation group (imatinib alone), and analyzed their survival, monitored for up to 36 months (38). OS was significantly better in the operation group compared with the nonoperation group (1- and 3-year OS; 100% and 89% versus 85% and 60%, respectively, P=0.03) (38). Du et al. conducted a multicenter RCT in China to assess whether cytoreductive surgery for patients with recurrent or metastatic GISTs responding to imatinib improves PFS compared with imatinib treatment alone (39). This RCT was closed early due to poor accrual and only 41 patients were enrolled. After a median follow-up of 23 months (range, 15–34 months), PFS did not significantly differ between the surgery arm (n=19) and imatinib alone arm (n=22; 2-year PFS: 88.4% vs. 57.7%, P=0.089) (39).
Because of the lack of RCTs, the impact of cytoreductive surgery on PFS and OS of patients with recurrent or metastatic GIST remains unclear. Although we cannot base evidence on the retrospective studies or results in prospective studies without statistical significance, cytoreductive surgery appears to be feasible and may be beneficial to some patients with recurrent or metastatic GISTs responding to imatinib (Figure 1). However, case selection is critical in ensuring cytoreductive surgery for those tumors. In a retrospective study of 239 patients with metastatic GIST who underwent metastasectomy and received imatinib therapy, long-term survival was observed in patients in whom complete macroscopic resection (R0 + R1) of metastatic disease can be achieved, and incomplete resection (R2) does not seem to prolong survival (36). Although this study enrolled GIST patients who were treated with imatinib either before or after metastasectomy, the results suggest that cytoreductive surgery may be indicated for metastatic GISTs responding to imatinib when complete resection can be obtained. In addition, it is important to restart imatinib as soon as the patient is able to tolerate oral medication after surgery (Figure 1). Further studies are needed to establish more detailed criteria to select patients to whom cytoreduction is beneficial, and cytoreductive surgery on imatinib treatment is being subjected to detailed investigation at special hospitals and institutions.
Cytoreductive surgery for imatinib-resistant GISTs
As described above, retrospective studies have indicated better outcomes after cytoreductive surgery for imatinib-responsive recurrent or metastatic GISTs than for imatinib-resistant GISTs. However, because effects of sunitinib and regorafenib beyond second-line treatment are considerably less than the huge survival benefit of imatinib in first-line treatment, cytoreductive surgery for imatinib-resistant GISTs warrants discussion. Retrospective studies of cytoreductive surgery during imatinib therapy indicate that PFS and OS were longer after surgery for patients with limited resistance to imatinib than for patients with systemic resistance (Table 1). In 2006 and 2007, two independent papers on surgical management of advanced GIST after TKI treatments were published from Brigham and Women’s Hospital and Dana-Farber Cancer Institute in Boston and Memorial Sloan-Kettering Cancer Center in New York, USA (24,25). These studies evaluated outcomes in their institution series of 69 and 40 consecutive patients, respectively, who were treated with TKIs and then underwent surgery for advanced or metastatic GISTs. These papers both concluded that patients with advanced or metastatic GISTs that respond, or show only focal resistance, to TKIs may benefit from elective resection, whereas surgery for patients with metastatic GIST who have multifocal resistance is generally not indicated. However, these studies included not only GIST patients treated with imatinib but also those treated with sunitinib, although 82 (75%) were treated with imatinib alone before surgery (24,25). In 2017, these two American institutes collected their data, analyzed clinicopathological data of 400 surgeries on 323 patients with TKI-treated metastatic GIST, and reported that surgery for metastatic imatinib-treated GIST in the absence of multifocal progressive disease was associated with outcomes at least comparable with second-line sunitinib, and may be considered in select patients (32). Kanda et al. retrospectively analyzed 48 patients with unresectable and metastatic GISTs who were diagnosed with imatinib secondary resistance (ISR) and/or underwent treatment for ISR (40). Of 24 patients who underwent surgical resection of progressive diseases (PD), 20 did so as second-line treatment after imatinib therapy. Long PFS in first-line imatinib therapy, small diameter of PD and surgical resection of PD were identified as favorable independent prognostic factors (40).
Although some retrospective studies of cytoreductive surgery for partially imatinib-resistant GIST have been conducted, their results may reflect patient-selection bias, and safety and efficiency of such therapy remain controversial. In addition, the survival benefit of cytoreductive surgery for imatinib-resistant GIST appears to be affected by postoperative course including the continuous administration of imatinib after complete resection of imatinib-resistant GIST and the administration of sunitinib and regorafenib after developing 2nd recurrence. Although there are only retrospective data and careful patient selection is needed, cytoreductive surgery may be indicated in limited disease progression refractory to imatinib if complete resection can be achieved (Figure 1). However, further studies are needed to establish criteria to select patients to whom cytoreduction is beneficial, and cytoreductive surgery for imatinib-resistant GIST warrants detailed investigations at hospitals and institution with significant experience of multidisciplinary treatment for advanced GISTs where all treatment options, including nonsurgical protocol therapies, can be discussed and performed.
Cytoreduction after second-line therapy
Surgical management following second-line treatment with sunitinib was the focus of wider discussion before regorafenib was introduced as a third-line therapy (33,41,42). In 2009, Ruka et al. reported four patients with inoperable and/or metastatic, imatinib-resistant GIST who had responded to sunitinib therapy and underwent surgical removal of residual disease (41). Macroscopically complete resection of residual disease was achieved in three of four cases; viable GIST cells were detected histologically in the resection specimens. In all cases, sunitinib treatment was resumed post-surgery, and none of the patients experienced any postoperative complications during 13–16 months of follow-up (41). In contrast, Raut et al. retrospectively reviewed 50 patients on sunitinib treatment who underwent surgery, and reported in 2010 that macroscopically complete resections were achieved only in 25 patients (50%) and completeness of resection did not correlate with response to sunitinib at time of surgery (33). Of importance, complication rate was high 54% and reoperations were required in 16% of cases. They concluded that rates of incomplete resections and complications are high, and benefits of surgery should be weighed against symptoms and alternative treatments (33). In a recent prospective cohort study, Yeh et al. investigated 26 patients who experienced local progression on sunitinib treatment and underwent surgeries, and reported that the complication rate was 15.3% and no additional operation was required (34). In this study, sunitinib-treated GIST patients with local progression who underwent cytoreductive surgery (n=26) gained significant PFS and OS benefits (P=0.003 and 0.02, respectively) compared with those not undergoing surgery (n=43), and the authors conclude that surgery is feasible for highly selected patients with metastatic GIST who are receiving sunitinib and experiencing local progression (34). The indication of cytoreductive surgery for GIST patients on sunitinib treatment is controversial. Notably, most of these retrospective studies of cytoreductive surgery for GIST patients treated with sunitinib occurred before regorafenib became clinically available. Furthermore, GIST treated with sunitinib at this relatively late phase tended to be biologically complex due to heterogeneity of genetic and epigenetic background in addition to baseline mutations in the c-kit gene that were acquired during first-line imatinib and second-line sunitinib treatment. We treated a patient who quickly relapsed after resection of a sunitinib-resistant GIST that harbored a secondary mutation at exon 13 of the c-kit gene. In this case, high proliferative activity of the recurrent foci was associated with sunitinib resistance and the perioperative withdrawal of sunitinib appeared to cause incomplete resection due to uncertain tumor burden at time of surgery and rapid postoperative growth of residual tumors (43). Taken together, although controversial, cytoreductive surgery for patients with metastatic GIST on sunitinib seem infeasible because of high rates of incomplete resections and complications, and more biologically complex and advanced disease, and may be indicated only for management of symptomatic bleeding or obstruction (Figure 1).
Individualization of multidisciplinary treatments based on c-kit and PDGFRA mutations
Although most patients with recurrent or metastatic GISTs treated with front-line imatinib achieve clinical benefit, approximately 10% progress within 6 months of initiating therapy (12). Response to imatinib depends on mutation of the c-kit or PDGFRA genes that occur in primary GIST (44). GISTs harboring primary mutations at exon 11 of the c-kit gene are likely to respond well to imatinib, whereas GISTs with mutations at exon 18 of the PDGFRA gene and those without mutations on c-kit or PDGFRA (wild-type GISTs) generally show primary resistance to imatinib. In vitro studies also revealed that GIST-associated KIT mutant isoforms including exon 9 and 11 were inhibited by imatinib with sensitivity similar to that of ligand-activated wild-type KIT, whereas the PDGFRA D842V mutant isoform was not inhibited by imatinib (44). In recurrent or metastatic GISTs harboring PDGFRA D842V mutation or wild-type GISTs that show primary resistance to imatinib, front-line surgery may be a treatment option. However, those tumors are reported to have more indolent disease courses (45,46). Surgery for such slowly growing metastases of wild-type GIST or those with PDGFRA mutations must be very carefully weighed against the risks.
Heinrich et al. have demonstrated that clinical activity of sunitinib is significantly influenced by both primary and secondary mutations in the predominant pathogenic kinases of imatinib-resistant GIST (47). In vitro studies have revealed that KIT double mutants, in which the second mutation occurred in the activation loop (V560D + D816H, V560D + D820G, V560D + N822K, and V560D + Y823D), were resistant to inhibition by sunitinib (47). Furthermore, primary and secondary c-kit or PDGFRA mutations were determined using biopsied specimens from patients with imatinib-refractory GIST who received sunitinib as part of a phase I/II trial. PFS and OS were longer and the clinical benefits were better in patients with imatinib-resistant GIST harboring secondary mutation at exon 13 or 14 (i.e., ATP-binding-pocket) than those with secondary mutation at exon 17 or 18 (i.e., activation loop) of the c-kit gene. Recurrent or metastatic GISTs on second-line therapy with sunitinib appear to have a more biologically complex and advanced nature than those on the first-line treatment with imatinib. Therefore, patient cohorts with such tumors are very heterogeneous, with different primary and secondary mutations that affect response to sunitinib. In addition, individual patients with different secondary mutations may show heterogeneity within multiple metastatic foci. The mutational status of primary and metastatic tumors is a critical consideration with regard to cytoreductive surgery for recurrent or metastatic GIST on sunitinib.
The principle treatment strategy of recurrent or metastatic GIST is sequential administration of imatinib, sunitinib and regorafenib, according to the results of RCTs. When considering cytoreductive surgery for recurrent or metastatic GISTs on imatinib therapy, postoperative courses are important determinants of PFS and OS. Imatinib should be reintroduced as immediately as possible after cytoreductive surgery. When postoperative recurrence appears, sunitinib should be introduced followed by regorafenib for sunitinib-resistant tumors (Figure 1). In such a treatment course after cytoreductive surgery, surgical complications often interfere with or delay TKI administration. Therefore, we need to carefully consider surgical procedures and indication, based on the patient’s comorbidities, general conditions and tumor status.
Taken together, individualization of multidisciplinary treatments needs to be planned based on c-kit and PDGFRA mutations in addition to the patient’s status so that cytoreductive surgery can be safely and appropriately performed.
Conclusions
Initial cytoreduction apparently offers no benefit in cases of recurrent or metastatic GISTs; the principle treatment strategy is imatinib administration. Although case selection is critical, cytoreductive surgery seems feasible in patients with recurrent or metastatic GISTs responding to imatinib or those with limited focal progression if complete resection can be achieved. Cytoreductive surgery for patients with metastatic GIST on sunitinib seems infeasible. Individualization of multidisciplinary treatments needs to be designed based on c-kit and PDGFRA mutations in addition to the patient’s status.
Acknowledgements
We thank Marla Brunker, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
References
- 1.Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998;152:1259-69. [PMC free article] [PubMed] [Google Scholar]
- 2.O'Leary T, Berman JJ. Gastrointestinal stromal tumors: answers and questions. Hum Pathol 2002;33:456-8. 10.1053/hupa.2002.124120 [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. 10.1126/science.279.5350.577 [DOI] [PubMed] [Google Scholar]
- 4.Hirota S, Ohashi A, Nishida T, et al. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology 2003;125:660-7. 10.1016/S0016-5085(03)01046-1 [DOI] [PubMed] [Google Scholar]
- 5.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003;299:708-10. 10.1126/science.1079666 [DOI] [PubMed] [Google Scholar]
- 6.Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74. 10.1016/S1470-2045(11)70299-6 [DOI] [PubMed] [Google Scholar]
- 7.Casali PG, Le Cesne A, Poveda Velasco A, et al. Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated With Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol 2015;33:4276-83. 10.1200/JCO.2015.62.4304 [DOI] [PubMed] [Google Scholar]
- 8.DeMatteo RP, Shah A, Fong Y, et al. Results of hepatic resection for sarcoma metastatic to liver. Ann Surg 2001;234:540-7; discussion 547-8. 10.1097/00000658-200110000-00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchdunger E, Cioffi CL, Law N, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther 2000;295:139-45. [PubMed] [Google Scholar]
- 10.Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 2001;344:1052-6. 10.1056/NEJM200104053441404 [DOI] [PubMed] [Google Scholar]
- 11.van Oosterom AT, Judson I, Verweij J, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 2001;358:1421-3. 10.1016/S0140-6736(01)06535-7 [DOI] [PubMed] [Google Scholar]
- 12.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620-5. 10.1200/JCO.2007.13.4403 [DOI] [PubMed] [Google Scholar]
- 13.O'Farrell AM, Abrams TJ, Yuen HA, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood 2003;101:3597-605. 10.1182/blood-2002-07-2307 [DOI] [PubMed] [Google Scholar]
- 14.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 2003;9:327-37. [PubMed] [Google Scholar]
- 15.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329-38. 10.1016/S0140-6736(06)69446-4 [DOI] [PubMed] [Google Scholar]
- 16.Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245-55. 10.1002/ijc.25864 [DOI] [PubMed] [Google Scholar]
- 17.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302. 10.1016/S0140-6736(12)61857-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Glabbeke M, Verweij J, Casali PG, et al. Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: a European Organisation for Research and Treatment of Cancer-Italian Sarcoma Group-Australasian Gastrointestinal Trials Group study. J Clin Oncol 2005;23:5795-804. 10.1200/JCO.2005.11.601 [DOI] [PubMed] [Google Scholar]
- 19.Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST) Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol 2010;28:1247-53. 10.1200/JCO.2009.24.2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.An HJ, Ryu MH, Ryoo BY, et al. The effects of surgical cytoreduction prior to imatinib therapy on the prognosis of patients with advanced GIST. Ann Surg Oncol 2013;20:4212-8. 10.1245/s10434-013-3279-9 [DOI] [PubMed] [Google Scholar]
- 21.Chang SC, Liao CH, Wang SY, et al. Feasibility and Timing of Cytoreduction Surgery in Advanced (Metastatic or Recurrent) Gastrointestinal Stromal Tumors During the Era of Imatinib. Medicine (Baltimore) 2015;94:e1014. 10.1097/MD.0000000000001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato S, Tsujinaka T, Yamamoto K, et al. Primary surgery as a frontline treatment for synchronous metastatic gastrointestinal stromal tumors: an analysis of the Kinki GIST registry. Surg Today 2016;46:1068-75. 10.1007/s00595-015-1282-4 [DOI] [PubMed] [Google Scholar]
- 23.Kanda T, Masuzawa T, Hirai T, et al. Surgery and imatinib therapy for liver oligometastasis of GIST: a study of Japanese Study Group on GIST. Jpn J Clin Oncol 2017;47:369-72. [DOI] [PubMed] [Google Scholar]
- 24.Raut CP, Posner M, Desai J, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol 2006;24:2325-31. 10.1200/JCO.2005.05.3439 [DOI] [PubMed] [Google Scholar]
- 25.DeMatteo RP, Maki RG, Singer S, et al. Results of tyrosine kinase inhibitor therapy followed by surgical resection for metastatic gastrointestinal stromal tumor. Ann Surg 2007;245:347-52. 10.1097/01.sla.0000236630.93587.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gronchi A, Fiore M, Miselli F, et al. Surgery of residual disease following molecular-targeted therapy with imatinib mesylate in advanced/metastatic GIST. Ann Surg 2007;245:341-6. 10.1097/01.sla.0000242710.36384.1b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sym SJ, Ryu MH, Lee JL, et al. Surgical intervention following imatinib treatment in patients with advanced gastrointestinal stromal tumors (GISTs). J Surg Oncol 2008;98:27-33. 10.1002/jso.21065 [DOI] [PubMed] [Google Scholar]
- 28.Yeh CN, Chen TW, Tseng JH, et al. Surgical management in metastatic gastrointestinal stromal tumor (GIST) patients after imatinib mesylate treatment. J Surg Oncol 2010;102:599-603. 10.1002/jso.21630 [DOI] [PubMed] [Google Scholar]
- 29.Mussi C, Ronellenfitsch U, Jakob J, et al. Post-imatinib surgery in advanced/metastatic GIST: is it worthwhile in all patients? Ann Oncol 2010;21:403-8. 10.1093/annonc/mdp310 [DOI] [PubMed] [Google Scholar]
- 30.Park SJ, Ryu MH, Ryoo BY, et al. The role of surgical resection following imatinib treatment in patients with recurrent or metastatic gastrointestinal stromal tumors: results of propensity score analyses. Ann Surg Oncol 2014;21:4211-7. 10.1245/s10434-014-3866-4 [DOI] [PubMed] [Google Scholar]
- 31.Rubió-Casadevall J, Martinez-Trufero J, Garcia-Albeniz X, et al. Role of surgery in patients with recurrent, metastatic, or unresectable locally advanced gastrointestinal stromal tumors sensitive to imatinib: a retrospective analysis of the Spanish Group for Research on Sarcoma (GEIS). Ann Surg Oncol 2015;22:2948-57. 10.1245/s10434-014-4360-8 [DOI] [PubMed] [Google Scholar]
- 32.Fairweather M, Balachandran VP, Li GZ, et al. Cytoreductive Surgery for Metastatic Gastrointestinal Stromal Tumors Treated With Tyrosine Kinase Inhibitors: A 2-institutional Analysis. Ann Surg 2017. [Epub ahead of print]. 10.1097/SLA.0000000000002281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raut CP, Wang Q, Manola J, et al. Cytoreductive surgery in patients with metastatic gastrointestinal stromal tumor treated with sunitinib malate. Ann Surg Oncol 2010;17:407-15. 10.1245/s10434-009-0784-y [DOI] [PubMed] [Google Scholar]
- 34.Yeh CN, Wang SY, Tsai CY, et al. Surgical management of patients with progressing metastatic gastrointestinal stromal tumors receiving sunitinib treatment: A prospective cohort study. Int J Surg 2017;39:30-6. 10.1016/j.ijsu.2017.01.045 [DOI] [PubMed] [Google Scholar]
- 35.Al-Batran SE, Hartmann JT, Heidel F, et al. Focal progression in patients with gastrointestinal stromal tumors after initial response to imatinib mesylate: a three-center-based study of 38 patients. Gastric Cancer 2007;10:145-52. 10.1007/s10120-007-0425-8 [DOI] [PubMed] [Google Scholar]
- 36.Bauer S, Rutkowski P, Hohenberger P, et al. Long-term follow-up of patients with GIST undergoing metastasectomy in the era of imatinib -- analysis of prognostic factors (EORTC-STBSG collaborative study). Eur J Surg Oncol 2014;40:412-9. 10.1016/j.ejso.2013.12.020 [DOI] [PubMed] [Google Scholar]
- 37.Le Cesne A, Van Glabbeke M, Verweij J, et al. Absence of progression as assessed by response evaluation criteria in solid tumors predicts survival in advanced GI stromal tumors treated with imatinib mesylate: the intergroup EORTC-ISG-AGITG phase III trial. J Clin Oncol 2009;27:3969-74. 10.1200/JCO.2008.21.3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia L, Zhang MM, Ji L, et al. Resection combined with imatinib therapy for liver metastases of gastrointestinal stromal tumors. Surg Today 2010;40:936-42. 10.1007/s00595-009-4171-x [DOI] [PubMed] [Google Scholar]
- 39.Du CY, Zhou Y, Song C, et al. Is there a role of surgery in patients with recurrent or metastatic gastrointestinal stromal tumours responding to imatinib: a prospective randomised trial in China. Eur J Cancer 2014;50:1772-8. 10.1016/j.ejca.2014.03.280 [DOI] [PubMed] [Google Scholar]
- 40.Kanda T, Ishikawa T, Kosugi SI, et al. Prognostic factors after imatinib secondary resistance: survival analysis in patients with unresectable and metastatic gastrointestinal stromal tumors. Int J Clin Oncol 2016;21:295-301. 10.1007/s10147-015-0903-7 [DOI] [PubMed] [Google Scholar]
- 41.Ruka W, Rutkowski P, Szawlowski A, et al. Surgical resection of residual disease in initially inoperable imatinib-resistant/intolerant gastrointestinal stromal tumor treated with sunitinib. Eur J Surg Oncol 2009;35:87-91. 10.1016/j.ejso.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 42.Pantaleo MA, Di Battista M, Catena F, et al. Surgical debulking of gastrointestinal stromal tumors: is it a reasonable option after second-line treatment with sunitinib? J Cancer Res Clin Oncol 2008;134:625-30. 10.1007/s00432-007-0347-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kikuchi H, Miyazaki S, Setoguchi T, et al. Rapid relapse after resection of a sunitinib-resistant gastrointestinal stromal tumor harboring a secondary mutation in exon 13 of the c-KIT gene. Anticancer Res 2012;32:4105-9. [PubMed] [Google Scholar]
- 44.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 2003;21:4342-9. 10.1200/JCO.2003.04.190 [DOI] [PubMed] [Google Scholar]
- 45.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. 10.1053/j.semdp.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 46.Lasota J, Miettinen M. KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs). Semin Diagn Pathol 2006;23:91-102. 10.1053/j.semdp.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 47.Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol 2008;26:5352-9. 10.1200/JCO.2007.15.7461 [DOI] [PMC free article] [PubMed] [Google Scholar]