Abstract
IMPORTANCE
B-cell depletion with the anti-CD20 antibody rituximab is highly effective for pemphigus vulgaris (PV) treatment. However, most patients experience relapse, and intravenous rituximab infusions are expensive. Therefore, cost-effective anti-CD20 therapies are desirable.
OBSERVATIONS
A compassionate-use investigational new drug protocol was approved to administer veltuzumab, a second-generation humanized anti-CD20 antibody, to a patient with refractory PV. Veltuzumab was administered as two 320-mg (188 mg/m2) subcutaneous doses 2 weeks apart, resulting in complete remission of disease off therapy. The disease relapsed 2 years after treatment. A second cycle of subcutaneous veltuzumab, using the same dosage regimen, again induced complete remission off therapy, which remained at 9 months. No serious adverse events occurred during 35 months of follow-up. Serum veltuzumab levels were 22 and 29 μg/mL 2 weeks after the first dose of each cycle, and the drug remained detectable in the serum for longer than 3 months. Relapse and response to veltuzumab generally correlated with desmoglein 3 enzyme-linked immunosorbent assay index values. Shortly after a relapse that occurred after a long-term remission, the patient demonstrated an elevated naive (CD19+CD27−) to memory (CD19+CD27+) B-cell ratio of 19.5 and transitional (CD19+CD24+CD38+) B-cell frequency of 12.5%.
CONCLUSIONS AND RELEVANCE
Subcutaneous veltuzumab may be a safe, effective, and more economical alternative to intravenous rituximab for PV therapy. Clinical trials of subcutaneous veltuzumab for PV are warranted.
Pemphigus vulgaris (PV) is a potentially fatal autoimmune blistering disease caused by antibodies to the keratinocyte adhesion protein desmoglein (Dsg) 3. B-cell depletion with the chimeric anti-CD20 antibody rituximab is effective in PV, with 95% to 100% of patients achieving short-term healing of mucocutaneous lesions and approximately 50% experiencing complete remission of disease off therapy.1–3 However, more than 80% of the patients experience relapse, suggesting that most patients with PV treated with rituximab may require multiple cycles of therapy. Additionally, intravenous administration of rituximab is expensive, and neutralizing human antichimeric antibodies to rituximab can occur.4 Therefore, the development of alternative anti-CD20 therapies is desirable.
We report successful treatment in a patient with refractory PV using veltuzumab, a novel second-generation humanized anti-CD20 antibody, administered by subcutaneous injection. Subcutaneous veltuzumab was safe and effective, resulting in complete remission of disease off therapy and no serious adverse events during 35 months of follow-up.
Report of a Case
A woman in her late 20s developed intermittent oral lesions initially attributed to herpes simplex virus. Two years later she developed vaginal erosions and focal areas of skin blistering. A skin biopsy demonstrated suprabasal acantholysis, and direct immunofluorescence analysis showed intercellular staining of IgG and complement C3, establishing a diagnosis of PV. The patient’s disease cleared with prednisone, 40 mg/d, but her mucosal disease flared when the dose was tapered. Adjunctive immunosuppression with azathioprine, 150 mg/d (2.25 mg/kg/d), for 3 months was unsuccessful, and dapsone was not tolerated because of cytopenias. Treatment with mycophenolate mofetil, 3000 mg/d (45 mg/kg/d), allowed prednisone to be tapered to 5 mg/d but not lower. The patient received her first cycle of rituximab (four 375-mg/m2 weekly intravenous doses), resulting in incomplete remission. Prednisone therapy was tapered to 3 mg/d while the mycophenolate mofetil dose remained 3000 mg/d, but mucosal blisters recurred 6 months later. The patient received a second cycle of the same dosage of rituximab 7 months after the first cycle, again resulting in incomplete remission; prednisone was discontinued and mycophenolate mofetil, 3000 mg/d, was continued, but the mucosal blisters recurred 6 months later. She received a third cycle of rituximab, with a modified regimen of two 1000-mg intravenous infusions 2 weeks apart, 8 months after the second cycle, with no response. Owing to the patient’s fatigue, the mycophenolate mofetil dose was decreased to 2000 mg/d, requiring a prednisone dose increase to 7.5 mg/d to achieve disease control, and doses could not be lowered further without disease flare. Infusion reactions and human antichimeric antibodies to rituximab were not observed.
Veltuzumab is a humanized anti-CD20 antibody that differs in sequence from rituximab, resulting in favorable pharmacokinetics and potent anti–B-cell activity in preclinical studies.5 Veltuzumab is currently under clinical development for the treatment of B-cell lymphomas6 and autoimmune diseases.7,8 A compassionate-use investigational new drug protocol to provide veltuzumab treatment for our patient with refractory PV was approved by the US Food and Drug Administration and a local institutional review board. The patient, in her early 40s at the time of treatment with veltuzumab (month 0 in Figure 1), received two 320-mg (188 mg/m2) subcutaneous doses of veltuzumab 2 weeks apart. She was observed for 1 hour after each injection without incident. No injection site reactions or serious adverse events were observed during long-term follow-up of 35 months.
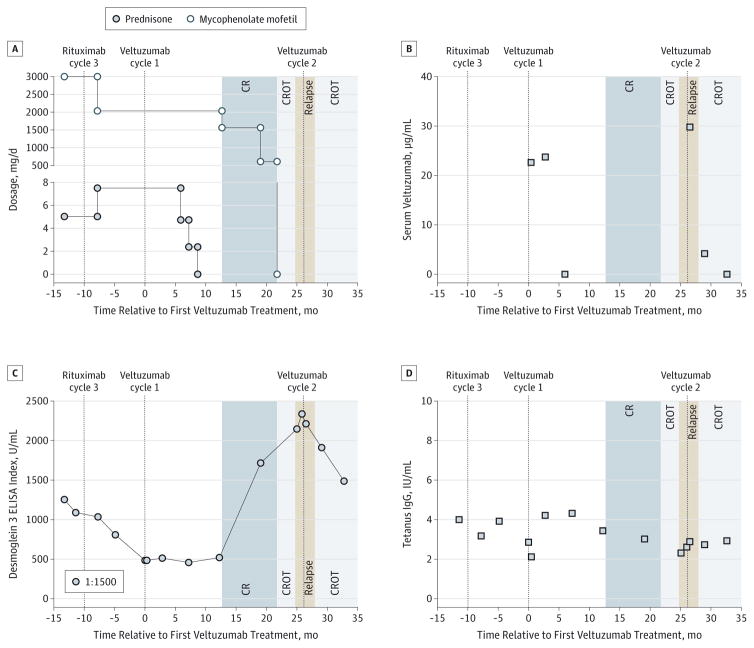
Figure 1. Evaluation of Clinical Course, Treatments, Pharmacokinetics, and Response to Therapy.
A, Immunosuppressant dosages and clinical outcomes. CR, complete remission (minimal therapy); CROT, CR off therapy. B, Pharmacokinetic analysis. Serum veltuzumab levels were 22 and 23 μg/mL and 29 and 4 μg/mL when measured at 0.5 and 3.0 months after veltuzumab cycles 1 and 2, respectively. C, Modified (1:1500) desmoglein 3 enzyme-linked immunosorbent assay (ELISA), which correlates with relapse and remission before and after veltuzumab cycle 2. D, Serum tetanus toxoid IgG remained stable.
After veltuzumab treatment, prednisone was successfully tapered to discontinuation, followed by mycophenolate mofetil, achieving the clinical end points of complete remission on minimal therapy9 at 13 months and complete remission off therapy at 22 months. At 24 months, her mucosal PV relapsed. She received a second cycle of veltuzumab 26 months after the first treatment, using the same veltuzumab dosing regimen without adjunctive use of daily systemic immunosuppressants. She achieved complete remission of disease off therapy within 2 months, which remains ongoing at 35 months follow-up.
Figure 1A details the patient’s clinical course and treatments. Human anti-veltuzumab antibodies were not detected during 35 months of follow-up (data not shown; eMethods in the Supplement). At 0.5 and 3.0 months, serum veltuzumab levels were 22 and 23 μg/mL, respectively, with the first cycle of treatment and 29 and 4 μg/mL, respectively, with the second cycle (Figure 1B). A modified Dsg3 enzyme-linked immunosorbent assay10 (1:1500 dilution to increase sensitivity by using the linear range of the assay) showed that Dsg3 index values generally correlated with treatment and disease activity (Figure 1C). Serum autoantibody levels decreased before the first cycle. Veltuzumab treatment correlated with a sustained decrease in Dsg3 index values. Relapse was preceded by increased autoantibody levels, and complete remission after the second treatment cycle correlated with reduced Dsg3 index values. Serum tetanus IgG remained stable above the protective limit of 0.1 IU/mL (Figure 1D). Epitope mapping experiments demonstrated that autoantibodies recognized Dsg3 extracellular domains 1 to 3, with no change in targeted epitopes throughout remission and relapse (data not shown).11
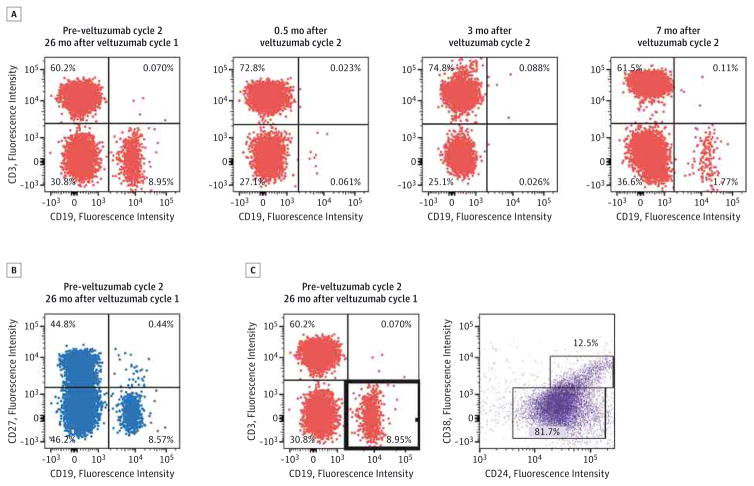
Multiparameter flow cytometry of whole blood was performed to evaluate the kinetics of B-cell depletion and repopulation (Figure 2A). Unexpectedly, the patient’s peripheral CD19+ B cells were almost undetectable just before the first veltuzumab treatment cycle, which precluded analysis of B-cell depletion during that cycle. The first veltuzumab injection occurred 10 months after the third cycle of rituximab, when the patient was taking 7.5 mg/d of prednisone and 2000 mg/d of mycophenolate mofetil. The levels of CD19+ B cells were 15/μL at 7 months and 294/μL at 25 months (during relapse). Two weeks after the first veltuzumab injection of cycle 2, peripheral blood CD19+ B-cell counts were undetectable, with repopulation first detected at 7 months after the second treatment cycle (42/μL).
Figure 2. Immunophenotype of Repopulating B Cells 26 Months After the First Cycle of Veltuzumab Therapy.
A, CD19+ peripheral blood B-cell depletion and repopulation during the second cycle of veltuzumab therapy. B-cell subsets were analyzed at 26 months, shortly after relapse from long-term remission. B, Long-term remission after the first cycle of veltuzumab correlates with the elevation of naive (CD19+CD27−) compared with memory (CD19+CD27+) B-cell frequencies. C, Long-term remission after the first cycle of veltuzumab correlates with the high levels (12.5%) of transitional (CD19+CD24+CD38+) B cells, as previously described.2
A previous study2 of patients with PV who were treated with rituximab reported B-cell repopulation characteristics for patients achieving long-term complete remission off therapy compared with patients in incomplete remission. In the present patient, 2 years after the first veltuzumab treatment and shortly after relapse, naive (CD19+CD27−) B cells constituted 95.1% of the total B-cell population, with a ratio of naive to memory (CD19+CD27+) B cells of 19.5 (absolute B-cell counts were 280/μL and 14/μL in the naive and memory pools, respectively) (Figure 2B). The transitional (CD19+CD24+CD38+) B-cell population frequency was 12.5% (37/μL) (Figure 2C).
Discussion
We report the first case of PV treated with the novel humanized anti-CD20 antibody veltuzumab. Veltuzumab is a second-generation anti-CD20 antibody with humanized framework regions and a single amino acid change in the heavy chain complementarity-determining region 3 compared with rituximab (aspartic acid at position 101 [D101] instead of asparagine). In preclinical studies,5 veltuzumab demonstrated a 2.7-fold higher half-life on the surface of human B-cell lines compared with rituximab, confirmed by site-directed mutagenesis studies to be due to the D101 substitution. Cynomolgus monkeys receiving low-dose subcutaneous veltuzumab demonstrated complete depletion of B cells in the peripheral blood, spleen, and mandibular and mesenteric lymph nodes, whereas rituximab effectively depleted B cells in the peripheral blood, but not consistently in the lymph nodes and/or spleen.12,13 Consistent with this enhanced anti–B-cell activity, low-dose subcutaneous veltuzumab has shown efficacy comparable to that of intravenous rituximab in phase 1 studies of patients with non-Hodgkin lymphoma6 and relapsed immune thrombocytopenia (ITP).7
Treatment with subcutaneous veltuzumab in our patient with refractory PV was effective, resulting in peripheral blood B-cell depletion within 2 weeks of the first subcutaneous injection and complete remission of the disease off therapy. Given the peripheral blood B-cell depletion and decreasing Dsg3 enzyme-linked immunosorbent assay index values at the start of veltuzumab therapy, we cannot rule out a delayed effect of rituximab in response to the first cycle of veltuzumab treatment. However, peripheral blood B-cell depletion has been reported14 in patients receiving high-dose mycophenolate mofetil and prednisone therapy who have never received rituximab, so we cannot definitively attribute the baseline B-cell depletion to prior rituximab therapy.
Serum levels of veltuzumab were sustained in our patient at 2 weeks and 3 months for the first treatment cycle (22 and 23 μg/mL, respectively) but not the second treatment cycle (29 and 4 μg/mL). In comparison, the mean veltuzumab serum level in patients with ITP who were receiving the same dosing regimen were 20 and 2 μg/mL at 2 weeks and 3 months, respectively.7 Although speculative, the sustained serum level of veltuzumab after the first treatment cycle in this patient might reflect her low baseline peripheral blood B-cell count (ie, low CD20 target antigen to deplete circulating veltuzumab), which recovered by the time of the second treatment cycle to values similar to those observed in the ITP population.
Repopulation of B cells began 7 months after the first treatment cycle, although relapse of the disease did not occur until 24 months after treatment. Patients achieving long-term complete remission after rituximab therapy have been reported2 to haveasignificantlyhighermeanfrequencyoftransitionalBcells compared with patients in incomplete remission (8.1% vs 1.9%). Similarly, our patient achieving long-term complete remission demonstrated a 12.5% transitional B-cell frequency 2 years after treatment, shortly after disease relapse.
Veltuzumab was well tolerated by our patient, with no serious adverse events, injection site reactions, or constitutional symptoms following treatment. No serious adverse events or serious infections have been observed in phase 1 studies of subcutaneous veltuzumab use in 34 patients with ITP7 and 17 patients with non-Hodgkin lymphoma.6 The most common adverse event was mild to moderate injection site reactions, experienced by 41% of the patients with ITP and 35% of those with lymphoma.
Conclusions
This single-patient experience suggests that subcutaneous veltuzumab may be a safe and effective alternative to rituximab for the treatment of PV, even in refractory cases. Subcutaneous administration is more convenient and likely cost-effective as it avoids the substantial costs of intravenous infusion. Clinical trials of veltuzumab in PV are warranted.
Acknowledgments
Funding/Support: This study was supported in part by the Penn Institute for Immunology (Drs Allman and Payne), Deutsche Forschungsgemeinschaft (grant EL711/1-1 to Dr Ellebrecht), and Immunomedics Inc, who provided the study drug and performed serum pharmacokinetic and human anti-veltuzumab antibody analyses.
Footnotes
Role of the Sponsor: Penn Institute for Immunology, Deutsche Forschungsgemeinschaft, and Immunomedics Inc had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: Heather Horne, AS, and Kirill Gordeyev, MS, both with Immunomedics, Inc, and Cynthia Clark, PhD, MSN, Perelman School of Medicine, University of Pennsylvania, provided administrative, technical, and/or regulatory assistance. No financial compensation was given.
Author Contributions: Drs Ellebrecht and Payne had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ellebrecht, Allman, Tsai, Goldenberg, Payne.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Ellebrecht, Tsai, Wegener, Goldenberg, Payne.
Critical revision of the manuscript for important intellectual content: Ellebrecht, Choi, Allman, Goldenberg, Payne.
Statistical analysis: Ellebrecht.
Obtained funding: Payne.
Administrative, technical, or material support: Ellebrecht, Choi, Allman, Wegener, Goldenberg, Payne.
Study supervision: Allman, Tsai, Payne.
Conflict of Interest Disclosures: Drs Wegener and Goldenberg are employed by and hold equity ownership of Immunomedics Inc, the manufacturer of veltuzumab. Drs Allman and Payne receive grant funding from the National Institutes of Health for research unrelated to the reported study. No other disclosures were reported.
References
- 1.Joly P, Mouquet H, Roujeau JC, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med. 2007;357(6):545–552. doi: 10.1056/NEJMoa067752. [DOI] [PubMed] [Google Scholar]
- 2.Colliou N, Picard D, Caillot F, et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013;5(175):175ra30. doi: 10.1126/scitranslmed.3005166. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed AR, Spigelman Z, Cavacini LA, Posner MR. Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med. 2006;355(17):1772–1779. doi: 10.1056/NEJMoa062930. [DOI] [PubMed] [Google Scholar]
- 4.Lunardon L, Payne AS. Inhibitory human antichimeric antibodies to rituximab in a patient with pemphigus. J Allergy Clin Immunol. 2012;130(3):800–803. doi: 10.1016/j.jaci.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldenberg DM, Rossi EA, Stein R, et al. Properties and structure-function relationships of veltuzumab (hA20), a humanized anti-CD20 monoclonal antibody. Blood. 2009;113(5):1062–1070. doi: 10.1182/blood-2008-07-168146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Negrea GO, Elstrom R, Allen SL, et al. Subcutaneous injections of low-dose veltuzumab (humanized anti-CD20 antibody) are safe and active in patients with indolent non-Hodgkin’s lymphoma. Haematologica. 2011;96(4):567–573. doi: 10.3324/haematol.2010.037390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liebman HA, Saleh MN, Bussel JB, et al. Low-dose anti-CD20 veltuzumab given intravenously or subcutaneously is active in relapsed immune thrombocytopenia: a phase I study. Br J Haematol. 2013;162(5):693–701. doi: 10.1111/bjh.12448. [DOI] [PubMed] [Google Scholar]
- 8.Tahir H, Rohrer J, Bhatia A, Wegener WA, Isenberg DA. Humanized anti-CD20 monoclonal antibody in the treatment of severe resistant systemic lupus erythematosus in a patient with antibodies against rituximab. Rheumatology (Oxford) 2005;44(4):561–562. doi: 10.1093/rheumatology/keh533. [DOI] [PubMed] [Google Scholar]
- 9.Murrell DF, Dick S, Ahmed AR, et al. Consensus statement on definitions of disease, end points, and therapeutic response for pemphigus. J Am Acad Dermatol. 2008;58(6):1043–1046. doi: 10.1016/j.jaad.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng SW, Kobayashi M, Kinoshita-Kuroda K, Tanikawa A, Amagai M, Nishikawa T. Monitoring disease activity in pemphigus with enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3. Br J Dermatol. 2002;147(2):261–265. doi: 10.1046/j.1365-2133.2002.04838.x. [DOI] [PubMed] [Google Scholar]
- 11.Ohyama B, Nishifuji K, Chan PT, et al. Epitope spreading is rarely found in pemphigus vulgaris by large-scale longitudinal study using desmoglein 2–based swapped molecules. J Invest Dermatol. 2012;132(4):1158–1168. doi: 10.1038/jid.2011.448. [DOI] [PubMed] [Google Scholar]
- 12.Schroder C, Azimzadeh AM, Wu G, Price JO, Atkinson JB, Pierson RN. Anti-CD20 treatment depletes B-cells in blood and lymphatic tissue of cynomolgus monkeys. Transpl Immunol. 2003;12(1):19–28. doi: 10.1016/S0966-3274(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 13.Mao CP, Brovarney MR, Dabbagh K, Birnböck HF, Richter WF, Del Nagro CJ. Subcutaneous versus intravenous administration of rituximab: pharmacokinetics, CD20 target coverage and B-cell depletion in cynomolgus monkeys. PLoS One. 2013;8(11):e80533. doi: 10.1371/journal.pone.0080533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horváth B, Huizinga J, Pas HH, Mulder AB, Jonkman MF. Low-dose rituximab is effective in pemphigus. Br J Dermatol. 2012;166(2):405–412. doi: 10.1111/j.1365-2133.2011.10663.x. [DOI] [PubMed] [Google Scholar]