Abstract
Many chronic inflammatory diseases can be improved by helminth infection, but the mechanisms are poorly understood. Allergy and helminthiasis are both associated with Th2-like immune responses; thus, defining how infection with parasites leads to reduced allergy has been particularly challenging. We sought to better understand this conundrum by evaluating host-parasite interactions involved in Th2 immunity in human schistosomiasis. Immune cells were cultured with schistosomes and the effect on CD23, an IgE receptor associated with resistance in schistosomiasis, was evaluated. Cells treated with schistosomes demonstrated reduced surface CD23 levels with a parallel accumulation of soluble (s) CD23 suggesting this IgE receptor is proteolytically cleaved by the parasite. Consistent with this hypothesis, a schistosome-generated (SG)-sCD23 fragment of 15kDa was identified. SG-sCD23 inhibited IgE from binding to CD23 and FcεRI, but lacked the ability to bind CD21. These results suggested that schistosomes target IgE-mediated immunity in immuno-evasive tactics. Based on its characteristics, we predicted that SG-sCD23 would function as an efficacious allergy preventative. Treatment of human FcεRI-transgenic mice with recombinant (r) SG-sCD23 reduced the ability of human IgE to induce an acute allergic response in vivo. In addition, an optimized form of rSG-sCD23 with an introduced point mutation at Asp258 (D258E) to stabilize IgE binding had increased efficacy compared to native rSG-sCD23. Schistosome infection may thus inhibit allergic-like protective immune responses by increasing soluble IgE decoy receptors. Allergy treatments based on this naturally occurring phenomenon may be highly effective and have fewer side effects with long-term use.
Keywords: Schistosomes, CD23, IgE, Host-parasite interactions
Graphical abstract
1.0 Introduction
Helminth infections are highly prevalent and cause significant morbidity worldwide (1). Paradoxically, parasitic worm infections have been shown to protect against the development of many chronic diseases in animal models, including inflammatory bowel disease, diabetes, and allergy, but the mechanisms are unclear (2). The effects of helminthiasis on allergy are particularly curious as both diseases are associated with a Th2-polarized immune response (3). Furthermore, the Th2 response is much more intense in helminth disease, though a higher concentration of parasite-specific IgE has not been shown to dilute functional allergen-specific IgE in vitro (4). These observations raise the question of how worms might play a role in reducing allergic responses in vivo. In this vein, it has been postulated that chronic helminth infection may dampen global Th2 immune responses through immuno-regulatory processes that allow for parasitism (5). However, this idea is in conflict with multiple published observations that demonstrate people infected with parasitic worms exhibit extremely high levels of IgE and circulating eosinophils— much higher than that reported in allergy, indicating that this issue is highly complex (6–11).
IgE is thought to have an important role in protective immunity to parasitic schistosomes in humans, but the functionality is not clear (12, 13). The human immune system has numerous IgE receptor-bearing cells suggesting that IgE has multiple functions in schistosomiasis (14). Effector functions of IgE have been demonstrated and include increasing the larvacidal activity of granulocytes (15). We previously reported that an increase in circulating FcεRII/CD23+ B cells was associated with the development of resistance in schistosome hyper-exposed populations from Kenya (16). Our findings suggested that CD23+ B cells may utilize surface bound IgE to capture and shuttle antigens from the bloodstream to the splenic follicles to augment immune responses (17, 18). Thus, IgE likely has diverse and unexpected roles in human immunity that remain to be defined.
CD23 has a broad cellular distribution in humans and is expressed by monocytes, resting eosinophils, and follicular dendritic cells in addition to B cells (19). CD23 is a type II integral membrane protein with a calcium-dependent lectin domain that binds IgE (20). A leucine zipper in the N- terminal stalk region allows CD23 molecules to form homo-trimers, which increase the affinity for IgE to the same level as the high affinity IgE receptor, FcεRI (20). CD23 also contains a CD21-binding C-terminal tail on the lectin head that amplifies certain functions, particularly inflammatory cytokine production and augmentation of antibody production (21). Cleavage of cell surface CD23 occurs in the N-terminal stalk by ADAM10 and other proteases to generate multiple soluble (s) forms of CD23 (22). Soluble fragments that are 29-, 33- and 37-kDa retain the ability to homo-trimerize and bind both IgE and CD21 (23). Smaller sCD23 fragments, 17- and 25- kDa, bind IgE and CD21 and are released from the cleavage of larger soluble molecules by several host and microbial proteases. For example, neutrophils secrete an elastase, which cleaves the 37kDa fragment into the 25kDa sCD23 fragment, which can be visualized in the serum (24). These smaller fragments lack the stalk region and generally exist as monomers.
The effects of sCD23 on the immune system depend upon whether the fragment is an oligomer, large or small fragment, and to which ligand it binds (CD23-bound IgE, B cell receptor (BCR)ε, CD21) (25–27). Larger, trimeric fragments have high affinity for BCRε and stimulate IgE secretion by memory ε+B cells (25). The 25–29 kDa fragments of sCD23 have been shown to promote differentiation of germinal center B cells and secretion of TNF-α through ligation of CD21 (20, 28, 29). In contrast, the smaller 17kDa polypeptide may compete with larger fragments to reduce IgE production and has direct anti-inflammatory effects (20, 23).
In this report, we describe a potential mechanism by which Schistosoma mansoni targets CD23 and IgE in immuno-evasive tactics. Schistosomes induce the release of a small, 15kDa isoform of sCD23 that both reduces the cell surface levels of the receptor and results in a soluble decoy receptor for IgE. These results suggest that schistosome infection may diminish protective immunity and by proxy, allergic responses, by regulating effector functions of IgE. We therefore developed the schistosome-generated (SG) sCD23 fragment into a potentially effective allergy treatment to regulate IgE in a physiologically relevant manner.
2.0 Materials and Methods
2.1 Study area and helminth-infected population
This study was approved by the Institutional Review Board of Boston University (BU IRB), the Scientific Steering Committee of the Kenya Medical Research Institute (KEMRI), and the National Ethics Review Committee of Kenya. A portion of the study was conducted along the shores of Lake Victoria in western Kenya with adult males (aged 18–38) exposed to infectious cercariae working as car washers (n =23) described in detail elsewhere (30). Upon informed consent, peripheral blood was drawn into heparinized tubes for the assays outlined below.
Levels of resistance to reinfection are presented as the Index of Susceptibility/Resistance (IoS/R) and indicate the current history of resistance and exposure for each individual: (number of times reinfected) × 100 / (amount of time followed [weeks]) × (mean number of cars washed per week [exposure]) = IoS/R (6).
S. mansoni is the primary schistosome species to infect humans in the region. Stool samples were examined for Schistosoma mansoni eggs and other helminth ova by the modified Kato-Katz method (Vestergaard Frandsen; 2 slides each, 3 stool specimens obtained over several days). Subjects positive for S. mansoni were treated with 40 mg/kg Praziquantel (PZQ); those positive for other helminth ova were treated with 400 mg of albendazole.
2.2 Flow cytometry on whole blood samples
B cells in fresh whole blood were evaluated for levels of surface CD23 by incubating 100 µl of heparinized blood with fluorescently labeled antibodies (anti-CD19; anti-CD23; BD Pharmingen, San Jose, CA) at 4°C for 30 min. Red blood cells were lysed with 2 ml of FACS lysing buffer® (BD Pharmingen) and the remaining leukocytes were washed and subjected to flow cytometic analyses. Assessment of surface expression on B cells was performed with gates generated with anti-CD19 and the appropriate isotype controls for each sample.
2.3 Parasites and parasite antigens
Biomphalaria glabrata snails infected with S. mansoni were supplied by Dr. Fred Lewis of the NIH-NIAID Schistosomiasis Resource Center (Rockville, MD). Cercariae were shed from snails under a bright light for two hours and transformed into schistosomulae. Briefly, cercariae were vortexed to shear tails followed by Percoll density gradient to separate the tail from the schistosomulae. Efficiency of the assay yielded about 80% of the larvae lacking tails. Schistosomulae were adapted to a modified serum-free Basch culture media containing 5% Lipid Concentrate (Gibco) in place of fetal bovine serum to eliminate exogenous serum proteins in media, such as bovine sCD23 and proteases (31). Worms were viable for approximately four days in this media. Excretory/secretory proteins (E/S) were collected from worm cultures after 24 hours and 3 days of incubation. Worms were harvested and homogenized in PBS after 24 hours in culture to collect somatic antigens. The crude somatic antigen mix was applied to 0.2µM syringe filters and the protein concentrations were quantified with the BCA Protein Assay (Fisher Scientific). S. mansoni egg antigen (SEA) were generous gifts from Drs. Edward Pearce (Washington University, St. Louis, MO) and W. Evan Secor (Centers for Disease Control and Prevention, Atlanta, GA). S. mansoni adult worm preparation (SWAP) was a generous gift from Dr. Secor.
2.4 Human cells
Leukopacks containing concentrated leukocytes from 500 ml of peripheral blood from unexposed/uninfected subjects (n =16) were purchased from NY Biologics, Inc (NY, NY.) Fresh, surgically discarded tonsils (n=10) and spleen (n=1) were purchased from the National Disease Research Institute (Philadelphia, PA). Leukocytes were isolated from lymphoid tissues by mincing in cold RPMI 1640 followed by gentle homogenization using the Closed Tissue Grinder System (Fisher Scientific, Houston, TX.) The homogenate was passed over a 70 µM cell strainer (Falcon) to obtain a single-cell suspension. Mononuclear cells were isolated by Ficoll density gradient (Histopaque; Sigma Aldrich). Tonsil mononuclear cells are a mix of approximately 60% B cells, 20% T cells and 10% macrophages. Blood and splenic mononuclear cells contain 10% B cells, 60% T cells and 20% monocytes. B cells were isolated from mononuclear cells by magnetic bead (negative) isolation kits (Miltenyi, Gladbach, Germany or Invitrogen, San Diego, CA).
The CD23+ Ramos B cell line and the THP-1 monocytic cell line were purchased from ATCC (Manassas, VA). Cells were cultured in 10% fetal bovine serum in RPMI 1640 supplemented with 2 mM L-glutamine and 1mM penicillin/streptomycin (Invitrogen.) CD23 was upregulated by B cells after activation with IL-4 (10 ng/ml; eBioscience) or stimulatory anti-CD40 (2 µg/ml; R&D Systems; Minneapolis, MN) for 18 hours and confirmed with anti-CD23 PE (BD Pharmingen). FcεRI α was upregulated on THP-1 monocytes after incubation with IL-10 (10 ng/ml; eBioscience) for 18 hours and confirmed with anti-FcεRIα FITC (eBioscience; see Fig. 3A).
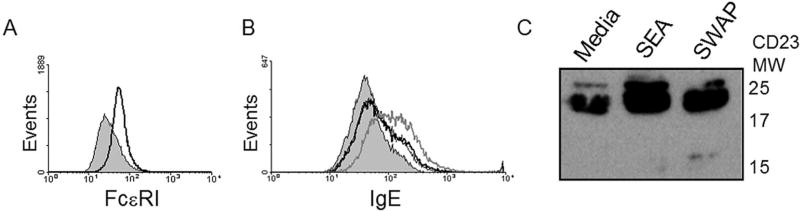
Figure 3. SG-sCD23 is an IgE decoy receptor.
A. IL-10 induced FcεRI α chain expression on THP-1 cells (10 ng/million cells); gray fill: isotype control; black line: anti-FcεRI. B. IgE is inhibited from binding to THP-1 cells in the presence of schistosome-treated cell-free supernatants (thick black line) compared to untreated supernatants (thick gray line). Recombinant SG-sCD23 also inhibits exogenous IgE from binding to THP-1 cells (thin black line; gray fill: isotype control). C. SG-sCD23 does not appear to bind CD21. Ramos B cells were treated with SEA or SWAP for 18 hrs. Supernatants were incubated with sCD21 polypeptide at 4°C for 2 hours and probed with anti-CD21 in Western blot (shown). Blots were stripped and re-probed with anti-CD23 and sCD23 molecular weight patterns are indicated by blot labels.
2.5 Host-parasite interactions
To determine the effect of S. mansoni on CD23 levels, mixed mononuclear cells, purified B cells, or immortalized cell lines were treated with schistosomula E/S (1:1 E/S to cell culture media) or 2 µg/ml of schistosomula crude somatic antigens, SEA, or SWAP from 5 minutes to 48 hours. Live worms were cultured with human cells at concentrations of one to 20 worms to 1 million cells in the modified Basch media and both cells and worms remained viable for up to four days. Protease inhibitors including, leupeptin, CA-074, and cathepsin L inhibitor, were used in cultures at 5 µg/ml (Calbiochem-EMD Millipore, Billerica, MA).
Recombinant (r) calpain/Sm-p80 and hyper-immune mouse sera to Sm-p80 were generated as previously described (32, 33). To block schistosomal calpain activity, cultures were treated with heat-inactivated hyper-immune anti-Sm-p80 serum. Following treatment, leukocytes were evaluated for surface levels of CD23 by flow cytometry (anti-CD23 PE) and cell-free supernatants were collected and stored at −20°C until evaluated.
Total sCD23 concentration was measured in serum and supernatants by ELISA (R&D Systems.) sCD23 fragments in serum and supernatants were evaluated by Western blot (antibodies from R&D Systems.) To better visualize small sCD23 fragments in serum, IgG and albumin were removed with ProteoPrep Blue Albumin & IgG Depletion Kit (Sigma, St. Louis, MO.) Larger sCD23 fragments were visualized on 8% or 10% acrylamide gels. Smaller fragments were visualized on 15% acrylamide gels. Densitometry of proteins on films was performed with ImageJ. sCD21 was measured in supernatants by ELISA (R&D Systems.)
2.6 Measurement of schistosomal calpain and calpain-specific IgG
Mouse anti-Sm-p80 was used to identify schistosomal calpain in serum from human subjects and from in vitro-generated schistosomulae by Western blot. To measure serum anti-Sm-p80 IgG, ELISA plates were coated with 5 µg/ml of rSm-p80 in PBS overnight at 4°C. The following day, plates were washed and blocked with 1% bovine serum albumin (BSA; Sigma) in PBS. Serum was applied at a dilution of 1:100 and incubated for two hours at room temperature. Bound IgG was measured with goat anti-human IgG (1:10,000; Southern Biotech).
2.7 Production of recombinant SG-CD23
Recombinant full-length sCD23 was purchased from R&D systems. Schistosome-generated (SG)-sCD23 and modified SG-sCD23 were cloned and expressed in E. coli by Genscript (Piscataway, NJ.) The following sequence was used to express rSG-sCD23 and assumed the native sequence with the presence of the lectin head and reduction of the C-terminal CD21-binding tail: 156-SGFVC NTCPEKWINF QRKCYYFGKG TKQWVHARYA CDDMEGQLVS IHSPEEQDFL TKHASHTGSW IGLRNLDLKG EFIWVDGSHV DYSNWAPGEP TSRSQGEDCV MMRGSGRWND AFCDRKLGAW VCDRLATCTP PA - 292 C TERMINUS.
The following sequence was used to generate modified SG-sCD23 (herein rSG-sCD23-A/G) by exchanging residue 258, an aspartic acid (D), for glutamic acid (E; bold and underlined): 156-SGFVC NTCPEKWINF QRKCYYFGKG TKQWVHARYA CDDMEGQLVS IHSPEEQDFL TKHASHTGSW IGLRNLDLKG EFIWVDGSHV DYSNWAPGEP TSRSQGEECV MMRGSGRWND AFCDRKLGAW VCDRLATCTP PA -192 C TERMINUS. rSG-sCD23 molecules contained a HIS-tag for purification. SDS-PAGE (not shown) and Western blot was used to validate the expressed SG-sCD23 (see Fig. 6A). Purity of rSG-sCD23 was about 75%.
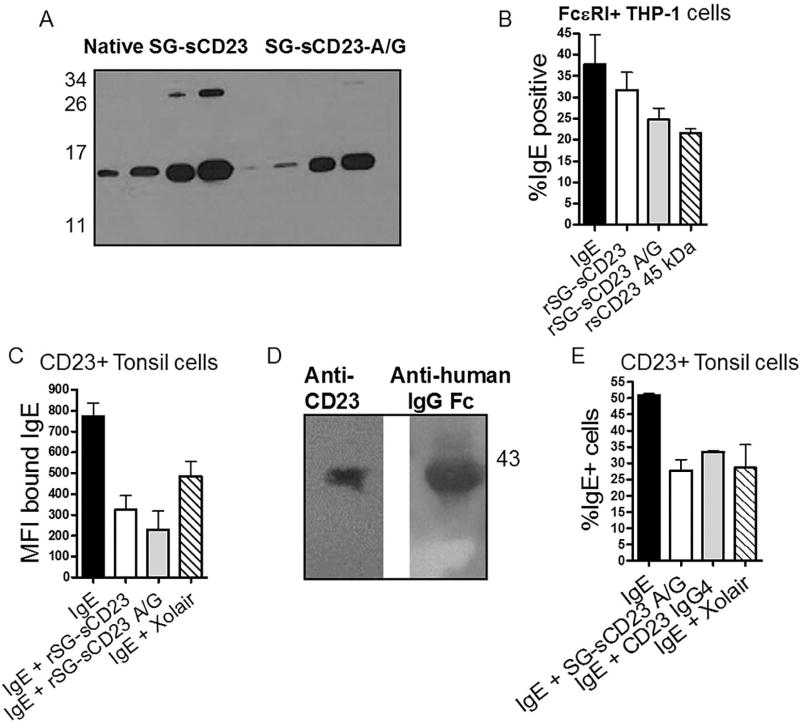
Figure 6. Development of an allergy preventative based on SG-sCD23.
A. Western blot of recombinant (r) sCD23. Native rSG-sCD23 is shown on the left and rSG-sCD23-A/G on the right. B. rSG-sCD23-A/G has increased ability to block IgE from binding to FcεRI+ THP-1 cells. Full-length, commercially available rsCD23 is used for comparison (n=4 separate experiments; P<0.05). C. The effectiveness of rSG-sCD23-A/G is similar to Omalizumab (Xolair) in preventing IgE from binding to IL-4-treated CD23+ tonsil MC measured by MFI (C) and percent positive (E); (n=4 tonsils; P<0.05 compared to IgE). D. rSG-sCD23-A/G expressed as a fusion protein with human IgG4 retained the ability to prevent IgE from binding to CD23+ tonsil cells (n=4 tonsils; P<0.05 compared to IgE.)
To increase the in vivo half-life of SG-sCD23-A/G, we created a SG-sCD23-A/G-IgG4 fusion protein. The mutated sCD23 (A/G) was cloned into the vector pcDNA3.3 containing the sequence for human IgG4 light chain Fc and expressed in HEK 293 cells (Iridium Biosciences, Walpole, MA). The fusion protein was purified using a Protein A column by Iridium Biosciences with ~85% purity (see Fig. 6D.)
2.8 IgE binding assay
CD23+ tonsil mononuclear cells and Ramos B cells and FcεRI+ THP-1 cells were subjected to an IgE-binding assay to load IgE receptors with exogenous IgE (Diatec Monoclonals; Olso, Norway). 1 million CD23+ cells were rotated in Tris-buffered saline (TBS) buffer containing 2 mM CaCl2 with 20 µg of IgE at 4°C. IgE binding to FcεRI+ THP-1 cells was performed similarly, but in PBS. Following rotation, cells were washed and surface IgE was measured by flow cytometry (anti-IgE PE; BD Pharmingen). To test if SG-sCD23 functioned as an IgE decoy, IgE binding assays were performed with one of the following: 50 µl of cell supernatants from cells treated with SEA or supernatants from untreated cells in 250 µl total volume, 2 µg/ml of SEA alone, or 10 µg/ml of one of the following: rSG-sCD23, rSG-sCD23-A/G, rSG-sCD23-A/G-IgG4, or Omalizumab (Xolair; Genentech)(34). IgE-bound cells were also utilized in host-parasite interactions assays described above to measure the effect of schistosome antigens on cell surface levels of CD23.
To test if SG-sCD23 was able to bind CD21, supernatants from schistosome-treated Ramos B cells (which contain an increased concentration of intermediate sized sCD23 fragments; See Fig. 2A rightmost) were treated with soluble sCD21 (XpressBio; Thurmont, MD) for two hours rotating in PBS at 4°C. The supernatants were subjected to a 15% acrylamide SDS-PAGE and evaluated by Western blot using anti-CD21-biotin (R&D Systems) to probe for bound sCD21 followed by stripping and re-probing for sCD23 (anti-CD23; R&D Systems.)
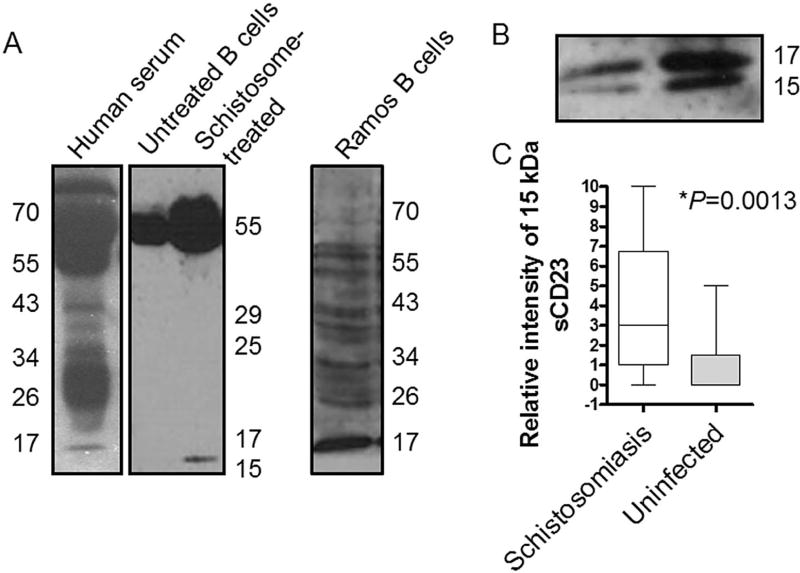
Figure 2. A small 15 kDa sCD23 fragment is increased in the presence of schistosomes.
A. Western blot of serum demonstrating the array of measureable sCD23 polypeptides (left panel). Supernatants from IL-4-treated B cells treated with SEA (18 hours; middle panel). A small 15kDa sCD23 fragment was increased in the presence of SEA. Supernatants from Ramos B cells contain multiple sCD23 fragments (right panel). B. Doublet of 17- and 15- kDa sCD23 fragments in the serum of subjects with schistosomiasis; 15% acrylamide gel. C. Subjects with schistosomiasis (n=23) have a higher concentration of the 15 kDa sCD23 fragment in serum compared to uninfected (n=16); P= 0.0013.
2.9 Induction of allergy in FcεRIα-transgenic mice
Mice transgenic for the human FcεRI α chain B6.CgFcer1a<tm1Knt>Tg (FCER1A)1Bhk/J were purchased from Jackson Laboratories (Bar Harbor, ME)(35). Mice were cared by the accredited Laboratory Animal Care Facility (LASC) at BU. Expression of human FcεRI on murine immune cells was validated with anti-human FcεRI (eBioscience; see Fig. 7A). To induce acute allergy, mice were injected intraperitoneally with 40 µg of human IgE (unknown specificity; Diatec Monoclonals) in 200 µl of PBS. Twenty-four to 48 hours later, mice were treated with 20 µg of anti-IgE (Sigma) in 200 µl of PBS to cross-link cell-bound IgE to induce an acute allergic response.
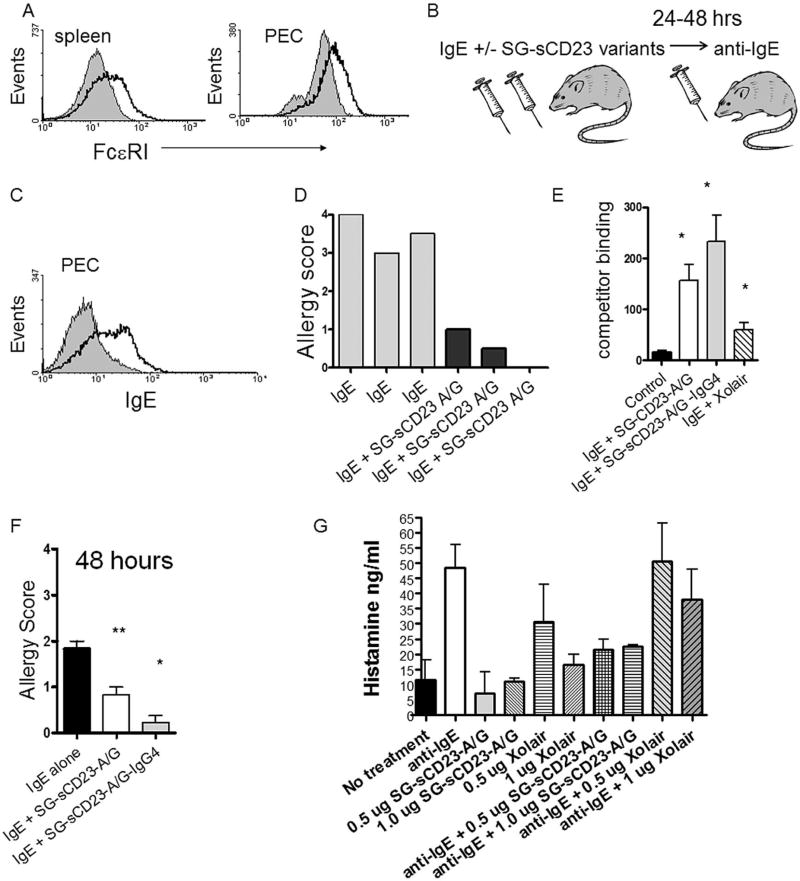
Figure 7. SG-sCD23-A/G prevents acute allergic responses.
A. Splenic leukocytes and peritoneal exudate cells (PEC; B) from mice transgenic for human FceRI express FcεRI α chain. B. Allergy induction protocol. C. Human IgE binds to peritoneal exudate cells (PEC) from transgenic mice. Mice were given 20 µg of human IgE and PEC were harvested 18 hours post-injection. D. rSG-sCD23-A/G prevents acute allergic response in mice. See Methods for scoring strategy. Each bar represents a single mouse. Representative of 7 experiments. E. rSG-sCD23-A/G and rSG-sCD23-A/G-IgG4 prevent acute allergic responses in mice as measured by histamine levels in serum. A higher level of competitive binding indicates a lower serum level of histamine. Mice were necropsied 20 minutes following anti-IgE injection (n=5 mice per group; representative of two separate experiments). F. rSG-rsCD23-A/G-IgG4 prevents allergy 48 hours after administration; (n=3 mice per group; representative of 3 separate experiments). G. SG-sCD23-A/G prevents basophil degranulation upon IgE cross-linking (n=4 blood samples; *P<0.05).
Baseline scratching and general activity were recorded for 10 minutes prior to injection with anti-IgE by an observer blind to the assigned groups (35). Following injection with anti-IgE, the allergic response generally had an onset of 10 minutes and was graded on a scale of 1–5 for 20 minutes or 50 observations per mouse. A score of 1 indicated no change in scratching frequency above baseline; a score of 2 indicated a mild increase in the frequency and intensity of scratching above baseline and scratching in the facial area, a score of 3 specified a moderate increase in the frequency and intensity of scratching above baseline, a score of 4 was characterized by intense scratching and self-biting with higher frequency and earlier onset, elevated respiration, and increased duration of the allergic response, and a score of 5 indicated anaphylactic shock and death.
The dosage of IgE and anti-IgE in our protocol largely induced an allergic response with an intensity of 3–4 that lasted about 20 minutes. All mice recovered from the acute allergic response. However, in some experiments, mice were necropsied 10 minutes following anti-IgE injection to measure histamine in the serum by ELISA (GenWay; San Diego, CA).
To prevent allergy, mice were given 20 µg of one of the following: SG-sCD23, SG-sCD23-A/G, SG-sCD23-A/G-IgG4, or Omalizumab/Xolair in 200 µl of PBS, or 200 µl PBS alone, at the same time as the IgE on day 0, but as a separate injection.
2.9 Basophil activation
To test the effect of IgE-binding proteins on basophil degranulation, whole blood from the leukopacks (NY Biologics, Inc) was diluted 1:3 in cell culture media and acclimated to 37°C for 2 hours. To induce histamine release, cultures were treated with 10 µg of anti-IgE (Sigma) +/−10 µg of one of the following added at the same time: SG-sCD23, SG-sCD23-A/G, SG-sCD23-A/G-IgG4, or Omalizumab/Xolair for 20 minutes. Supernatants were evaluated for histamine concentrations by ELISA as above.
2.10 Statistical analyses
Statistical analyses were performed using GraphPad Prism (GraphPad Software). A one-way analysis of variance with Dunn’s post-test and the Mann-Whitney U test were used for multiple- or single-group comparisons, respectively. Possible correlations were examined using Spearman’s rank correlation test. Group sample sizes differ among the tests for human subjects because some samples were unavailable.
3.0 Results
3.1 Schistosomes reduce surface CD23 on B cells and monocytes
CD23 expression by B cells is associated with resistance to schistosomiasis (16, 36). We recently reported that schistosome antigens did not alter the surface level of CD23 on B cells (18), but these experiments did not test whether there were changes to the soluble form of CD23. We thus measured levels of sCD23 in cell-free supernatants following treatment of B cells with schistosome antigens and found a modest increase (Fig. 1A). As sCD23 is normally generated from proteolytic reduction of cell surface CD23, we hypothesized that schistosomes might cleave CD23.
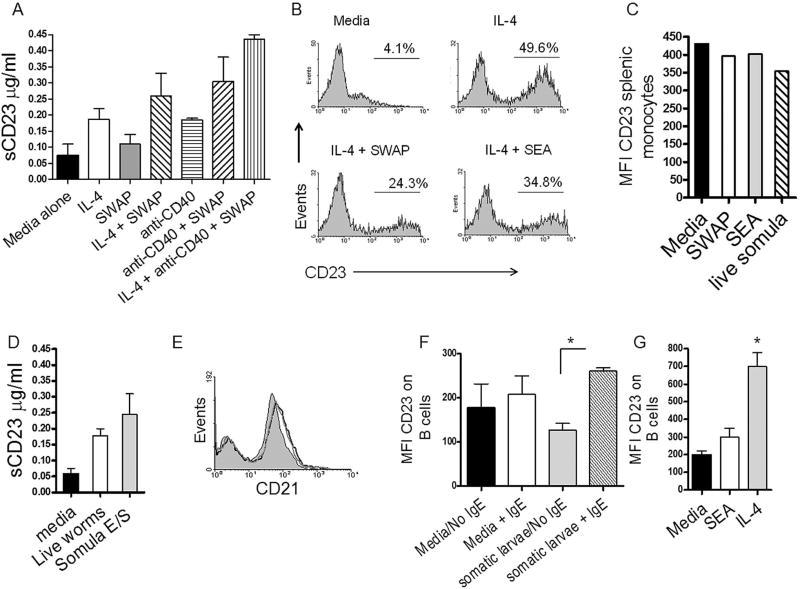
Figure 1. Schistosomes reduce surface CD23 from IL-4 treated cells.
A. Crude schistosome antigens from adult worms (SWAP; 2 µg/million cells) increase the level of soluble (s) CD23 in cell-free supernatants from IL-4 (10 ng/ml)- or anti-CD40 (2 µg/ml)- treated tonsil B cells; 5 separate experiments; *P<0.05 compared to media alone; 18 hour culture. B. SEA (soluble egg antigen; 2 µg/million cells) and SWAP reduce the surface level of CD23 on IL-4-treated tonsil B cells (representative of 5 separate experiments.) C. Mean fluorescence intensity (MFI) of CD23 is reduced on IL-4-treated tonsil mononuclear cells (MC) within 45 minutes of treatment with SEA, SWAP or live schistosomula (somula). 500,000 cells were cultured with five mechanically-transformed schistosomula. D. Incubation of IL-4-treated splenic MC with live schistosomula or E/S from schistosomula (5 µg/million cells) increases measurable sCD23. 18 hour culture; n=4; *P<0.05 compared to media alone. E. Levels of CD21 on B cells are not affected by schistosomes; splenic macrophages. F. Reduction of CD23 by schistosomes is inhibited by the presence of CD23-bound IgE; n=4; P<0.05. G. Levels of surface CD23 rise following a 48 hour culture in mixed cell cultures of tonsil MC; n=4; *P<0.05 compared to media and SEA alone.
To test this possibility, purified tonsil B cells were pre-treated with IL-4 to increase the production of nascent surface CD23 (Fig. 1B). Twenty-four hours later, IL-4-treated B cells were incubated with schistosome antigens for an additional 18 hours. In the presence of SWAP, sCD23 levels increased to levels above IL-4 treatment alone (Fig. 1A). In addition, we noted a reduction of surface CD23 by SWAP on IL-4-treated B cells (Fig. 1B). Reduced surface levels of CD23 were evident as early as 45 minutes post-exposure in the presence of live schistosomulae at ratios of 10 worms/1 million cells or higher (Fig. 1C). Crude egg (SEA), schistosomular somatic antigens, and schistosomular E/S had similar effects on the levels of surface and sCD23 illustrating that all three mammalian life stages may promote the release of sCD23 (Fig. 1B–D). In contrast, schistosomes did not appear to have an effect on cell surface CD21 (Fig. 1E) or on levels of sCD21 (not shown)(26).
We next sought to reconcile our previously published findings demonstrating that schistosome antigens do not affect surface levels of CD23, including those on circulating B cells from schistosome hyper-exposed Kenyans (18). We hypothesized that this would be explained by the observation that peripheral blood CD23+ B cells are pre-loaded with bound IgE, which might block cleavage sites (17). To test this, nascent CD23 was upregulated on tonsillar and splenic B cells with IL-4 and B cells were then loaded with exogenous IgE at 4°C. CD23-bound IgE on B cells is stable in culture for up to three days (17). Fig. 1F shows that B cells bound by IgE retained their surface levels of CD23 in the presence of schistosome antigens suggesting that IgE may protect or block CD23 from schistosome-mediated cleavage.
Although schistosome antigens reduced levels of CD23 on IL-4-treated B cells at time points earlier than 18 hours in culture, surface expression of CD23 began to recover over time and was elevated above untreated cells by 48 hours (Fig. 1G) with a parallel accumulation in sCD23 (not shown). This observation was evident in mixed cell cultures, including PBMC and splenic- and tonsil- MC, and is consistent with parasitic worms inducing Th2 cytokines, which increase CD23 expression (37).
3.2 Generation of a 15kDa sCD23 fragment
Host-derived proteases generate sCD23 fragments that are heterogeneous in size and function (20, 38). In contrast to what is observed in human serum (Fig 2A; left), purified B cell- or mixed mononuclear cell- culture supernatants contained primarily 50–70 kDa sCD23 polypeptides, which are likely dimers of the lectin head and stalk (Fig. 2A; middle.) Supernatants from Ramos B cells, however, contained multiple fragments of CD23, the presence of which varied between batches of cells (Fig. 2A; rightmost).
In supernatants from primary B cells treated with schistosomes, we detected the accumulation of a small fragment of sCD23, approximately 15 kDa (Fig. 2A; middle), which is slightly smaller than the naturally found fragment of 17 kDa observed in serum (Fig. 2A; left). We also noted an increase in the 50–70kDa sCD23 in schistosome-treated supernatants (Fig. 2A; middle), although our previous results indicate that schistosomes do not increase mRNA of inducible CD23 (18). Both the 17- and 15kDa sCD23 fragments were identified in serum from individuals infected with schistosomes (Fig. 2B). Furthermore, a higher concentration of the 15 kDa sCD23 was found in serum from schistosome-infected subjects compared to serum from uninfected subjects of which small amounts of 15 kDa sCD23 were noted (Fig. 2C).
3.3 Schistosome-generated (SG)-sCD23 inhibits IgE from binding to cells
The commercially available anti-CD23 antibody (clone 138628; R&D Systems) utilized in the Western blot (Fig. 2A) recognizes the lectin head portion of the molecule. This suggested that the schistosome-generated (SG)-sCD23 fragment contained the IgE-binding portion of the molecule. Indeed, schistosome-treated cell supernatants reduced the ability of exogenous IgE to bind IgE-receptor positive cells (Fig. 3A) compared to supernatants from untreated cells (Fig. 3B; which contain primarily 50–70 kDa sCD23 polypeptide fragments; see Fig. 2A middle.) These results suggest that SG-sCD23 retains the ability to bind IgE and may be more potent in capturing IgE than the 50–70 kDa native soluble fragments, despite the apparent low concentration of the 15 kDa SG-sCD23 present.
3.4 SG-sCD23 does not bind CD21
Full length CD23 (both cell-associated and soluble) contains a C-terminal tail on the globular lectin head, which binds CD21 (20). Dust mite cysteine protease, Derp-1, cleaves CD23 to release a 16 kDa fragment that contains the lectin head and 10 (out of ~39) amino acids of the CD21-binding tail and retains the ability to bind both IgE and CD21 (39). We thus predicted that the 15 kDa SG-sCD23 fragment contained a smaller, non-functional portion of the CD21-binding tail. Figure 3C indicates that, whereas native 17 kDa sCD23 from untreated Ramos B cells bound exogenous CD21, the 15kDa sCD23 from cells treated with SWAP and SEA had reduced binding.
Based on the size of the SG-sCD23 fragment and the lack of co-localization with sCD21, we hypothesized that the majority of the CD21 C-terminal tail was removed or degraded in SG-sCD23 and that the IgE binding lectin head was intact with the ability to fold properly, but not homo-trimerize.
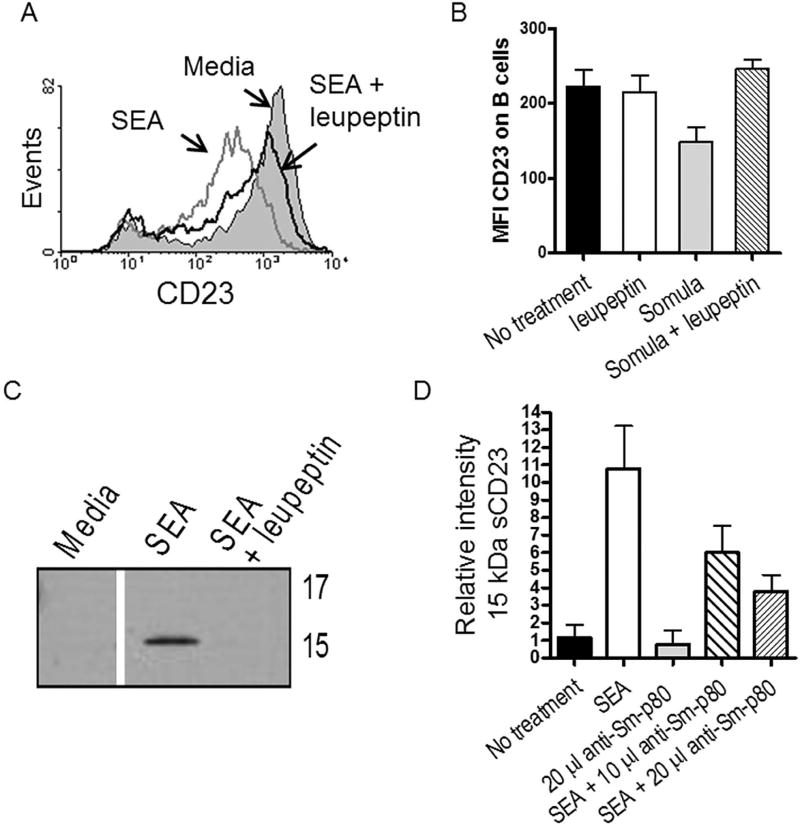
3.5 Leupeptin inhibits generation of SG-sCD23
CD23 is cleaved by a variety of host proteases, such as the metalloproteinase, ADAM10. In addition, cysteine proteases from dust mites and Leishmania spp. have been shown to generate sCD23 fragments (40–42). Schistosomes use a plethora of proteases during parasitism, including cysteine proteases, elastases, calpain, thioredoxin and peroxidases, some of which cleave IgE (43–46). To determine if a schistosomal protease might cleave CD23, we incubated purified, IL-4-treated B cells with schistosome antigens in the presence of inhibitors to classes of proteases previously shown to cleave CD23 (24, 39, 42). Our results demonstrate that, whereas inhibitors of cathepsin L and capthesin B did not change the level of sCD23 generated in the presence of schistosome antigens, leupeptin inhibited the accumulation of total sCD23 in cell supernatants (Fig. 4A&B). Figure 4C reveals a reduction in the release of SG-sCD23 in the presence of leupeptin suggesting that a schistosomal calpain-like protease cleaves CD23.
Figure 4. Schistosomal calpain-like protease generates SG-sCD23.
A. Leupeptin (5µg/ml) inhibits SEA-mediated reduction of surface CD23 on tonsil MC (18 hr culture). Shown are CD23 levels on monocytes. B. Leupeptin inhibits live schistosomula from reducing surface CD23 on purified tonsil B cells (representative of 5 separate experiments; 18 hr culture; *P<0.05 compared to no treatment). C. Leupeptin inhibits the generation of SG-sCD23 from tonsil B cells; 18 hr culture. D. High titer polyclonal anti-Sm-p80 from immunized mice inhibits the generation of SG-sCD23 from PBMC (18 hr culture; n=6 blood samples; *P<0.01 compared to no treatment). Plot depicts densitometry from Western blot probed with anti-CD23.
Calpains are calcium-activated neutral proteases with broad endopeptidase specificity. Schistosomal calpain, or Sm-p80, is a neutral protease shown to be present in the gut of adult worms (47). Leupeptin has broad protease specificity and may also inhibit cathepsin B; thus we tested the effect that Sm-p80 might have in CD23 cleavage. Figure 4D demonstrates a reduction in the accumulation of SG- sCD23 in the presence of hyper-immune mouse anti-Sm-p80, further suggesting that schistosomal calpain plays a role in the production of SG-sCD23.
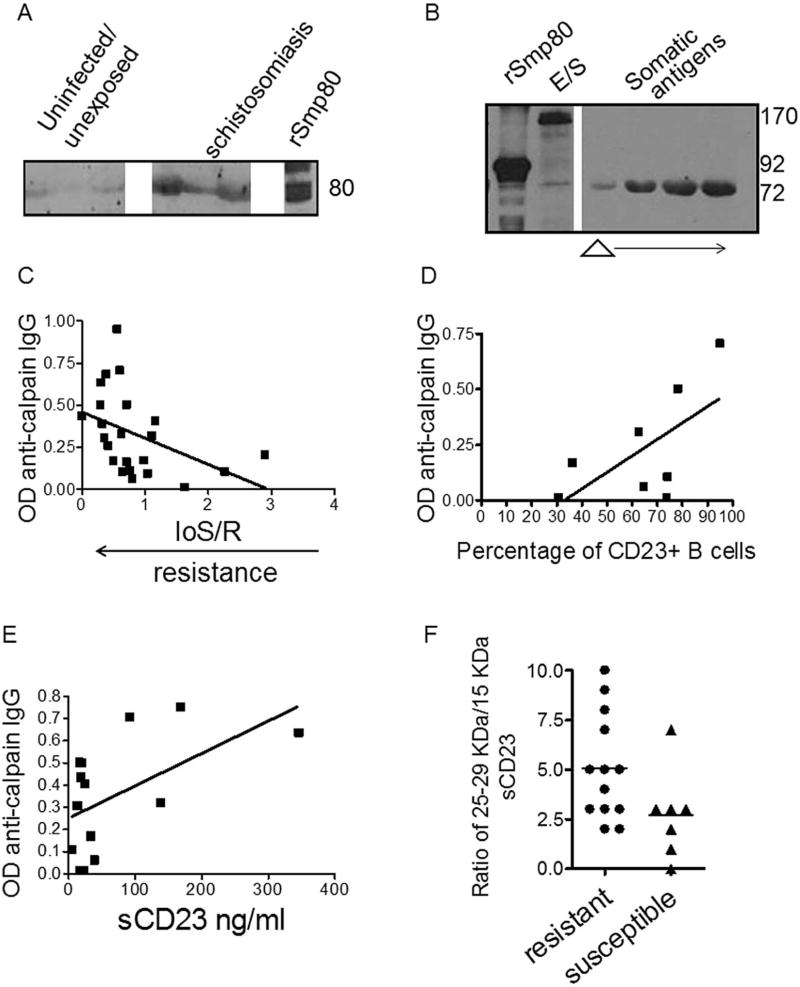
3.6 Host immunity to calpain/Sm-p80
Calpain/Sm-p80 is detectable in the serum of individuals infected with schistosomes indicating that the protease is systemic during infection at relatively high concentrations (Fig. 5A). Both somatic antigens and E/S of schistosomula contain high levels of calpain/Sm-p80 implying a role in parasitism (Fig. 5B). Sm-p80 is a vaccine candidate for schistosomiasis under development for Phase I clinical trials (33, 47–50). Figure 5C demonstrates that increased levels of anti-Sm-p80 IgG are associated with a history of resistance to reinfection with Schistosoma mansoni in occupationally-exposed car washers in Kenya. Interestingly, we found a positive correlation between increased levels of anti-calpain IgG and surface levels of CD23 on B cells ex vivo suggesting that immunity to calpain/Sm-p80 may help to reduce in vivo changes in CD23 (Fig. 5D).
Figure 5. Immunity to schistosomal calpain correlates with resistance and higher surface levels of CD23.
A. Schistosomal calpain can be detected in the bloodstream of subjects with schistosomiasis (middle panel). B. Calpain can be detected in E/S products (left panel) and in somatic antigens from mechanically-processed schistosomula (right panel). Higher MW calpain in E/S may be due to aggregation of calpain with host or parasite-derived proteins. rSm-p80 is shown in leftmost panel. C. Anti-calpain IgG levels in serum from Kenyan car washers positively correlate with the level of resistance over time (n=23; P=0.04; r=−0.423). D. Anti-calpain IgG levels correlate with the percentage of CD23+ B cells in blood. CD23+ B cells were evaluated in fresh, unprocessed whole blood samples (n=8; P=0.09; r=0.629). E. Anti-calpain IgG levels positively correlate with total levels of sCD23 measured in serum (n=14; P=0.04); r=0.545). F. The ratio of 25–29 kDa sCD23 to 15 kDa sCD23 levels is higher in patients resistant to reinfection with schistosomes (n=13; 95% CI=3.5 to 6.7) compared to more susceptible subjects (n=7; CI=0.66 to 4.7; P=0.05)
Nevertheless, it should be noted that anti-Sm-p80 antibody levels were positively associated with total levels of sCD23 (Fig. 5E). We previously reported that total levels of serum sCD23 were correlated with resistance to schistosomiasis in HIV-negative hyper-exposed individuals (51). Based on the multiple effects of sCD23 on immunity, larger fragments of sCD23 most likely have protective roles in schistosomiasis, particularly the 25–29kDa fragments which can increase IgE (25). To better clarify the role of heterogeneous soluble fragments in schistosomiasis, we evaluated the relationship between the concentration of 25–29 kDa and 15 KDa sCD23 serum fragments and found that an increased ratio of the 25–29 kDa to the 15 kDa fragment (see Fig. 2A; rightmost and Fig. 2B) was associated with higher levels of resistance to reinfection (Fig. 5F).
3.7 SG-sCD23 as an allergy preventative
Parasitic worm infection is associated with a low incidence of allergic disease despite the presence of increased IgE and cellular mediators in the bloodstream (52). Nevertheless, reduction of systemic IgE is a goal for several therapeutics aimed at controlling allergy (34, 53). We hypothesized that SG-sCD23 would represent an ideal mechanism to regulate IgE in allergy in a more natural manner to mimic the potential beneficial effects of parasite infection. Based on its size and functional characteristics, we expressed amino acids 156–292 of CD23 in E. coli to produce a fragment containing the lectin head with a reduction in the C-terminal tail (rSG-sCD23; Fig. 6A). Existing data strongly suggest that this E. coli-expressed fragment will fold properly and bind IgE (25). We demonstrate that rSG-sCD23 reduced the ability of exogenous IgE to bind to both CD23+ and FcεRI+ cells (Fig. 6 B&C).
Because monomers of CD23 have been shown to have low affinity for IgE, we aimed to increase IgE-binding ability of rSG-sCD23 without the requirement of the trimeric form, which increases IgE production (54). Calcium augments the binding of IgE to CD23, which bears significant sequence similarity to other calcium binding lectins, such as the human asialoglycoprotein receptor, mannose-binding protein (MBP), and DC-SIGN (55, 56). The best characterized of these proteins, MBP, is known to chelate calcium using 8 atoms to form a square pyramidal chelation structure (56). In contrast, CD23 appears to bind calcium using only 7 atoms. Because regions associated with calcium chelation are also associated with IgE binding (57), we posited that stabilizing this region on CD23 would increase binding to IgE. To promote the stability of calcium binding, we mutated residue 258 (an aspartic acid) to a glutamic acid on SG-sCD23 (SG-sCD23-A/G) changing the heptavalent calcium chelation site on the lectin head to an octavalent site.
The presence of rSG-sCD23-A/G in IgE-binding assays reduced the level of detectable surface IgE on cells (Fig. 6B & C). The reduction was greater than native rSG-sCD23 and similar to levels noted with Xolair®, a monoclonal antibody used to reduce IgE in severe asthma (Fig. 6C)(11).
The same blocking ability was demonstrated when rSG-sCD23-A/G was expressed as a fusion protein with IgG4 Fc (rSG-sCD23-A/G-IgG4; Fig. 6D & E.) The IgG4 Fc fusion increases the half-life of protein-based biologics (58), but binds Fc receptors poorly in vivo.
3.8 SG-sCD23-A/G prevents allergy in vivo
We next tested the ability of rSG-sCD23-A/G and rSG-sCD23-A/G-IgG4 to prevent acute allergic responses in vivo in FcεRI α chain transgenic mice (Fig. 7A) using a protocol that mimicked the increase in IgE following allergen exposure (Fig. 7B & C.) This protocol induces an acute allergic response in mice characterized by histamine release and intense scratching that lasts approximately 20 minutes. We demonstrate that rSG-sCD23-A/G and rSG-sCD23-A/G-IgG4 reduce allergic reactions in vivo as measured by allergic score and histamine levels, similar to levels reduced by Xolair (Fig. 7E). In addition, rSG-sCD23-A/G-IgG4 fusion protein was able to reduce the effect of the bolus IgE injection for at least 48 hours (Fig. 7F).
Finally, because schistosomes are intravascular parasites that appear to cause chronic Th2-responses, we tested the influence of SG-sCD23-A/G on circulating basophil IgE-mediated activation. We show that SG-sCD23-A/G inhibited IgE-mediated histamine release (Fig. 7G) suggesting that schistosomes may reduce intravascular IgE-mediated degranulation of basophils through SG-sCD23.
4.0 Discussion
It is remains unknown why people infected with parasitic worms do not display frank allergic responses to, for example, local pollen, despite the exceedingly increased immune components that cause allergy. Observations of intense eosinophilia or elevated IgE levels noted in humans with helminthiasis are in conflict with current hypotheses that worm infection may play a role in reducing allergic responses through IL-10 or T regulatory cells (59). We present a possible additional mechanism by which trematodes regulate protective IgE-mediated responses, namely by cleaving a cell-associated IgE-receptor, CD23, and releasing an IgE decoy receptor that inhibits IgE from binding to target cells. In addition, SG-sCD23 may be inhibitory to cell-bound IgE-mediated basophil degranulation. Others have shown that sCD23 and FcεRI α bind to different regions of IgE hypothesizing that this shields the host from sCD23-mediated IgE cross-linking on FcεRI+ cells (60, 61). Our results suggest that sCD23 may in fact actively prevent FcεRI-bound IgE from cross-linking. Bearing in mind that schistosomiasis is a disease caused by intravascular worms, inhibition of active basophil degranulation may be necessary to prevent excessive allergic-like responses in the bloodstream. Interestingly, suppression of basophil function may be vital during PZQ treatment, which induces a massive allergic-like response upon the death of adult worms, with relatively few clinical symptoms (8, 62).
Small sCD23 monomers have also been shown to directly reduce IgE secretion by BCRε+ memory B cells and decrease pro-inflammatory cytokine secretion by macrophages suggesting multiple anti-inflammatory and perhaps immuno-regulatory mechanisms of SG-sCD23 (25, 63). SG-sCD23 likely further dampens inflammatory responses during infection due to the reduction of the CD21-binding tail. In contrast, Derp-1, which cleaves CD23 to release a 16/17 kDa fragment, is an immunodominant antigen for the stimulation of specific IgE in allergic responses to dust mites. We detected only small amounts of anti-calpain IgE in schistosomiasis suggesting that Derp-1 and schistosomal calpain induce host-parasite interactions that affect IgE regulation differently.
Although increased absolute levels of serum sCD23 correlate with a history of protection against reinfection (51), we report here that a higher ratio of the 15kDa fragment to the 25–29 kDa is associated with reduced protection. Thus, competition for binding sites between the different sized sCD23 isoforms may influence protective immunity or allergy pathways. By targeting CD23, as well as downstream pathways involving CD21, schistosomes may use a multi–pronged attack on IgE-mediated protective responses in addition to those directly cleaving IgE molecules (46).
The molecular mechanism by which schistosomes cleave CD23 remains to be defined. Derp-1, which is a cysteine protease, cleaves CD23 at two sites (Ser155–Ser156 and Glu298-Ser299) to produce a fragment (der-sCD23) containing the lectin domain and part of the C-terminal tail (amino acids 156–298) (40). This 16/17-kDa fragment contains the minimum structural requirement for binding to both IgE and CD21, but lacks the leucine zipper portion and cannot oligomerize (39). SG-sCD23 appears to be similar to der-sCD23 and another described sCD23 fragment, but differs slightly in molecular weight and does not bind CD21 (64). As the generation of 25- and 16/17- kDa sCD23 fragments is thought to occur from cleavage of the larger, soluble fragments from both microbial- and host- proteases, SG-sCD23 may be cleaved from larger, soluble fragments. However, our observations that cell surface levels of CD23 were reduced in the presence of schistosomes and protected by bound IgE indicate a more complex host-parasite interaction. The precise cleavage sites will be determined in future studies.
Our results support a recent publication demonstrating that total levels of sCD23 were associated with reduced atopy in schistosome-infected individuals, though the report did not distinguish between the different sCD23 fragments or the level of immunity to schistosomiasis (65). Based on its functional characteristics, we hypothesized that SG-sCD23 would be ideal for inhibiting IgE-mediated responses in atopic patients. Our allergy drug was expressed using the amino acid sequence 156–292 of CD23, which includes the IgE-binding lectin head and sufficient residues for folding, but is devoid of those required for CD21 interaction (25). Because previous iterations of sCD23 as an allergy treatment required trimerization for increased efficacy, we sought to improve the efficacy of the rSG-sCD23-based drug by introducing a mutation at position Asp258, changing the aspartic acid to a glutamic acid (rSG-sCD23-A/G). This mutation allows the sCD23 fragment to carry an additional carboxyl group, thereby altering the heptavalent calcium chelation site to an octavalent chelation site, which we predicted would stabilize calcium and thus IgE binding. Published reports indicate that position Asp258 on CD23 is indeed critical for IgE-binding (61). rSG-sCD23-A/G was demonstrated to have an increased efficacy in inhibiting IgE from binding to its target cells. Furthermore, SG-sCD23-A/G - IgG4 fusion protein was equally effective in preventing allergy in vivo, which opens the door for a variety of treatment regimens and allergic conditions treatable with our drug.
In conclusion, schistosomes appear to target CD23 in immuno-evasive tactics that could be exploited for effective allergy treatments. From a schistosomiasis disease perspective, our findings suggest that all three mammalian life stages (schistosomula, adult, egg) result in SG-CD23. Because eggs are not killed by PZQ treatment, residual tissue eggs might continue to modulate CD23- mediated immunity even after successful worm clearance, further confirming the need for a vaccine to reduce the accumulation of eggs in the human host. Although published results indicate high levels of anti-Sm-p80 IgG develop during resistance, we must reconcile how a vaccine against calpain/Smp80, would affect systemic allergic responses, the potential role in immuno-regulatory pathways observed by others, and the risk associated with PZQ treatment with a higher number of samples from infected and uninfected subjects.
Highlights.
Schistosoma mansoni appears to cleave CD23 to release a 15 kDa soluble (s) CD23 fragment (SG-sCD23)
SG-sCD23 can prevent IgE from binding to its receptors and prevent acute allergy
A point mutation in the IgE-binding head, D258E, increased efficacy of SG-sCD23
SG-sCD23 may be an effective allergy treatment
Immunity to calpain may inhibit CD23 cleavage and improve protection against schistosomiasis
Acknowledgments
Funding for this proposal was provided by NIAID AI074843 (LG), The Walter H. Coulter Foundation and Boston University (LG), The Boston University SURF program, and NIAID AI090533 (AS). We like to thank Dr. Vincent Ling and Lisa Orecchio (both of Iridium Biosciences) for helpful discussions on sCD23-IgG4; Dr. George Vlasuk (GSK) for suggestions on constructing the fusion protein; Ashley Cruz and Daniel Onguru for technical assistance, and Balvinder Dhaliwal, King's College London, London for helpful discussions on Asp258.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hotez PJ, Bundy DAP, Beegle K, Brooker S, Drake L, de Silva N, Montresor A, Engels D, Jukes M, Chitsulo L, Chow J, Laxminarayan R, Michaud C, Bethony J, Correa-Oliveira R, Shuhua X, Fenwick A, Savioli L. Helminth Infections: Soil-transmitted Helminth Infections and Schistosomiasis. In: Jamison DT, Breman JG, Measham AR, et al., editors. Disease Control Priorities in Developing Countries. Washington (DC): 2006. [Google Scholar]
- 2.Elliott DE, Weinstock JV. Helminth-host immunological interactions: prevention and control of immune-mediated diseases. Ann N Y Acad Sci. 2012;1247:83–96. doi: 10.1111/j.1749-6632.2011.06292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337(6093):431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitre E, Norwood S, Nutman TB. Saturation of immunoglobulin E (IgE) binding sites by polyclonal IgE does not explain the protective effect of helminth infections against atopy. Infect Immun. 2005;73(7):4106–4111. doi: 10.1128/IAI.73.7.4106-4111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broide DH. Immunomodulation of allergic disease. Annu Rev Med. 2009;60:279–291. doi: 10.1146/annurev.med.60.041807.123524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganley-Leal LM, Mwinzi PN, Cetre-Sossah CB, Andove J, Hightower AW, Karanja DM, Colley DG, Secor WE. Correlation between eosinophils and protection against reinfection with Schistosoma mansoni and the effect of human immunodeficiency virus type 1 coinfection in humans. Infection and Immunity. 2006;74(4):2169–2176. doi: 10.1128/IAI.74.4.2169-2176.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunne D, Butterworth A, Fulford A, Kariuki H, Langley J, Ouma J, Capron A, Pierce R, Sturrock R. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992;22(6):1483–1494. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- 8.Satti MZ, Cahen P, Skov PS, Joseph S, Jones FM, Fitzsimmons C, Hoffmann KF, Reimert C, Kariuki HC, Kazibwe F, Mwatha JK, Kimani G, Vennervald BJ, Ouma JH, Kabatereine NB, Dunne DW. Changes in IgE- and antigen-dependent histamine-release in peripheral blood of Schistosoma mansoni-infected Ugandan fishermen after treatment with praziquantel. BMC Immunol. 2004;5(1):6. doi: 10.1186/1471-2172-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glovsky MM. Measuring allergen-specific IgE: where have we been and where are we going? Methods Mol Biol. 2007;378:205–219. doi: 10.1007/978-1-59745-323-3_15. [DOI] [PubMed] [Google Scholar]
- 10.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8(3):205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton RG, Marcotte GV, Saini SS. Immunological methods for quantifying free and total serum IgE levels in allergy patients receiving omalizumab (Xolair) therapy. J Immunol Methods. 2005;303(1–2):81–91. doi: 10.1016/j.jim.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Dunne DW, B A, Fulford AJ, Ouma JH, Sturrock RF. Human IgE responses to Schistosoma mansoni and resistance to reinfection. Memorias do Instituto Oswaldo Cruz. 1992;4:99–103. doi: 10.1590/s0074-02761992000800014. [DOI] [PubMed] [Google Scholar]
- 13.Jiz M, Friedman JF, Leenstra T, Jarilla B, Pablo A, Langdon G, Pond-Tor S, Wu HW, Manalo D, Olveda R, Acosta L, Kurtis JD. Immunoglobulin E (IgE) responses to paramyosin predict resistance to reinfection with Schistosoma japonicum and are attenuated by IgG4. Infect Immun. 2009;77(5):2051–2058. doi: 10.1128/IAI.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gould HJ, Sutton BJ, Beavil AJ, Beavil RL, McCloskey N, Coker HA, Fear D, Smurthwaite L. The biology of IGE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 15.Gounni AS, Lamkhioued B, Ochiai K, Tanaka Y, Delaporte E, Capron A, Kinet JP, Capron M. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature. 1994;367(6459):183–186. doi: 10.1038/367183a0. [DOI] [PubMed] [Google Scholar]
- 16.Mwinzi PN, Ganley-Leal L, Black CL, Secor WE, Karanja DM, Colley DG. Circulating CD23(+) B Cell Subset Correlates with the Development of Resistance to Schistosoma mansoni Reinfection in Occupationally Exposed Adults Who Have Undergone Multiple Treatments. J Infect Dis. 2009;199(2):272–279. doi: 10.1086/595792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffith QK, Liang Y, Onguru DO, Mwinzi PN, Ganley-Leal LM. CD23-bound IgE augments and dominates recall responses through human naive B cells. J Immunol. 2011;186(2):1060–1067. doi: 10.4049/jimmunol.1002709. [DOI] [PubMed] [Google Scholar]
- 18.Onguru D, Liang Y, Elliot J, Mwinzi P, Ganley-Leal L. CD23b isoform expression in human schistosomiasis identifies a novel subset of activated B cells. Infect Immun. 2011;79(9):3770–3777. doi: 10.1128/IAI.05094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burton OT, Oettgen HC. Beyond immediate hypersensitivity: evolving roles for IgE antibodies in immune homeostasis and allergic diseases. Immunol Rev. 2011;242(1):128–143. doi: 10.1111/j.1600-065X.2011.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hibbert R, Teriete P, Grundy G, Beavil R, Reljic R, Holers V, Hannan J, Sutton B, Gould H, McDonnell J. The structure of human CD23 and its interactions with IgE and CD21. Journal of Experimental Medicine. 2005;202(6):751–760. doi: 10.1084/jem.20050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aubry J, Pochon S, Graber P, Jansen K, Bonnefoy J. CD21 is a ligand for CD23 and regulates IgE production. Nature. 1992;358(6386):505–507. doi: 10.1038/358505a0. [DOI] [PubMed] [Google Scholar]
- 22.Lemieux GA, Blumenkron F, Yeung N, Zhou P, Williams J, Grammer AC, Petrovich R, Lipsky PE, Moss ML, Werb Z. The low affinity IgE receptor (CD23) is cleaved by the metalloproteinase ADAM10. J Biol Chem. 2007;282(20):14836–14844. doi: 10.1074/jbc.M608414200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowles SL, Jaeger C, Ferrara C, Fingeroth J, Van De Venter M, Oosthuizen V. Comparative binding of soluble fragments (derCD23, sCD23, and exCD23) of recombinant human CD23 to CD21 (SCR 1–2) and native IgE, and their effect on IgE regulation. Cell Immunol. 2011;271(2):371–378. doi: 10.1016/j.cellimm.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Brignone C, Munoz O, Batoz M, Rouquette-Jazdanian A, Cousin JL. Proteases produced by activated neutrophils are able to release soluble CD23 fragments endowed with proinflammatory effects. Faseb J. 2001;15(11):2027–2029. doi: 10.1096/fj.00-0773fje. [DOI] [PubMed] [Google Scholar]
- 25.McCloskey N, Hunt J, Beavil RL, Jutton MR, Grundy GJ, Girardi E, Fabiane SM, Fear DJ, Conrad DH, Sutton BJ, Gould HJ. Soluble CD23 monomers inhibit and oligomers stimulate IGE synthesis in human B cells. J Biol Chem. 2007;282(33):24083–24091. doi: 10.1074/jbc.M703195200. [DOI] [PubMed] [Google Scholar]
- 26.Fremeaux-Bacchi V, Fischer E, Lecoanet-Henchoz S, Mani J, Bonnefoy J, Kazatchkine M. Soluble CD21 (sCD21) forms biologically active complexes with CD23: sCD21 is present in normal plasma as a complex with trimeric CD23 and inhibits soluble CD23-induced IgE synthesis by B cells. Int Immunol. 1998;10(10):1459–1466. doi: 10.1093/intimm/10.10.1459. [DOI] [PubMed] [Google Scholar]
- 27.Kwon HS, Park MC, Kim DG, Cho K, Park YW, Han JM, Kim S. Identification of CD23 as a functional receptor for the proinflammatory cytokine AIMP1/p43. J Cell Sci. 2012;125(Pt 19):4620–4629. doi: 10.1242/jcs.108209. [DOI] [PubMed] [Google Scholar]
- 28.Cooper AM, Hobson PS, Jutton MR, Kao MW, Drung B, Schmidt B, Fear DJ, Beavil AJ, McDonnell JM, Sutton BJ, Gould HJ. Soluble CD23 controls IgE synthesis and homeostasis in human B cells. J Immunol. 2012;188(7):3199–3207. doi: 10.4049/jimmunol.1102689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu YJ, Cairns JA, Holder MJ, Abbot SD, Jansen KU, Bonnefoy JY, Gordon J, MacLennan IC. Recombinant 25-kDa CD23 and interleukin 1 alpha promote the survival of germinal center B cells: evidence for bifurcation in the development of centrocytes rescued from apoptosis. Eur J Immunol. 1991;21(5):1107–1114. doi: 10.1002/eji.1830210504. [DOI] [PubMed] [Google Scholar]
- 30.Karanja DM, Hightower AW, Colley DG, Mwinzi PN, Galil K, Andove J, Secor WE. Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and effect of HIV-1 co-infection on susceptibility to schistosomiasis: a longitudinal study. Lancet. 2002;360(9333):592–596. doi: 10.1016/S0140-6736(02)09781-7. [DOI] [PubMed] [Google Scholar]
- 31.Basch PF, DiConza JJ. In vitro development of Schistosoma mansoni cercariae. J Parasitol. 1977;63(2):245–249. [PubMed] [Google Scholar]
- 32.Siddiqui AA, Phillips T, Charest H, Podesta RB, Quinlin ML, Pinkston JR, Lloyd JD, Paz M, Villalovos RM, Pompa J. Induction of protective immunity against Schistosoma mansoni via DNA priming and boosting with the large subunit of calpain (Sm-p80): adjuvant effects of granulocyte-macrophage colony-stimulating factor and interleukin-4. Infect Immun. 2003;71(7):3844–3851. doi: 10.1128/IAI.71.7.3844-3851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmad G, Zhang W, Torben W, Haskins C, Diggs S, Noor Z, Le L, Siddiqui AA. Prime-boost and recombinant protein vaccination strategies using Sm-p80 protects against Schistosoma mansoni infection in the mouse model to levels previously attainable only by the irradiated cercarial vaccine. Parasitol Res. 2009;105(6):1767–1777. doi: 10.1007/s00436-009-1646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacGlashan D., Jr Therapeutic efficacy of omalizumab. J Allergy Clin Immunol. 2009;123(1):114–115. doi: 10.1016/j.jaci.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 35.Dombrowicz D, Brini AT, Flamand V, Hicks E, Snouwaert JN, Kinet JP, Koller BH. Anaphylaxis mediated through a humanized high affinity IgE receptor. J Immunol. 1996;157(4):1645–1651. [PubMed] [Google Scholar]
- 36.Black CL, Muok EM, Mwinzi PN, Carter JM, Karanja DM, Secor WE, Colley DG. Increases in levels of schistosome-specific immunoglobulin E and CD23(+) B cells in a cohort of Kenyan children undergoing repeated treatment and reinfection with Schistosoma mansoni. J Infect Dis. 2010;202(3):399–405. doi: 10.1086/653828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delespesse G, S M, Peleman R. Influence of recombinant IL-4, IFN-alpha, and IFN-gamma on the production of human IgE-binding factor (soluble CD23) Journal of Immunology. 1989;142(1):134–138. [PubMed] [Google Scholar]
- 38.Letellier M, Sarfati M, Delespesse G. Mechanisms of formation of IgE-binding factors (soluble CD23)--I. Fc epsilon R II bearing B cells generate IgE-binding factors of different molecular weights. Mol Immunol. 1989;26(12):1105–1112. doi: 10.1016/0161-5890(89)90054-0. [DOI] [PubMed] [Google Scholar]
- 39.John RJ, Rusznak C, Ramjee M, Lamont AG, Abrahamson M, Hewitt EL. Functional effects of the inhibition of the cysteine protease activity of the major house dust mite allergen Der p 1 by a novel peptide-based inhibitor. Clin Exp Allergy. 2000;30(6):784–793. doi: 10.1046/j.1365-2222.2000.00840.x. [DOI] [PubMed] [Google Scholar]
- 40.Hewitt CR, Brown AP, Hart BJ, Pritchard DI. A major house dust mite allergen disrupts the immunoglobulin E network by selectively cleaving CD23: innate protection by antiproteases. J Exp Med. 1995;182(5):1537–1544. doi: 10.1084/jem.182.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulz O, Laing P, Sewell HF, Shakib F. Der p I, a major allergen of the house dust mite, proteolytically cleaves the low-affinity receptor for human IgE (CD23) Eur J Immunol. 1995;25(11):3191–3194. doi: 10.1002/eji.1830251131. [DOI] [PubMed] [Google Scholar]
- 42.Pollock KG, McNeil KS, Mottram JC, Lyons RE, Brewer JM, Scott P, Coombs GH, Alexander J. The Leishmania mexicana cysteine protease, CPB2.8, induces potent Th2 responses. J Immunol. 2003;170(4):1746–1753. doi: 10.4049/jimmunol.170.4.1746. [DOI] [PubMed] [Google Scholar]
- 43.Hansell E, Braschi S, Medzihradszky KF, Sajid M, Debnath M, Ingram J, Lim KC, McKerrow JH. Proteomic analysis of skin invasion by blood fluke larvae. PLoS Negl Trop Dis. 2008;2(7):e262. doi: 10.1371/journal.pntd.0000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knudsen GM, Medzihradszky KF, Lim KC, Hansell E, McKerrow JH. Proteomic analysis of Schistosoma mansoni cercarial secretions. Mol Cell Proteomics. 2005;4(12):1862–1875. doi: 10.1074/mcp.M500097-MCP200. [DOI] [PubMed] [Google Scholar]
- 45.Brindley PJ, Kalinna BH, Wong JY, Bogitsh BJ, King LT, Smyth DJ, Verity CK, Abbenante G, Brinkworth RI, Fairlie DP, Smythe ML, Milburn PJ, Bielefeldt-Ohmann H, Zheng Y, McManus DP. Proteolysis of human hemoglobin by schistosome cathepsin D. Mol Biochem Parasitol. 2001;112(1):103–112. doi: 10.1016/s0166-6851(00)00351-0. [DOI] [PubMed] [Google Scholar]
- 46.Aslam A, Quinn P, McIntosh RS, Shi J, Ghumra A, McKerrow JH, Bunting KA, Dunne DW, Doenhoff MJ, Morrison SL, Zhang K, Pleass RJ. Proteases from Schistosoma mansoni cercariae cleave IgE at solvent exposed interdomain regions. Mol Immunol. 2008;45(2):567–574. doi: 10.1016/j.molimm.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 47.Siddiqui AA, Zhou Y, Podesta RB, Karcz SR, Tognon CE, Strejan GH, Dekaban GA, Clarke MW. Characterization of Ca(2+)-dependent neutral protease (calpain) from human blood flukes, Schistosoma mansoni. Biochim Biophys Acta. 1993;1181(1):37–44. doi: 10.1016/0925-4439(93)90087-h. [DOI] [PubMed] [Google Scholar]
- 48.Ahmad G, Zhang W, Torben W, Noor Z, Siddiqui AA. Protective effects of Sm-p80 in the presence of resiquimod as an adjuvant against challenge infection with Schistosoma mansoni in mice. Int J Infect Dis. 14(9):e781–787. doi: 10.1016/j.ijid.2010.02.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hota-Mitchell S, Siddiqui AA, Dekaban GA, Smith J, Tognon C, Podesta RB. Protection against Schistosoma mansoni infection with a recombinant baculovirus-expressed subunit of calpain. Vaccine. 1997;15(15):1631–1640. doi: 10.1016/s0264-410x(97)00081-9. [DOI] [PubMed] [Google Scholar]
- 50.Siddiqui AA, Pinkston JR, Quinlin ML, Kavikondala V, Rewers-Felkins KA, Phillips T, Pompa J. Characterization of protective immunity induced against Schistosoma mansoni via DNA priming with the large subunit of calpain (Sm-p80) in the presence of genetic adjuvants. Parasite. 2005;12(1):3–8. doi: 10.1051/parasite/2005121003. [DOI] [PubMed] [Google Scholar]
- 51.Mwinzi PN, Ganley-Leal L, Black CL, Secor WE, Karanja DM, Colley DG. Circulating CD23+ B cell subset correlates with the development of resistance to Schistosoma mansoni reinfection in occupationally exposed adults who have undergone multiple treatments. J Infect Dis. 2009;199(2):272–279. doi: 10.1086/595792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Renz H, Blumer N, Virna S, Sel S, Garn H. The immunological basis of the hygiene hypothesis. Chem Immunol Allergy. 2006;91:30–48. doi: 10.1159/000090228. [DOI] [PubMed] [Google Scholar]
- 53.Spiess C, Bevers J, 3rd, Jackman J, Chiang N, Nakamura G, Dillon M, Liu H, Molina P, Elliott JM, Shatz W, Scheer JM, Giese G, Persson J, Zhang Y, Dennis MS, Giulianotti J, Gupta P, Reilly D, Palma E, Wang J, Stefanich E, Scheerens H, Fuh G, Wu LC. Development of a Human IgG4 Bispecific Antibody for Dual Targeting of Interleukin-4 (IL-4) and Interleukin-13 (IL-13) Cytokines. J Biol Chem. 2013;288(37):26583–26593. doi: 10.1074/jbc.M113.480483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelly AE, Chen BH, Woodward EC, Conrad DH. Production of a chimeric form of CD23 that is oligomeric and blocks IgE binding to the Fc epsilonRI. J Immunol. 1998;161(12):6696–6704. [PubMed] [Google Scholar]
- 55.Wurzburg BA, Tarchevskaya SS, Jardetzky TS. Structural changes in the lectin domain of CD23, the low-affinity IgE receptor, upon calcium binding. Structure. 2006;14(6):1049–1058. doi: 10.1016/j.str.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 56.Weis WI, Drickamer K, Hendrickson WA. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature. 1992;360(6400):127–134. doi: 10.1038/360127a0. [DOI] [PubMed] [Google Scholar]
- 57.Bettler B, Texido G, Raggini S, Ruegg D, Hofstetter H. Immunoglobulin E-binding site in Fc epsilon receptor (Fc epsilon RII/CD23) identified by homolog-scanning mutagenesis. J Biol Chem. 1992;267(1):185–191. [PubMed] [Google Scholar]
- 58.Goldenberg MM. Etanercept, a novel drug for the treatment of patients with severe, active rheumatoid arthritis. Clin Ther. 1999;21(1):75–87. doi: 10.1016/S0149-2918(00)88269-7. discussion 71–72. [DOI] [PubMed] [Google Scholar]
- 59.McSorley HJ, Hewitson JP, Maizels RM. Immunomodulation by helminth parasites: defining mechanisms and mediators. Int J Parasitol. 2013;43(3–4):301–310. doi: 10.1016/j.ijpara.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 60.Dhaliwal B, Yuan D, Pang MO, Henry AJ, Cain K, Oxbrow A, Fabiane SM, Beavil AJ, McDonnell JM, Gould HJ, Sutton BJ. Crystal structure of IgE bound to its B-cell receptor CD23 reveals a mechanism of reciprocal allosteric inhibition with high affinity receptor FcepsilonRI. Proc Natl Acad Sci U S A. 2012;109(31):12686–12691. doi: 10.1073/pnas.1207278109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borthakur S, Hibbert RG, Pang MO, Yahya N, Bax HJ, Kao MW, Cooper AM, Beavil AJ, Sutton BJ, Gould HJ, McDonnell JM. Mapping of the CD23 binding site on immunoglobulin E (IgE) and allosteric control of the IgE-Fc epsilonRI interaction. J Biol Chem. 2012;287(37):31457–31461. doi: 10.1074/jbc.C112.397059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reimert CM, Fitzsimmons CM, Joseph S, Mwatha JK, Jones FM, Kimani G, Hoffmann KF, Booth M, Kabatereine NB, Dunne DW, Vennervald BJ. Eosinophil activity in Schistosoma mansoni infections in vivo and in vitro in relation to plasma cytokine profile pre- and posttreatment with praziquantel. Clin Vaccine Immunol. 2006;13(5):584–593. doi: 10.1128/CVI.13.5.584-593.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Punnonen J, G A, Cocks BG, de Vries JE. Role of interleukin-4 and interleukin-13 in synthesis of IgE and expression of CD23 by human B cells. Allergy. 1994;49(8):576–586. doi: 10.1111/j.1398-9995.1994.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 64.Sarfati M, Bettler B, Letellier M, Fournier S, Rubio-Trujillo M, Hofstetter H, Delespesse G. Native and recombinant soluble CD23 fragments with IgE suppressive activity. Immunology. 1992;76(4):662–667. [PMC free article] [PubMed] [Google Scholar]
- 65.Rujeni N, Nausch N, Midzi N, Gwisai R, Mduluza T, Taylor DW, Mutapi F. Soluble CD23 levels are inversely associated with atopy and parasite-specific IgE levels but not with polyclonal IgE levels in people exposed to helminth infection. Int Arch Allergy Immunol. 2013;161(4):333–341. doi: 10.1159/000346545. [DOI] [PMC free article] [PubMed] [Google Scholar]