ABSTRACT
Oxygen is essential for most animals, and exposure to a complete lack of oxygen, i.e. anoxia, can result in irreparable damage to cells that can extend up to the organismal level to negatively affect performance. Although it is known that brief anoxia exposure may confer cross-tolerance to other stressors, few data exist on the biochemical and organismal consequences of repeated intermittent bouts of anoxia exposure. In nature, the Caribbean fruit fly, Anastrepha suspensa (Diptera: Tephritidae), is frequently exposed to heavy tropical rainfall while pupating in the soil, equating to multiple exposures to hypoxia or anoxia during development. Here, we tested whether prior anoxia exposures during pupal development can induce a beneficial acclimation response, and we explored the consequences of prior exposure for both whole-organism performance and correlated biochemical metrics. Pharate adults (the last developmental stage in the pupal case) were most sensitive to anoxia exposure, showing decreased survival and fertility compared with controls. These negative impacts were ameliorated by exposure to anoxia in earlier pupal developmental stages, indicating a hormetic effect of prior anoxia exposure. Anoxia exposure early in pupal development reduced the oxygen debt repaid after anoxia exposure relative to pharate adults experiencing anoxia for the first time. Lipid levels were highest in all pupal stages when exposed to prior anoxia. Prior anoxia thus benefits organismal performance and relocates resources towards lipid storage throughout pupal–adult development.
KEY WORDS: Antioxidants, Fruit fly, Hormesis, Hypoxia, Metabolism, Oxidative stress
Summary: Prior anoxia exposure benefits organismal performance, increases lipid levels and reduces the oxygen debt compared with a singular exposure to anoxia in the Caribbean fruit fly.
INTRODUCTION
Throughout their lives, organisms are repeatedly exposed to stress, and the ability to mitigate stressful conditions is crucial for maintaining performance. Substantial changes in temperature, humidity or food availability, for example, can severely impact performance-related traits, including growth, survival and reproduction (Beaulieu et al., 2015; Benoit et al., 2011; King and MacRae, 2015; Romero, 2012). However, stress does not inevitably lower an individual's performance. Rapid cold hardening, in which individuals receive a brief, mild cold exposure, increases hardiness to subsequent cold exposures (Lee et al., 1987), as has been observed in the vinegar fly, Drosophila melanogaster (Shreve et al., 2004), and the migratory locust, Locusta migratoria (Findsen et al., 2013). Furthermore, preconditioning to mild heat exposure (Hercus et al., 2003; Olsen et al., 2006) or irradiation (Ina and Sakai, 2004, 2005) can even increase performance during or after exposure to subsequent heat or irradiation stress. A mild exposure to stress may thus induce a conditional hormetic response (Calabrese et al., 2007; Costantini et al., 2010), where physiological acclimation leads to improved resistance or tolerance to the benefit of organismal performance when subsequently exposed to additional stress.
Oxygen-dependent cellular respiration in mitochondria yields high levels of ATP required to sustain cellular functions; hence a complete lack of oxygen, i.e. anoxia, can have catastrophic consequences for an organism (Hochachka, 1980; Lutz, 1992). Most mammals and birds are able to withstand anoxia no longer than a few minutes (but see Hermes-Lima and Zenteno-Savín, 2002), but many invertebrates have evolved an array of adaptations preventing severe damage from oxygen deprivation (Harrison et al., 2006). Tiger beetle larvae, for example, can survive up to 6 days immersed in water under anoxic conditions (Hoback et al., 1998). Anoxia tolerance differs among species and also among life stages as a result of adaptations to variation in environmental hypoxia exposure (Wegener and Moratzky, 1995; Woods and Lane, 2016). Exposure to anoxia can further provide cross-tolerance to other stressors. In the cactus moth, Cactoblastis cactorum, for example, a brief anoxia exposure decreased irradiation-induced oxidative damage, while increasing mating success and survival (López-Martínez et al., 2014). Similarly, brief anoxia exposure in the housefly, Musca domestica, significantly increased subsequent cold hardiness (Coulson and Bale, 1991). Anoxic conditions can thus result in cross-tolerance when organisms are exposed to a different stressor, but relatively little is known about the consequences of repeated exposure to anoxia (but see Lighton and Schilman, 2007; Van Voorhies, 2009). Repeated stress exposures frequently invoke fundamentally different responses compared with single stress exposures (Benoit et al., 2010; Marshall and Sinclair, 2012), so understanding the extent to which organisms are affected by single versus multiple low-oxygen exposures is a crucial gap in our understanding of anoxia responses.
Metabolically, insects respond to anoxia by sharply reducing metabolic rate, shutting down oxidative phosphorylation in the electron transport chain, and increasing glycolytic flux to maintain energy supply anaerobically (Kölsch et al., 2002; Van Voorhies, 2009). This results in an accumulation of anaerobic by-products such as lactate, alanine or other organic acids that acidify body fluids and alter bicarbonate buffering ability of the hemolymph (Kölsch et al., 2002; Storey and Storey, 1990; Woods and Lane, 2016). Reduced metabolic flux and loss of oxidative phosphorylation cause loss of ATP homeostasis (Kölsch et al., 2002). Loss of energetic homeostasis increases in severity with increasing time of anoxia exposure, leading to failure of transmembrane ion pumps, catastrophic ion influx into the cell, loss of membrane potential, and cellular damage (Storey and Storey, 1990; Wegener, 1993). During reperfusion and recovery from anoxia, ionic homeostasis must be restored, accumulated anaerobic by-products metabolized and high-energy adenylate levels replenished (Ellington, 1983). These energetically expensive processes result in a temporary increase in metabolic rate during recovery that corresponds to the repayment of the oxygen debt incurred during anoxia (Kölsch et al., 2002). This oxygen debt can be reduced, and organismal performance under anoxia improved, by reducing metabolic energy demands during anoxia and thus reducing the oxygen debt accumulated (Wegener and Moratzky, 1995). During reperfusion, the influx of oxygen back into the systems can cause a burst of reactive oxygen species (ROS) production. ROS damage is a major cause of reperfusion injury that becomes worse with subsequent reperfusions if insufficient time is available to fully recover in between (Lighton and Schilman, 2007). To counteract ROS damage, insects increase antioxidant defences during anoxia, in anticipation of reperfusion (Forcella et al., 2007; López-Martínez and Hahn, 2012).
Some insects live in habitats where exposure to anoxic conditions can recur frequently (Hoback and Stanley, 2001). Tropical fruit fly pests in the genus Anastrepha, for example, pupate in the soil and take several weeks to complete development, during which time they may be exposed to frequent, heavy rainfall (Hou et al., 2006; Montoya et al., 2008). Oxygen uptake is limited in submerged insects; hence, submersion stress mimics anoxia exposure (Kölsch et al., 2002; Woods and Lane, 2016). Soil-dwelling insect pupae may thus be adapted to survive hypoxia or anoxia. Here, we tested for responses to anoxia during the course of the metamorphic pupal life stage. Using Anastrepha suspensa pupae, we investigated whether prior anoxia exposure induces a hormetic response by: (1) determining how (repeated) anoxia affects performance, and (2) describing biochemical changes associated with the (repeated) anoxia stress response. We expected that prior anoxia exposure would induce a hormetic response that will increase performance-related traits through physiological mechanisms such as increased antioxidant levels and metabolic depression. Metabolic rates during pupae–adult development typically follow a U-shaped curve (Denlinger et al., 1972; Merkey et al., 2011), suggesting that anoxia sensitivity may be highest early or late in development. We monitored oxygen consumption and carbon dioxide production during reperfusion to describe the dynamics of metabolic recovery in individuals exposed to single versus repeated anoxia throughout the prepupal, pupal and pharate adult periods.
MATERIALS AND METHODS
Insects
Anastrepha suspensa (Loew 1862) (Diptera: Tephritidae) larvae were obtained from a rearing facility at the Florida Department of Agriculture and Consumer Services in Gainesville, FL, USA, and maintained in the laboratory in a climate-controlled incubator and room at a temperature of 25°C, a relative humidity of 60% and a photoperiod of 14 h:10 h light:dark.
Experimental design
Metamorphosis in higher flies, such as A. suspensa, is characterized by an additional metamorphic event, the formation of the puparium, which consists of the rigid cuticle of the third-instar larva (Denlinger and Zdarek, 1994). Pupariation is followed by pupation, pupal development, pupal–pharate adult metamorphosis, and finally adult emergence. Wandering larvae thus develop into prepupae, pupae and pharate adults while encased within the puparium. Therefore, it is important to distinguish between developmental stages within the puparium in higher flies, which we will refer to as prepupae, pupae and pharate adults throughout.
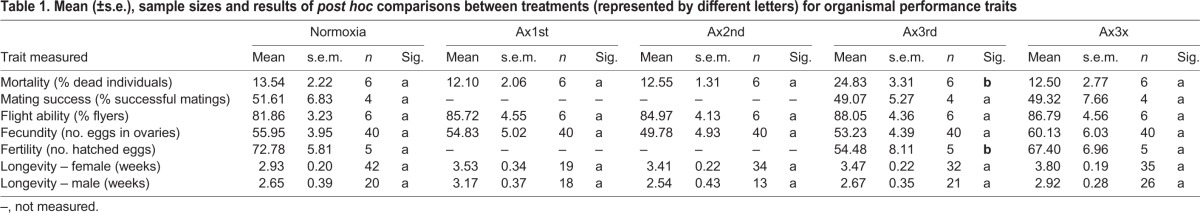
Larvae were collected just prior to pupariation. After pupariation, prepupae were divided into five treatment groups: (1) control (Nx), (2) single anoxia exposure as a prepupa (Ax1st), (3) single anoxia exposure as a pupa (Ax2nd), (4) single anoxia exposure as a pharate adult (Ax3rd) and (5) repeated anoxia exposure, once in each of the three stages (Ax3x). Each treatment group was exposed to anoxia by placing individuals in a polypropylene bag that was flushed with nitrogen for 1 min, replacing the volume of gas in the bag several times over that time period. The bag was then placed inside a second bag, nitrogen was flushed similarly and the bag was sealed for 3 h (to approximate the length of soil saturation following a short rain inundation) on day 1 (Ax1st, Ax3x), day 7 (Ax2nd, Ax3x) and/or day 14 (Ax3rd, Ax3x) after pupariation. Pupal stages that did not undergo anoxia treatment (i.e. under normoxia) were sealed in similar polypropylene bags that were heavily perforated to sustain air exchange as a handling control. Pupae were either (1) frozen in liquid nitrogen directly after exposure to normoxic or anoxic conditions and stored at −70°C until further biochemical analyses, or (2) used to measure resting metabolic rate (RMR) and whole-organism performance-related traits (Tables 1 and 2 provide an overview of all measured traits).
Table 1.
Mean (±s.e.), sample sizes and results of post hoc comparisons between treatments (represented by different letters) for organismal performance traits
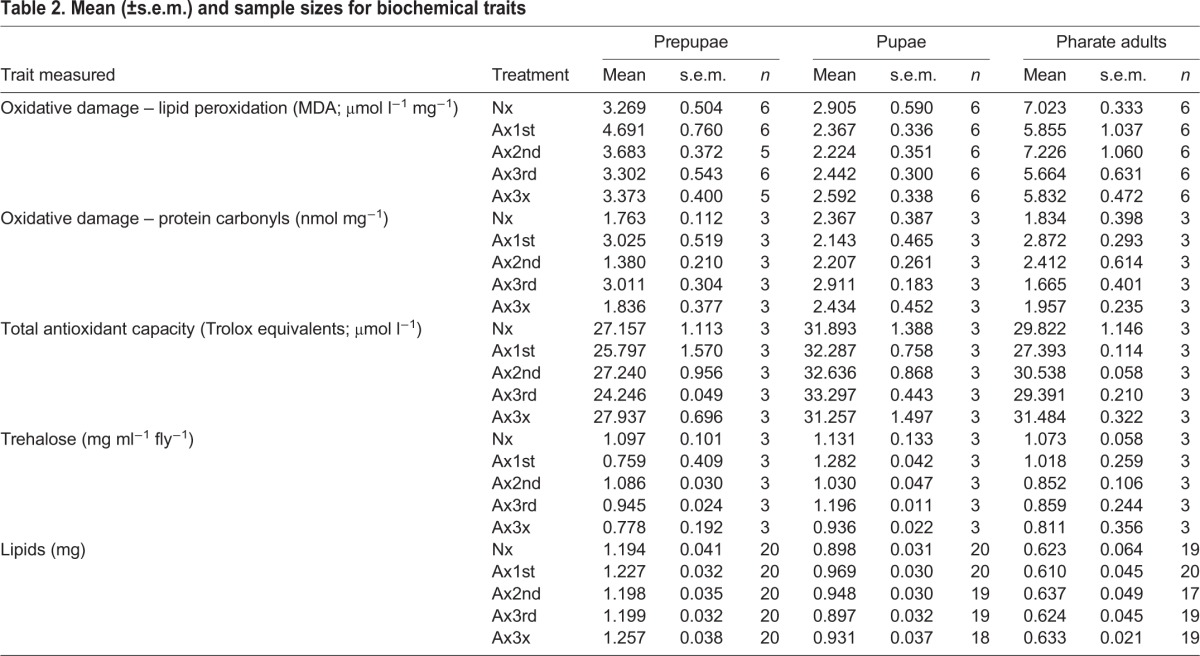
Table 2.
Mean (±s.e.m.) and sample sizes for biochemical traits
Effect of anoxia on desiccation
Heavy rainfall leads to repeated submersion of A. suspensa pupae in the soil under natural conditions. For our experiments, we assumed that submersion in soil reduces oxygen availability similarly to dry anoxic conditions, in which atmospheric air is completely replaced by nitrogen. By exposing pupae to anoxic conditions within a dry rather than wet environment, anoxia exposure could lead to desiccation stress that would skew our performance and biochemical assays. To test whether our experimental protocol within a dry environment led to desiccation, an experiment was performed comparing the masses of all three life stages before and after exposure to anoxia or normoxia treatments performed as above (∼72 individuals per treatment, total N=504). We compared water loss rates by weighing A. suspensa before and after treatment on a Mettler Toledo xp6 analytical balance (Columbus, OH, USA). We analyzed our water loss data using ANOVA with developmental stage, anoxia treatment and their interaction as factors. Water loss over the 3-h period was higher in prepupae (0.501±0.0173 mg) compared with in pupae (0.103±0.0153 mg) or pharate adults (0.0893±0.00099 mg; F2,497=278, P<0.00001), probably because the recently pupated larvae lose water rapidly after pupation before reaching a steady state. Thus high water losses in recently pupariated individuals likely represent a natural water loss over this period of development. Water loss rates were generally low across all life stages and treatments, averaging 1.13±0.0964% of the initial mass. The effects of anoxia on water loss depended on the developmental stage (developmental stage×anoxia: F2,497=4.1, P=0.0429): in the pre-pupal stage, anoxia treatment slightly reduced rates of water loss (0.458±0.0263 mg in anoxia exposed prepupae versus 0.545±0.0263 mg in control prepupae; Tukey’s post hoc test, P=0.037), possibly because development was slowed, whereas in the pupal and pharate adult stages, anoxia treatment did not affect rates of water loss (P=0.889 and 0.999, respectively). There is thus no evidence that our anoxia treatments caused appreciable desiccation stress.
Organismal performance
Organismal performance-related traits were determined following protocols described in López-Martínez and Hahn (2012). In short, mortality was determined by placing one pupa per well in a 96-well plate, after which the number of individuals emerging as adults were counted. Mating success was determined by performing mate-choice assays where untreated females were allowed to choose between two differently treated males. Flight ability was tested by placing 100 pupae inside a paper ring within a Petri dish, which was covered with a black PVC cylinder containing talcum powder on the inside (to prevent climbing and stimulate flying). Flies that were found outside the tube were scored as having flown out. Fecundity was determined by dissecting females and counting the number of eggs 10 days after emergence (when females become sexually receptive). Fertility was measured in a separate experiment by counting the number of hatched eggs laid by five females per sample over 6 days. Longevity was determined by inspecting cages weekly and counting dead individuals during 10 consecutive weeks. Based on preliminary mortality data (revealing the highest differences in mortality between Ax3rd and Ax3x treated pupae), mating success and fertility were only compared between Nx, Ax3rd and Ax3x groups. Sample sizes for all traits and treatments can be found in Table 1.
Biochemistry
Oxidative damage
Lipid peroxidation was estimated by measuring malondialdehyde using the thiobarbituric acid reactive substances (TBARS) test as described in López-Martínez and Hahn (2012) using pools of five pupae per sample with five to six samples (i.e. n=5–6; see Table 2). Oxidation of proteins was measured using the method of Levine et al. (1990), adapted for A. suspensa as described in López-Martínez and Hahn (2012) using pools of five pupae per sample with three samples (Table 2).
Total antioxidant capacity
Antioxidant capacity was determined using the ABTS radical cation decolorization assay, which measures trolox-equivalent antioxidant capacity (TEAC; Re et al., 1999) according to the protocol described in López-Martínez and Hahn (2012) using pools of five pupae per sample with three samples (Table 2).
Trehalose
Trehalose concentration was determined by homogenizing pupae (pools of five pupae per sample with three samples) in 2% sodium sulfate and extracting sugars with methanol. Concentrated extracted sugars were treated with hydrochloric acid (which hydrolyzes sucrose to glucose and fructose) and sodium hydroxide (which destroys the anthrone reactivity of glucose and fructose) and then reacted with anthrone (van Handel, 1985). Trehalose was only compared between Nx (pharate adults), Ax1st, Ax2nd, Ax3rd and Ax3x samples, as no significant difference was found between developmental stages on Nx trehalose levels (Ptreatment=0.8069, Pstage=0.2091, Ptreatment×stage=0.8584; i.e. no effect of stage in Nx treated samples).
Lipid
Pupae were freeze-dried for 3 days, after which dry mass was determined. Lipids were then extracted by placing pupae in a Soxhlet apparatus containing diethyl ether for 24 h. Following lipid extraction, pupae were placed in a freeze-dryer for another 3 days, after which the lipid-free dry mass was determined. Lipid quantities were calculated by subtracting dry mass after ether extraction from dry mass before ether extraction (Table 2). Ether extraction removes predominantly neutral lipids (e.g. triglycerides, sterols and free fatty acids), and gives quantitatively similar results to chromatography-based methods of lipid quantitation (Williams et al., 2011).
Resting metabolic rate (RMR)
Once per week for each cohort starting at the first pupal stage, one individual was haphazardly chosen from each group in the evening (16:00–18:00 h), weighed and placed into a respirometry chamber. Chambers were 5–8 cm lengths of Bev-A-Line-V tubing (3.175 mm inner diameter), with quick-lock connector fittings shielded by small squares of Dacron Chiffon mesh, to prevent the pupa from falling out. After being placed into the respirometry chambers, individuals were returned to the incubator overnight to recover from handling stress. In the morning at 08:00 h, pupae in respirometry chambers were placed in a Peltier controlled-temperature chamber set to 25°C (equivalent to rearing conditions), in configuration with a flow multiplexer [RM8, Sable Systems International (SSI), Las Vegas, NV, USA] that routed air through an empty chamber or one of the five animal chambers. Chambers were flushed sequentially with dry, carbon dioxide (CO2)-free air for 2 min at a flow rate of 200 ml min−1, then sealed for 30 min before being passed through oxygen (O2) (Oxzilla II, SSI) and CO2 detectors (Licor7000, Li-Cor, Lincoln, NE, USA), equivalent to stop-flow respirometry. The multiplexer was programmed to repeat this cycle every 30 min over the following 2.5 h, to obtain baseline gas exchange values for each individual. After these baseline measurements were taken, individuals were either exposed to anoxia or kept under normoxia for 3 h, after which they were returned to the respirometer and had their respiration rates measured every 30 min over the next 6.5 h of recovery (14:00–20:30 h). Metabolic rates were measured once per week (corresponding to the pre-pupal, pupal and pharate adult developmental stages), and this was repeated for five separate cohorts (three stages×five cohorts×five treatments, n=75). Four individuals died during the experiments (two anoxia and two normoxia), leaving 71 individuals with both V̇CO2 and V̇O2 measured at 18 time points (six before and 12 after anoxia). There were 60 missing values (evenly split across treatment groups), mostly owing to an analyzer malfunction (5.6% of total data).
Statistics
Mortality, mating success, flight ability and fecundity were analyzed using one-way ANOVAs. Fertility and trehalose were analyzed using a two-way ANOVA with treatment and day as factors. The effects of developmental stage (prepupa, pupa, pharate adult) and treatment (Ax1st, Ax2nd, Ax3rd, Ax3x, Nx) on oxidative damage and total antioxidant capacity were analyzed using two-way ANOVAs. Post hoc tests were performed using Tukey's HSD. A proportional hazards model was used to determine the effect of treatment on longevity. ANOVAs and proportional hazards analyses were performed using the statistical package JMP version 11.
To test for differences in lipid levels between developmental stages and treatments, a general linear effects model was fitted with developmental stage and treatment as fixed effects and the neutral lipid-free dry mass as a covariate. For the metabolic rate measurements, we first fit linear models with time of measurement (0–2.5 h) and individual as fixed effects, and used these models to predict metabolic rates at each measurement time point for each individual (effectively smoothing the baseline data). Metabolic rates initially decreased but reached a minimum by 1.5 h and did not decrease further, so we extrapolated out to the full time course of the ‘after anoxia’ measurements, i.e. out to 6 h in 30 min increments. This gave us a baseline time series of metabolic rate for each individual that was then subtracted from all ‘after anoxia’ measurements to account for variation in individual metabolic rates and response to handling, giving us the new variables ΔV̇CO2 and ΔV̇O2. To assess the effects of past anoxia exposure, developmental stage and mass on baseline metabolic rates, we took the average of the last three time points (when metabolic rate had stabilized) and fit general linear mixed-effects models using the nlme package (R package version 3.1-128). To assess the effects of anoxia and past anoxia exposure on ΔV̇CO2 and ΔV̇O2, we fit models with fixed effects of time, anoxia, past anoxia, stage and all interactions, with individual as a random factor. For all these models, we started with saturated models and removed non-significant terms and factor levels stepwise, confirming improved fit using Akaike's information criterion, until the minimal adequate model was reached (Crawley, 2007). Lipid levels and RMR data were analyzed using R project version 3.3.3 (https://www.r-project.org/).
RESULTS
Organismal performance
Survival
Anoxia exposure in the pharate adult stage induced substantially greater mortality (∼25% mortality) than anoxia exposure in the prepupal or pupal stages, neither of which suffered more mortality than the normoxic control group (∼14% mortality in the normoxic group; F4,25=4452, P=0.025; post hoc P<0.05; Table 1). Interestingly, prior exposure to anoxia during the prepupal and pupal stages prevented deleterious effects of anoxia in the pharate adult stage. Individuals exposed to three bouts of anoxia – with one exposure in the prepupal stage, followed by a second exposure in the pupal stage and a third exposure in the pharate adult stage – suffered substantially less mortality than individuals just exposed to a single bout of anoxia in the pharate adult stage, and were not different in survival from normoxic controls.
Mating success
Treated males exposed to a single bout of anoxia as pharate adults (Ax3rd), as well as those exposed to repeated bouts of anoxia (Ax3x), were as successful in obtaining mates as males kept under normoxic conditions (F2,9=1.085, P=0.378; Table 1). Here, only Ax3rd and Ax3x treatments were compared with controls because preliminary data on mortality revealed an effect of anoxia for these treatments.
Flight ability
Flight ability did not differ between any of the treatments (F4,25=0.646, P=0.642; Table 1).
Fecundity
Fecundity did not differ between females exposed to normoxia, anoxia as prepupae, anoxia as pupae, anoxia as pharate adults and repeated anoxia exposure (F4,195=0.531, P=0.713; Table 1).
Fertility
Fertility was highest when adult females were repeatedly exposed to anoxia (mean of ∼67% of eggs hatched) and under normoxia (mean of ∼73% of eggs hatched), and was lowest when females experienced a single bout of anoxia as pharate adults (mean of ∼54% of eggs hatched; F2,12=2.2927, Ptreatment=0.0055; F4,10=3.477, Pday=0.0199; post hoc P<0.0055; Table 1).
Longevity
Longevity did not differ between treatments for males (χ2=0.688, P=0.953) or females (χ2=4.376, P=0.358; Table 1).
Biochemistry
Oxidative damage
Lipid peroxidation did not differ between treatments (F14,73=1.012, P=0.407; Table 2). Levels of lipid peroxidation did, however, differ between developmental stages (F14,73=54.746, P<0.0001), with pharate adults showing the highest level of malondialdehyde, followed by prepupae and pupae, which did not differ from each other (post hoc P<0.05). Protein oxidation did not differ between treatments or developmental stages (F14,31=1.996, P=0.055; Table 2).
Total antioxidant capacity
Neither anoxia treatment nor developmental stage affected TEAC (F4,40=0.996, P=0.481; Table 2).
Trehalose
Trehalose levels were not different between treatments (F14,29=0.7411, P=0.7097; Table 2).
Lipid
Lipid levels corrected for lean dry mass differed between treatments (F2,287=7.030, P=0.001) and stage (F1,288=244.956; P<0.0001) and there was an interaction between dry mass and stage (F1,289=19.472, P<0.0001). The effect of anoxia on lipids was dosage dependent, with lipid levels being lowest in controls (Nx), higher in individuals following a single exposure (Ax1st, Ax2nd and Ax3rd) and highest in individuals that were repeatedly exposed to anoxia (Ax3x) (Table 2, Fig. 1).
Fig. 1.
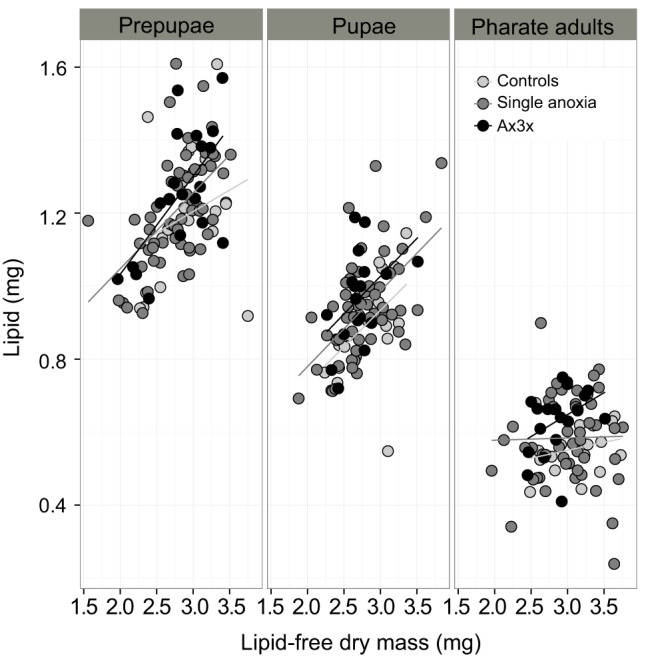
Lipid-free dry mass plotted against lipid quantities for Anastrepha suspensa pupae for normoxia (controls), single exposure and repeated exposure (Ax3x) at three stages in pupal development (prepupae, pupae and pharate adults). N=94–1007 per pupal stage.
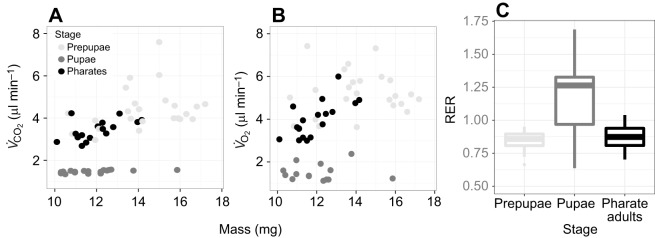
Baseline metabolic rates
There was no effect of past anoxia on baseline metabolic rates or the respiratory exchange ratio (RER; P>0.05). The baseline RER (V̇CO2/V̇O2) was 0.86±0.08 (mean±s.d.) in prepupae and pharate adults, and was significantly higher and more variable in pupae (1.17±0.30; F1,73=45.7, P<0.0001; Fig. 2C). Prepupae were significantly heavier than pupae and pharate adults (F1,73=55.2, P<0.0001), and past anoxia exposure did not affect mass (P>0.05). Metabolic rate (V̇CO2 and V̇O2) increased strongly with mass for prepupae and pharate adults, but was only weakly mass dependent in pupae (mass×stage F1,70=13.9, P=0.0004; statistics given for V̇O2 but qualitatively identical for V̇CO2 unless otherwise noted; Fig. 2A,B). Pupae had considerably lower metabolic rates for their mass compared with prepupae and pharate adults (F2,70=90.0, P<0.0001).
Fig. 2.
Respiratory gas exchange rates (proxy for metabolic rate) of prepupal, pupal and pharate adult Anastrepha suspensa. (A,B) Mass-scaling of baseline (normoxia) gas exchange rates for each life stage, and (C) respiratory gas exchange ratios (RER). N=25 per stage.
Metabolic response to anoxia
For handling controls that did not experience anoxia, V̇O2 and V̇CO2 remained at baseline levels throughout the experiment (Fig. 3). Immediately following anoxia, V̇O2 was elevated above baseline values (time×anoxia, F1,779=183.0, P<0.00001; Fig. 3A) and V̇CO2 was suppressed (stage×time×anoxia, F2,720=44.0, P<0.0001; Fig. 3B). The magnitude of perturbations in both measures gradually decreased back towards baseline levels over the subsequent 6 h. V̇O2 returned to baseline within 3 to 4 h. The absolute oxygen consumption increase in response to anoxia was slightly less in pupae compared with prepupae or pharate adults (stage×anoxia, F2,63=4.2, P=0.016). However, when expressed as a percentage of initial oxygen consumption, the absolute oxygen consumption increase was considerably larger in pupae than prepupae and pharate adults owing to their originally much lower metabolic rates (Fig. 3). Increases in oxygen consumption above baseline were 170±61% in pupae, 51±21% in prepupae and 66±22% in pharate adults (mean±s.d.). Compared with the other developmental stages, V̇CO2 in prepupae was suppressed to the greatest degree following anoxia exposure, and then increased to above control levels after 2.5 h of recovery and remained elevated for the rest of the experiment (Fig. 3B, left panel). For pupae and pharate adults, V̇CO2 dropped after anoxia exposure to a more modest degree, and returned to control levels within 2 h during recovery (Fig. 3B, middle and right panels).
Fig. 3.
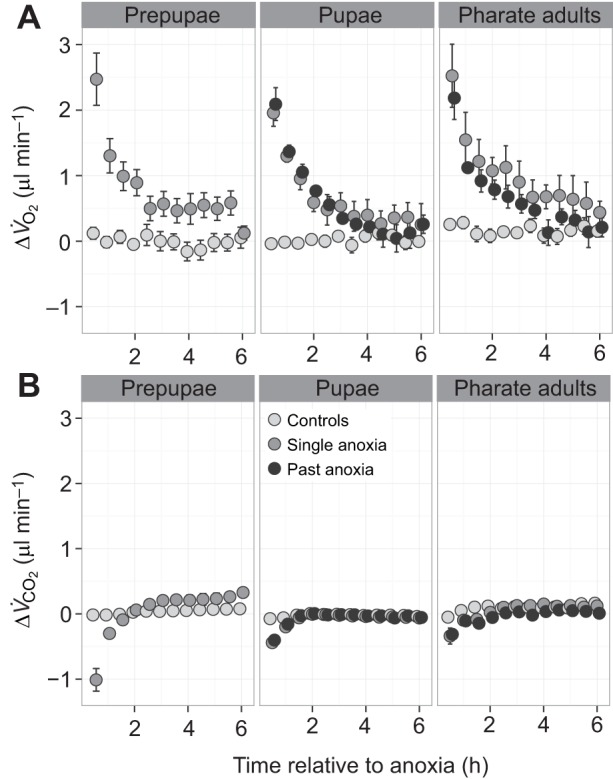
Metabolic response to anoxia for Anastrepha suspensa pupae at three stages in pupal development (prepupae, pupae and pharate adults). Data are differences from starting metabolic rates (A, VO2; B, VCO2) for each individual (means±s.e.m.). Sample sizes were as follows: prepupae: controls=13 (Nx, Ax2nd, Ax3rd), single anoxia=8 (Ax1st+Ax3x); pupae: controls=15 (Nx, Ax1st, Ax3rd), single anoxia=5 (Ax2nd), past anoxia=5 (Ax3x); pharate adults: controls=15 (Nx, Ax1st, Ax2nd), single anoxia=5 (Ax3rd), past anoxia=5 (Ax3x).
Effects of past anoxia on metabolic response to anoxia
Pupae that had experienced a past anoxia exposure had a similar V̇O2 increase after anoxia, relative to pupae experiencing anoxia for the first time (P>0.05; Fig. 3A, middle panel). However, pharate adults that had experienced prior anoxia exposures had a blunted V̇O2 increase after anoxia, relative to adults experiencing anoxia for the first time (F1,64=12.5, P=0.000;4 Fig. 3A, right panel). Past anoxia had no effect on V̇CO2 during recovery from anoxia (P>0.05).
DISCUSSION
The goal of this study was to determine whether repeated oxygen deprivation, i.e. anoxia, would induce a hormetic response in the soil-pupating Caribbean fruit fly, A. suspensa, a species that is recurrently exposed to heavy rainfall during pupal development under natural conditions (Hou et al., 2006; Montoya et al., 2008). Our results revealed that pharate adult pupae experiencing prior anoxia over the course of pupal–adult development had greater survival and higher female fertility compared with pharate adults experiencing a single bout of anoxia. The oxygen debt accumulated during anoxia exposure was lower in pharate adults with prior anoxia exposure, whereas lipid levels were enhanced, which could underlie the observed improvement in survival and fertility. Increased carbohydrate, glycogen and lipid levels have indeed been implicated in higher stress resistance in selection lines of the fruit fly D. melanogaster (Djawdan et al., 1997; Gibbs et al., 1997; Chippindale et al., 1998). Prior anoxia exposure thus increases investment in lipid synthesis during pupal development, and mitigates negative effects of anoxia on organismal performance of pharate adults.
We characterized the metabolic response to reperfusion after 3 h of anoxia at three time points from pre-pupal to adult development, and determined the effect of prior anoxia exposure on that response. Individuals at all three developmental stages increased oxygen consumption during reperfusion, consistent with repaying an oxygen debt (Ellington, 1983). Increased metabolic rate following hypoxia or anoxia exposure has been demonstrated in beetles, locusts and moths, and the magnitude of percent increases we observed were within ranges previously found by microcalorimetry (Kölsch et al., 2002; Wegener and Moratzky, 1995; but see Van Voorhies, 2009, who found no increase in oxygen consumption following hypoxia in D. melanogaster). Our data thus reinforce that insects incur an oxygen debt during anoxia. We further found that this oxygen debt was partially mitigated by prior anoxia exposure, mirroring the findings of Wegener and Moratzky (1995), who showed that a hypoxia-tolerant insect (M. sexta) incurs a lower oxygen debt during anoxia compared with a hypoxia-sensitive insect (L. migratoria). Acclimatization to anoxia or hypoxia may thus work through mechanisms similar to those occurring during evolutionary adaptation to low oxygen environments. This decrease in oxygen debt may result from a decreased accumulation of anaerobic substrates or improved adenylate homeostasis, perhaps resulting from increased metabolic suppression during anoxia.
Concurrent with the increase in oxygen consumption, pupae showed a marked suppression of CO2 emission immediately after reperfusion that returned to baseline levels within ∼2 h. Anoxia is a strong stimulus for spiracle opening (Förster and Hetz, 2010), so spiracles of the pupae were likely open during anoxia, causing loss of CO2 that must then be replaced during reperfusion (Matthews, 2016). Depressed CO2 emission during reperfusion could also reflect increased CO2 buffering in the hemolymph as lactate, acetate or other organic acids are removed from circulation when mitochondrial metabolism is reinitiated. Manduca sexta pupae and adult rhinoceros beetles also experience transient decreases in CO2 emission during reperfusion (Matthews, 2016; Woods and Lane, 2016), suggesting that this may be a general phenomenon (but see Lighton and Schilman, 2007). Drosophila melanogaster and M. sexta show an initial spike in CO2 release immediately upon reperfusion (Lighton and Schilman, 2007; Woods and Lane, 2016). In our experiment, respirometry chambers were flushed with CO2-free air after they were put in line with the gas analyzer, so between 4 and 12 min passed before gas was collected and analyzed for each individual. We could thus have missed an initial CO2 spike after reperfusion in our experiments with A. suspensa. High respiratory exchange ratios of pupae may indicate lipid synthesis (Melampy and Willis, 1939).
Biochemical changes differed markedly between pupal developmental stages. Oxidative damage to cell lipid membranes was highest for pharate adults, regardless of treatment, consistent with the substantial metabolic demand required for undergoing metamorphosis. Pupae may be better able to reduce damage to cell lipid membranes through the action of antioxidants, potentially priming this developmental stage for hormetic effects after repeated exposures that could emerge in later developmental stages, as in our study. Little has been published about oxidative damage and the antioxidant response throughout pupal–adult development in other insects, but in Tenebrio molitor (Gulevsky et al., 2006), lipid peroxidation was found to be highest at the pupal stage compared with other life stages. Antioxidant enzymes seem to show a species-specific response throughout ontogeny, with levels of the enzyme superoxide dismutase progressively increasing with each stage of the life cycle of T. molitor, staying stable across life stages in M. domestica (Allen et al., 1991) and following a U-shaped curve from egg to adult in D. melanogaster (Nickla et al., 1983). In A. suspensa, mass decreased from prepupal to pharate adult development, but lipid levels in pharate adults exposed to a single bout of anoxia were not elevated with increasing dry mass (i.e. lipid levels remained stable rather than increased), as observed in prepupae and pupae. Larger pharate adults in the single exposure treatments thus do not seem to contain a higher quantity of lipids, potentially because of the high energetic demands of completing the final stages of metamorphosis (Nestel et al., 2003; Merkey et al., 2011).
Unlike larvae and pupae, adult insects can more easily disperse away from low oxygen environments and may be less resistant to anoxic stress (Wegener, 1993; but see Callier et al., 2015). Some tiger beetle larvae, for example, can survive up to 13 times longer under anoxia compared with adults (Brust and Hoback, 2009). As late pharate adults have largely completed adult development and undergo only minor developmental changes before emergence as free-living adults, large effects of anoxia exposure on pharate adults can be expected. Anastrepha suspensa pharate adults indeed proved to be most vulnerable to anoxia exposure, because they had lower survival into adulthood and lower female fertility after a single anoxic stress exposure. Similar results in terms of survival were found in the beetle Callosobruchus subinnotatus, where late pharate adults exposed to controlled atmospheres (varying in the amount of N2 versus O2) had higher mortality compared with pupae and early pharate adults (Mbata et al., 2000). In our experiments on A. suspensa, prior anoxia exposure alleviated the negative effects on survival and fertility observed in individuals experiencing a single exposure as pharate adults, a clear hormetic effect wherein exposure earlier in development primed greater stress hardiness later in development. Lipid storage was increased following prior exposure, and these additional energy stores may help to sustain survival and fertility in multiply stressed individuals at levels similar to those observed in unstressed normoxia controls. The hormetic response after multiple exposures observed in oxygen consumption and lipid levels may thus benefit organismal performance by mitigating the negative effects of a single exposure on survival and fertility.
Acknowledgements
We are grateful to three reviewers for their suggestions on a previous version of the manuscript. We would like to thank Andre Szejner Sigal, Sabrina White and Theodore R. Cogley for help during experiments. The authors further wish to thank George Schneider from the Florida Department of Agriculture and Consumer Services for providing the flies for all our experiments. This is publication BRC 398 of the Biodiversity Research Centre.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: B.V., C.M.W., D.A.H., G.L.-M.; Methodology: B.V., C.M.W., D.A.H., C.A.S., G.L.-M.; Formal analysis: B.V., C.M.W., G.L.-M.; Investigation: B.V., C.M.W., G.L.-M.; Writing - original draft: B.V., C.M.W.; Writing - review & editing: B.V., C.M.W., D.A.H., C.A.S., G.L.-M.; Supervision: D.A.H.; Funding acquisition: B.V., C.M.W., D.A.H., G.L.-M.
Funding
This work was supported by the Fonds de la Recherche Scientifique - FNRS [24905063 to B.V.], the National Science Foundation division of Integrated Organismal Systems [1051890 to D.A.H.; 1558159 to C.M.W.], the Florida Agricultural Experiment Station and the IAEA/FAO CRP in Dormancy Management to D.A.H., the United States Department of Agriculture - National Institute of Food and Agriculture [2011-67012-30671 to G.L.-M.], and the National Institute of General Medical Sciences of the National Institutes of Health [P20GM103451 to G.L.-M.]. Deposited in PMC for release after 12 months.
References
- Allen R. G., Oberley L. W., Elwell J. H. and Sohal R. S. (1991). Developmental patterns in the antioxidant defenses of the housefly, Musca domestica. J. Cell. Physiol. 146, 270-276. 10.1002/jcp.1041460212 [DOI] [PubMed] [Google Scholar]
- Beaulieu M., Geiger R. E., Reim E., Zielke L. and Fischer K. (2015). Reproduction alters oxidative status when it is traded-off against longevity. Evolution 69, 1786-1796. 10.1111/evo.12697 [DOI] [PubMed] [Google Scholar]
- Benoit J. B., Patrick K. R., Desai K., Hardesty J. J., Krause T. B. and Denlinger D. L. (2010). Repeated bouts of dehydration deplete nutrient reserves and reduce egg production in the mosquito Culex pipiens. J. Exp. Biol. 213, 2763-2769. 10.1242/jeb.044883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit J. B., Lopez-Martinez G., Patrick K. R., Phillips Z. P., Krause T. B. and Denlinger D. L. (2011). Drinking a hot blood meal elicits a protective heat shock response in mosquitoes. Proc. Natl. Acad. Sci. USA 108, 8026-8029. 10.1073/pnas.1105195108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust M. L. and Hoback W. W. (2009). Hypoxia tolerance in adult and larval Cicindela tiger beetles varies by life history but not habitat association. Ann. Entomol. Soc. Am. 102, 462-466. 10.1603/008.102.0316 [DOI] [Google Scholar]
- Calabrese E. J., Bachmann K. A., Bailer A. J., Bolger P. M., Borak J., Cai L., Cedergreen N., Cherian M. G., Chiueh C. C., Clarkson T. W. et al. (2007). Biological stress response terminology: integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol. 222, 122-128. 10.1016/j.taap.2007.02.015 [DOI] [PubMed] [Google Scholar]
- Callier V., Hand S. C., Campbell J. B., Biddulph T. and Harrison J. F. (2015). Holometabolic transformation of hypoxic exposure and responses to anoxia in Drosophila melanogaster. J. Exp. Biol. 218, 2927-2934. 10.1242/jeb.125849 [DOI] [PubMed] [Google Scholar]
- Chippindale A. K., Gibbs A. G., Sheik M., Yee K. J., Djawdan M., Bradley T. J. and Rose M. R. (1998). Resource acquisition and the evolution of stress resistance in Drosophila melanogaster. Evolution 52, 1342-1352. 10.1111/j.1558-5646.1998.tb02016.x [DOI] [PubMed] [Google Scholar]
- Costantini D., Metcalfe N. B. and Monaghan P. (2010). Ecological processes in a hormetic framework. Ecol. Lett. 13, 1435-1447. 10.1111/j.1461-0248.2010.01531.x [DOI] [PubMed] [Google Scholar]
- Coulson S. J. and Bale J. S. (1991). Anoxia induces rapid cold hardening in the housefly Musca domestica (Diptera: Muscidae). J. Insect Physiol. 37, 497-501. 10.1016/0022-1910(91)90026-V [DOI] [Google Scholar]
- Crawley M. (2007). The R Book. West Sussex: John Wiley and Sons Ltd. [Google Scholar]
- Denlinger D. L. and Zdarek J. (1994). Metamorphosis behavior of flies. Annu. Rev. Entomol. 39, 243-266. 10.1146/annurev.en.39.010194.001331 [DOI] [PubMed] [Google Scholar]
- Denlinger D. L., Willis J. H. and Fraenkel G. (1972). Rates and cycles of oxygen consumption during pupal diapause in Sarcophaga flesh flies. J. Insect Physiol. 18, 871-882. 10.1016/0022-1910(72)90026-1 [DOI] [PubMed] [Google Scholar]
- Djawdan M., Rose M. R. and Bradley T. J. (1997). Does selection for stress resistance lower metabolic rate? Ecology 78, 828-837. 10.1890/0012-9658(1997)078[0828:DSFSRL]2.0.CO;2 [DOI] [Google Scholar]
- Ellington W. R. (1983). The recovery from anaerobic metabolism in invertebrates. J. Exp. Zool. 228, 431-444. 10.1002/jez.1402280305 [DOI] [Google Scholar]
- Findsen A., Andersen J. L., Calderon S. and Overgaard J. (2013). Rapid cold hardening improves recovery of ion homeostasis and chill coma recovery time in the migratory locust, Locusta migratoria. J. Exp. Biol. 216, 1630-1637. 10.1242/jeb.081141 [DOI] [PubMed] [Google Scholar]
- Forcella M., Berra E., Giacchini R. and Parenti P. (2007). Antioxidant defenses preserve membrane transport activity in Chironomus riparius larvae exposed to anoxia. Arch. Insect Biochem. Physiol. 65, 181-194. 10.1002/arch.20197 [DOI] [PubMed] [Google Scholar]
- Förster T. D. and Hetz S. K. (2010). Spiracle activity in moth pupae—the role of oxygen and carbon dioxide revisited. J. Insect Physiol. 56, 492-501. 10.1016/j.jinsphys.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Gibbs A. G., Chippindale A. K. and Rose M. R. (1997). Physiological mechanisms of evolved desiccation resistance in Drosophila melanogaster. J. Exp. Biol. 200, 1821-1832. [DOI] [PubMed] [Google Scholar]
- Gulevsky A. K., Relina L. I. and Grishchenkova Y. A. (2006). Variations of the antioxidant system during development of the cold-tolerant beetle, Tenebrio molitor. Cryo Lett. 27, 283-290. [PubMed] [Google Scholar]
- Harrison J., Frazier M. R., Henry J. R., Kaiser A., Klok C. J. and Rascón B. (2006). Responses of terrestrial insects to hypoxia or hyperoxia. Respir. Physiol. Neurobiol. 154, 4-17. 10.1016/j.resp.2006.02.008 [DOI] [PubMed] [Google Scholar]
- Hercus M. J., Loeschcke V. and Rattan S. I. S. (2003). Lifespan extension of Drosophila melanogaster through hormesis by repeated mild heat stress. Biogerontology 4, 149-156. 10.1023/A:1024197806855 [DOI] [PubMed] [Google Scholar]
- Hermes-Lima M. and Zenteno-Savín T. (2002). Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 133, 537-556. 10.1016/S1532-0456(02)00080-7 [DOI] [PubMed] [Google Scholar]
- Hoback W. W. and Stanley D. W. (2001). Insects in hypoxia. J. Insect Physiol. 47, 533-542. 10.1016/S0022-1910(00)00153-0 [DOI] [PubMed] [Google Scholar]
- Hoback W. W., Stanley D. W., Higley L. G. and Barnhart M. C. (1998). Survival of immersion and anoxia by larval tiger beetles, Cicindela togata. Am. Midl. Nat. 140, 27-33. 10.1674/0003-0031(1998)140[0027:SOIAAB]2.0.CO;2 [DOI] [Google Scholar]
- Hochachka P. W. (1980). Living Without Oxygen. Cambridge, MA: Harvard University Press. [Google Scholar]
- Hou B., Xie Q. and Zhang R. (2006). Depth of pupation and survival of the Oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae) pupae at selected soil moistures. Appl. Entomol. Zool. 41, 515-520. 10.1303/aez.2006.515 [DOI] [Google Scholar]
- Ina Y. and Sakai K. (2004). Prolongation of life span associated with immunological modification by chronic low-dose-rate irradiation in MRL-lpr/lpr mice. Radiat. Res. 161, 168-173. 10.1667/RR3120 [DOI] [PubMed] [Google Scholar]
- Ina Y. and Sakai K. (2005). Further study of prolongation of life span associated with immunological modification by chronic low-dose-rate irradiation in MRL-lpr/lpr mice: effects of whole-life irradiation. Radiat. Res. 163, 418-423. 10.1667/RR3316 [DOI] [PubMed] [Google Scholar]
- King A. M. and MacRae T. H. (2015). Insect heat shock proteins during stress and diapause. Annu. Rev. Entomol. 60, 59-75. 10.1146/annurev-ento-011613-162107 [DOI] [PubMed] [Google Scholar]
- Kölsch G., Jakobi K., Wegener G. and Braune H. J. (2002). Energy metabolism and metabolic rate of the alder leaf beetle Agelastica alni (L.) (Coleoptera, Chrysomelidae) under aerobic and anaerobic conditions: a microcalorimetric study. J. Insect Physiol. 48, 143-151. 10.1016/S0022-1910(01)00158-5 [DOI] [PubMed] [Google Scholar]
- Lee R. E., Chen C.-P. and Denlinger D. L. (1987). A rapid cold-hardening process in insects. Science 238, 1415-1417. 10.1126/science.238.4832.1415 [DOI] [PubMed] [Google Scholar]
- Levine R. L., Garland D., Oliver C. N., Amici A., Climent I., Lenz A.-G., Ahn B.-W., Shaltiel S. and Stadman E. R. (1990). Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 186, 464-479. 10.1016/0076-6879(90)86141-H [DOI] [PubMed] [Google Scholar]
- Lighton J. R. B. and Schilman P. E. (2007). Oxygen reperfusion damage in an insect. PLoS ONE 2, e1267 10.1371/journal.pone.0001267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Martínez G. and Hahn D. A. (2012). Short-term anoxic conditioning hormesis boosts antioxidant defenses, lowers oxidative damage following irradiation and enhances male sexual performance in the Caribbean fruit fly, Anastrepha suspensa. J. Exp. Biol. 215, 2150-2161. 10.1242/jeb.065631 [DOI] [PubMed] [Google Scholar]
- López-Martínez G., Carpenter J. E., Hight S. D. and Hahn D. A. (2014). Low-oxygen atmospheric treatment improves the performance of irradiation-sterilized male cactus moths used in SIT. J. Econ. Entomol. 107, 185-197. 10.1603/EC13370 [DOI] [PubMed] [Google Scholar]
- Lutz P. L. (1992). Mechanisms for anoxic survival in the vertebrate brain. Annu. Rev. Physiol. 54, 601-618. 10.1146/annurev.ph.54.030192.003125 [DOI] [PubMed] [Google Scholar]
- Marshall K. E. and Sinclair B. J. (2012). The impacts of repeated cold exposure on insects. J. Exp. Biol. 215, 1607-1613. 10.1242/jeb.059956 [DOI] [PubMed] [Google Scholar]
- Matthews P. G. D. (2016). Acid-base regulation in insect haemolymph. In Acid-Base Balance and Nitrogen Excretion in Invertebrates: Mechanisms and Strategies in Various Invertebrate Groups with Considerations of Challenges Caused by Ocean Acidification (ed. Weihrauch D. and O'Donnell M.), pp. 219-238. Switzerland: Springer International Publishing. [Google Scholar]
- Mbata G. N., Hetz S. K., Reichmuth C. and Adler C. (2000). Tolerance of pupae and pharate adults of Callosobruchus subinnotatus Pic (Coleoptera: Bruchidae) to modified atmospheres: a function of metabolic rate. J. Insect Physiol. 46, 145-151. 10.1016/S0022-1910(99)00110-9 [DOI] [PubMed] [Google Scholar]
- Melampy R. M. and Willis E. R. (1939). Respiratory metabolism during larval and pupal development of the female honeybee (Apis mellifica L.). Physiol. Zool. 12, 302-311. 10.1086/physzool.12.3.30151505 [DOI] [Google Scholar]
- Merkey A. B., Wong C. K., Hoshizaki D. K. and Gibbs A. G. (2011). Energetics of metamorphosis in Drosophila melanogaster. J. Insect Physiol. 57, 1437-1445. 10.1016/j.jinsphys.2011.07.013 [DOI] [PubMed] [Google Scholar]
- Montoya P., Flores S. and Toledo J. (2008). Effect of rainfall and soil moisture on survival of adults and immature stages of Anastrepha ludens and A. obliqua (Diptera: Tephritidae) under semi-field conditions. Florida Entomol. 91, 643-650. [Google Scholar]
- Nestel D., Tolmasky D., Rabossi A. and Quesada-Allué L. A. (2003). Lipid, carbohydrates and protein patterns during metamorphosis of the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 96, 237-244. 10.1603/0013-8746(2003)096[0237:LCAPPD]2.0.CO;2 [DOI] [Google Scholar]
- Nickla H., Anderson J. and Palzkill T. (1983). Enzymes involved in oxygen detoxification during development of Drosophila melanogaster. Experientia 39, 610-612. 10.1007/BF01971122 [DOI] [PubMed] [Google Scholar]
- Olsen A., Vantipalli M. C. and Lithgow G. J. (2006). Lifespan extension of Caenorhabditis elegans following repeated mild hormetic heat treatments. Biogerontology 7, 221-230. 10.1007/s10522-006-9018-x [DOI] [PubMed] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M. and Rice-Evans C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26, 1231-1237. 10.1016/S0891-5849(98)00315-3 [DOI] [PubMed] [Google Scholar]
- Romero L. M. (2012). Using the reactive scope model to understand why stress physiology predicts survival during starvation in Galápagos marine iguanas. Gen. Comp. Endocrinol. 176, 296-299. 10.1016/j.ygcen.2011.11.004 [DOI] [PubMed] [Google Scholar]
- Shreve S. M., Kelty J. D. and Lee R. E. (2004). Preservation of reproductive behaviors during modest cooling: rapid cold-hardening fine-tunes organismal response. J. Exp. Biol. 207, 1797-1802. 10.1242/jeb.00951 [DOI] [PubMed] [Google Scholar]
- Storey K. B. and Storey J. M. (1990). Metabolic rate depression and biochemical adaptation in anaerobiosis, hibernation and estivation. Q. Rev. Biol. 65, 145-174. 10.1086/416717 [DOI] [PubMed] [Google Scholar]
- van Handel E. (1985). Rapid determination of glycogen and sugars in mosquitoes. J. Am. Mosq. Control Assoc. 1, 299-301. [PubMed] [Google Scholar]
- Van Voorhies W. A. (2009). Metabolic function in Drosophila melanogaster in response to hypoxia and pure oxygen. J. Exp. Biol. 212, 3132-3141. 10.1242/jeb.031179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener G. (1993). Hypoxia and post-hypoxic recovery in insects: physiological and metabolic aspects. In Surviving Hypoxia: Mechanisms of Control and Adpation (ed. Hochachka P., Lutz P., Sick T., Rosenthal M. and van den Thillart G.), pp. 417-434. Boca Raton, FL: CRC Press. [Google Scholar]
- Wegener G. and Moratzky T. (1995). Hypoxia and anoxia in insects: microcalorimetric studies on two species (Locusta migratoria and Manduca sexta) showing different degrees of anoxia tolerance. Thermochim. Acta 251, 209-218. 10.1016/0040-6031(94)02009-D [DOI] [Google Scholar]
- Williams C. M., Thomas R. H., MacMillan H. A., Marshall K. E. and Sinclair B. J. (2011). Triacylglyceride measurement in small quantities of homogenised insect tissue: comparisons and caveats. J. Insect Physiol. 57, 1602-1613. 10.1016/j.jinsphys.2011.08.008 [DOI] [PubMed] [Google Scholar]
- Woods H. A. and Lane S. J. (2016). Metabolic recovery from drowning by insect pupae. J. Exp. Biol. 219, 3126-3136. 10.1242/jeb.144105 [DOI] [PubMed] [Google Scholar]