ABSTRACT
Membrane transporters and sequestration mechanisms concentrate metal ions differentially into discrete subcellular microenvironments for use in protein cofactors, signalling, storage or excretion. Here we identify zinc storage granules as the insect's major zinc reservoir in principal Malpighian tubule epithelial cells of Drosophila melanogaster. The concerted action of Adaptor Protein-3, Rab32, HOPS and BLOC complexes as well as of the white-scarlet (ABCG2-like) and ZnT35C (ZnT2/ZnT3/ZnT8-like) transporters is required for zinc storage granule biogenesis. Due to lysosome-related organelle defects caused by mutations in the homologous human genes, patients with Hermansky–Pudlak syndrome may lack zinc granules in beta pancreatic cells, intestinal paneth cells and presynaptic vesicles of hippocampal mossy fibers.
KEY WORDS: AP-3 complex, Eye color mutants, ICP-OES, Malpighian tubules, Synchrotron, Zincosomes
Summary: Drosophila genes required for the biogenesis of lysosome-related organelles (eye pigment granules) also function in the formation of zinc storage granules in the insect's excretory organ, the Malpighian tubules.
INTRODUCTION
Metal ions are cofactors of enzymes (Warner and Finnerty, 1981; Kirby et al., 2008; Gonzalez-Morales et al., 2015; Llorens et al., 2015; Dow, 2017). Iron and copper are required for mitochondrial respiration and cuticle formation (Villee, 1948; Anderson et al., 2005; Binks et al., 2010; Kroll et al., 2014), manganese for superoxide and arginine (nitrogen) turnover (Duttaroy et al., 1997; Samson, 2000), and molybdenum for cysteine and methionine (sulfur) metabolism, and purine and aldehyde catabolism (Bogaart and Bernini, 1981; Marelja et al., 2014). The shared chemical property that turns iron, copper, manganese and molybdenum into essential cofactors of enzymes is the physicochemical stability of their ions in different oxidation states. In contrast, zinc ions do not readily change their valence and are therefore preferentially used as structural binding elements in zinc-finger transcription factors (Schuh et al., 1986; Redemann et al., 1988). Alternatively, the strong Lewis acid activity of the zinc cation is utilized in proteolytic enzymes and carbonic anhydrase (Wessing et al., 1997; Llano et al., 2000).
The development, growth and reproduction of all animals depend on the physiological regulation of metal ions: specific protein metallation is achieved in specialized cellular compartments, facilitated by metal chaperones (Lye et al., 2013; Qin et al., 2013; Southon et al., 2013). Such physiological regulation takes place both systemically through circulating factors secreted from specialized organs and at the cellular level through metal sensing coupled to gene and protein responses (Cyert and Philpott, 2013; Bird, 2015) and is highly relevant in disease (Esposito et al., 2013; Xiao et al., 2013; Zhu et al., 2014; Chi et al., 2015; Ott et al., 2015; Calap-Quintana et al., 2017; Mercer et al., 2017).
These systems are well understood in human iron physiology. At the systemic level, the liver senses transferrin iron saturation (i.e. sufficient iron availability), stores excess iron in ferritin, and secretes hepcidin as a response; hepcidin binds to and internalizes the iron exporter ferroportin from cell membranes, reducing iron efflux at intestinal basolateral membranes, and spleen macrophages recycle iron from senescent red blood cell hemoglobin (Drakesmith et al., 2015; Camaschella et al., 2016; Muckenthaler et al., 2017). At the cellular level, cytosolic iron deficiency results in the stabilization of the transferrin receptor for iron uptake from circulation and in the translational inhibition of ferritin for iron storage through the action of iron regulatory proteins; the opposite effects occur under cytosolic iron overload, and these processes can be viewed as a balancing act (Hentze et al., 2004; Zhang et al., 2014; Kühn, 2015). The similarities and differences between iron regulation in Drosophila melanogaster and mammals have been reviewed (Mandilaras et al., 2013; Tang and Zhou, 2013). In Drosophila, iron availability is linked to key developmental signals, such as ecdysone synthesis (Llorens et al., 2015; Palandri et al., 2015), and to processes such as the formation of epithelial septate junctions (Tiklová et al., 2010), the functionality of the circadian clock (Mandilaras and Missirlis, 2012), and the induction of mitotic events (Li, 2010). So far, a single iron transporter moving iron into the cytosol has been identified in flies (Orgad et al., 1998; Bettedi et al., 2011) and a single iron exporter has been suggested to traffic iron from the cytosol into the endoplasmic reticulum and Golgi apparatus (Xiao et al., 2014), where insect ferritin resides (Missirlis et al., 2007; Rosas-Arellano et al., 2016).
In contrast to iron, the systemic regulation of zinc homeostasis is not well understood in either human or insect biology. There is a growing appreciation of the specific, directional membrane transport functions provided by the Zrt- and Irt-like proteins (ZIPs) and Zn transporters (ZnTs) and of the metal sequestration properties of the cytosolic metallothioneins (Plum et al., 2010; Babula et al., 2012; Kimura and Kambe, 2016). Zinc-responsive gene regulation is largely mediated through Metal Transcription Factor-1 (MTF-1) (Günther et al., 2012). The metallothionein genes are major targets of MTF-1 because the encoded proteins sequester zinc and other metals such as copper or cadmium. Nevertheless, no humoral factor has been described responding to zinc deficiency, or to zinc overload. Nor is a tissue reserve known from which zinc is mobilized to meet functional requirements under conditions of dietary deprivation. The same considerations apply to Drosophila zinc physiology (Richards and Burke, 2016; Xiao and Zhou, 2016; Navarro and Schneuwly, 2017). Drosophila ZIPs and ZnTs are phylogenetically conserved, with different members of each family localizing to separate subcellular compartments (Lye et al., 2012; Dechen et al., 2015), enabling zinc absorption at the intestine (Wang et al., 2009; Qin et al., 2013; Richards et al., 2015) and zinc excretion from the Malpighian tubules (Yepiskoposyan et al., 2006; Chi et al., 2015; Yin et al., 2017). Cellular responses to zinc are coordinated via MTF-1 and metallothioneins (Egli et al., 2003, 2006; Atanesyan et al., 2011; Sims et al., 2012; Merritt and Bewick, 2017; Mohr et al., 2017; Qiang et al., 2017). MTF-1 also regulates the ferritin subunit genes, for reasons that are unclear (Yepiskoposyan et al., 2006; Gutiérrez et al., 2010, 2013). Little is known about the mechanism of zinc homeostasis in the organism as a whole (Richards et al., 2017), particularly how zinc is stored in Drosophila (Schofield et al., 1997).
We came to the question of physiological zinc storage by studying poco-zinc, a previously identified recessive X-linked mutation that causes a threefold reduction of total body zinc accumulation in laboratory strains of D. melanogaster (Afshar et al., 2013). By genetic mapping, we show that mutants in the white gene (Morgan, 1910) have a threefold reduction in zinc content. The white protein encodes an ATP-binding cassette sub-family G2 (ABCG2) transporter that is best known for its function in the transport of two types of pigment precursors in the pigment granules of the eye, functioning as a dimer with either of two other members of the Drosophila ABCG2 protein family, brown and scarlet (Dreesen et al., 1988; Mackenzie et al., 2000). Two types of pigment granules have been identified in wild-type animals on the basis of ultrastructure morphology (Nolte, 1961; Shoup, 1966). Many eye color mutants affect enzymes of biosynthetic pathways for the brown ommochromes (Wiley and Forrest, 1981) and bright red drosopterins (Kim et al., 2013), but a subset, known as transport mutants (Sullivan and Sullivan, 1975), affect the formation of the pigment granules per se. Amongst these transport mutants, we have also analysed total body zinc accumulation in the adaptin protein complex-3 (AP-3) mutants garnet (g) (Ooi et al., 1997), carmine (cm) (Mullins et al., 1999), ruby (rb) (Kretzschmar et al., 2000) and orange (or) (Mullins et al., 2000), in lightoid (ltd) and claret (ca) that encode for Rab32 and its Guanine Exchange Factor (Ma et al., 2004), in pink (p), which encodes for the Hermansky–Pudlak syndrome 5 homologue (Falcón-Pérez et al., 2007; Syrzycka et al., 2007) and in light (lt), which encodes for the VPS41 HOPS complex homologue (Warner et al., 1998). Collectively, all these proteins are required for the biogenesis of lysosome-related organelles (LROs) – specialized low-pH subcellular compartments that accumulate a variety of complex metabolites (Lloyd et al., 1998; Krämer, 2002; Dell'Angelica, 2009; Cheli et al., 2010; Harris et al., 2011). Here we describe a LRO in the Malpighian tubules of Drosophila that forms the major physiological zinc storage site in this animal. This zinc storage granule concentrates the entire chelatable pool of total body zinc in flies, and is distinct from the previously described riboflavin-containing granules that give the wild-type tubule its characteristic yellow-orange color (Nickla, 1972; van Breugel, 1987).
MATERIALS AND METHODS
Drosophila melanogaster stocks
In this study, w* and w+ refer to isogenic stocks generated in the laboratory using the w* mutant and the Tan3 wild-type strains, respectively (Afshar et al., 2013). First, single crosses between w* siblings were set for 20 generations. A single Tan3 male was then crossed to a w* isogenic female. For 20 further generations, a w* male (always taken from the w* isogenic stock) was backcrossed to a w*/w+ female. Finally, a w+ male from the heterozygous mothers was backcrossed to a w*/w+ female to re-establish the isogenic w+ stock.
A new allele of ste01330 resulting from a piggy-Bac insertion into the open reading frame of the st gene (Thibault et al., 2004) was crossed into the w+ background and used in this study. All strains were obtained from the Bloomington Drosophila Stock Center and are listed along with the respective stock numbers (Table 1) except for X-chromosome meiotic recombination mapping stocks cm1, m74f, sd1, oss and wa, cv1, t1 corresponding to #1282 and #121, respectively, and y1,w67c23;ZnT35CMI07746−GFSTF.1/SM6a, a GFP protein-trap line (Nagarkar-Jaiswal et al., 2015), corresponding to #59419. The latter chromosome was also introduced into the w+ background. All flies were fed on molasses and yeast and kept at 25°C (Rempoulakis et al., 2014).
Table 1.
Metal determinations by AAS and ICP-OES in different strains of Drosophila melanogaster
Metal measurements
Both flame atomic absorption spectrometry (AAS) and inductively coupled plasma optic emission spectrometry (ICP-OES) were used for metal determinations. Adult fruit flies, 4–8 days old, of mixed sex were collected and stored at –80°C. They were freeze-dried for 8 h to remove water. For the experiments with AAS, 200 mg of dry sample was digested with metal-free nitric acid (Fluka, Hampshire, IL, USA) at 60°C for 48 h, whereas for ICP-OES, 20 mg of dry sample was digested at 200°C for 15 min in closed vessels of MARS6 microwave digestion system (CEM Corporation, Matthews, NC, USA). Metal determinations in individual tissues were performed with ICP-OES; five pairs of Malpighian tubules (anterior and posterior), five pairs of ovaries, five pairs of testes, five heads and five intestines were dissected in phosphate-buffered saline (PBS), transferred to 400 µl water in MARS6 vessels, where 400 µl nitric acid was added for the digestion step and samples were diluted to 1.2 ml final volume with water. Total Zn, Fe, Mn and Cu concentrations were measured against calibration curves and a digestion blank in the Avanta M System 300 GF 3000 AAS (Dandenong, Victoria, Australia) and the PerkinElmer Optima 8300 ICP-OES (Shelton, CT, USA) instruments, respectively.
Confocal fluorescence microscopy
Malpighian tubules were dissected from adult female flies in PBS (130 mmol l−1 NaCl, 7 mmol l−1 Na2HPO4, 3 mmol l−1 NaH2PO4; pH 7.0). The tissue was fixed with ice-cold methanol for 5 min and rinsed three times for 3 min with PBS. Fluozin-3AM (Invitrogen, Carlsbad, CA, USA) was dissolved in dimethyl sulfoxide (DMSO) at 5 µmol l−1, stored in frozen aliquots (Groth et al., 2013) and protected against exposure to direct light at all times. For each experiment, a fresh aliquot was diluted at 2.5 µmol l−1 in PBS containing 0.02% Triton X-100 and 0.001% Tween 20. The fixed tissues were incubated with the Fluozin-3AM solution for 45 min at 38°C in a humid heat chamber. After three washes with PBS, the tissues were carefully mounted in Vecta Shield with DAPI (Vector H-1200, Burlingame, CA, USA) and observed without delay under a TCS SP8 Leica confocal system coupled to a DMI6000 inverted microscope (Leica Microsystems, Wetzlar, Germany). Methanol-fixation procedure and corresponding mounting was also used for direct visualization of the ZnT35CGFP construct.
Synchrotron X-ray fluorescence microscopy
Malpighian tubules were dissected from adult female flies in PBS, washed three times with 0.1 mol l−1 ammonium acetate (Jones et al., 2015), placed on microscope slide coverslips (Thermo Scientific Nunc Thermanox) and air-dried at 4°C. X-ray fluorescence images were collected at the Stanford Synchrotron Radiation Lightsource using beam line 2–3. The incident X-ray energy was set to 11 keV using a Si (111) double crystal monochromator with a storage ring (Stanford Positron Electron Accelerating Ring) containing 500 mA at 3.0 GeV. The fluorescence lines of the elements of interest, as well as the intensity of the total scattered X-rays, were monitored using a silicon drift Vortex detector (SII NanoTechnology USA, Northridge, CA, USA) mounted at 90 deg to the incident beam. Photon processing was accomplished with Xpress3 signal processing electronics (Quantum Detectors, Chilton, Oxfordshire, UK). In addition to these regions of interest, the entire fluorescence spectrum was also collected at each data point. The microfocused beam of 3×3 microns was provided by an Rh-coated Kirkpatrick-Baez mirror pair (Xradia, Pleasanton, CA, USA). The incident and transmitted X-ray intensities were measured with nitrogen-filled ion chambers. Samples were mounted at 45 deg to the incident X-ray beam and were spatially rastered in the microbeam using a Newport VP-25XA-XYZ stage. Beam exposure was 100 ms per pixel. Fluorescence signals were normalized against the incident X-ray beam intensity to take into account its fluctuations. Data analysis was performed using the MicroAnalysis Toolkit computer program (Webb, 2011). No smoothing or related data manipulations were performed.
RESULTS
Mapping the mutant that caused threefold reduction in total body zinc
We refer to the X-linked recessive mutant with 3-fold reduction in total zinc accumulation as poco-zinc (Afshar et al., 2013). The X-chromosome meiotic recombination mapping stock cm1, m74f, sd1, oss accumulated 0.07 mg Zn g−1 dry mass, suggesting that it also carried the poco-zinc allele. Flies from this stock were crossed to counterparts from wild-type Tan3, accumulating 0.20 mg Zn g−1 dry mass. A total of 121 recombinants were established arising from single or double crossovers between the parental chromosomes and were screened for the presence of poco-zinc. The cm mutant was present in 48 recombinants, all of which also carried poco-zinc, whereas in the remaining 73 recombinants neither poco-zinc nor cm was present (Fig. 1A). To estimate the distance between poco-zinc and cm, we generated a new recombinant y1, cm1 stock (low in zinc) and outcrossed it with wild-type flies. We were unable to dissociate poco-zinc from cm: 259 single cm1 mutants derived from this cross segregated with poco-zinc, whereas 221 single y1 mutants were normal (Fig. 1B). To confirm the proximity between poco-zinc and cm, we also used the strain wa, cv1, t1. Surprisingly, all recombinants carrying the wa allele (irrespectively of whether they carried either cv1, or t1, or neither of the two) were associated with poco-zinc, and, conversely, all w+ flies were normal. As cv1 lies between w and cm, the findings pointed to a new chromosomal location for poco-zinc, this time in the vicinity of w and distant from cm (Fig. 1C).
Fig. 1.
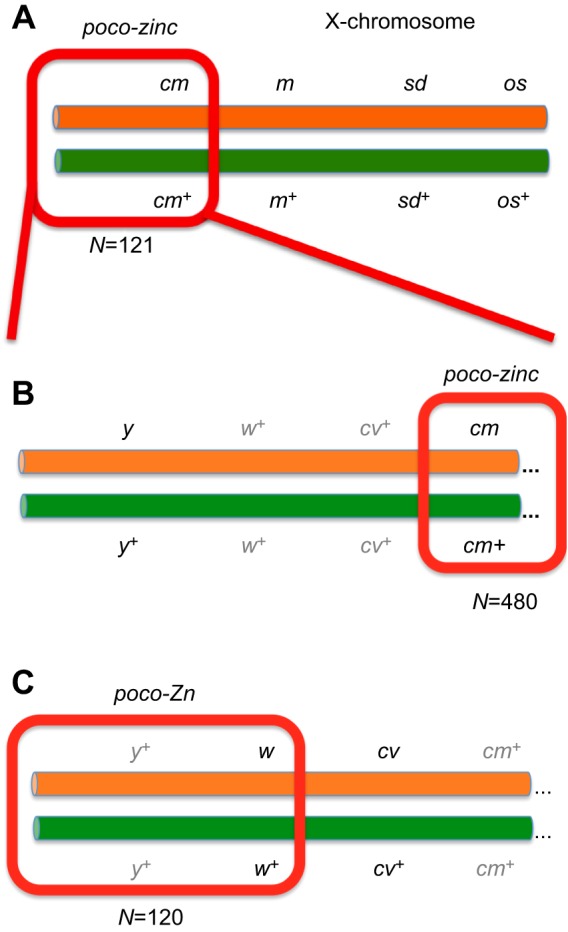
Meiotic recombination mapping strategy for poco-zinc in Drosophila melanogaster. The wild-type Tan3 chromosome is represented in green, whereas the mapping stock chromosome, carrying recessive alleles with visible phenotypes, is orange. (A) The first set of recombinant analysis situated the poco-zinc allele on the left part of the X-chromosome as it segregated 100% together with the cm gene. (B) Efforts to dissociate poco-zinc from cm were not successful, suggesting that poco-zinc is tightly linked (or identical) to the cm gene. (C) Efforts to map poco-zinc using a different mapping stock resulted in joint segregation of poco-zinc together with the w gene and far away from the cm gene. N is the total number of recombinants analysed.
The cm gene encodes for the μ3 subunit of the AP-3 complex (Mullins et al., 1999; Rodriguez-Fernandez and Dell'Angelica, 2015). In mammalian cells, the AP-3 complex is required for the formation of LROs, organelles known to accumulate zinc (Kantheti et al., 1998; Salazar et al., 2004; McAllister and Dyck, 2017). Furthermore, and despite the generally held idea that w mutants lack pigment because of defective transport of 3-hydroxy-kynurenine and 6-pyruvoyl tetrahydropterin into pigment granules (Sullivan et al., 1979; Evans et al., 2008; Green et al., 2012; Hersh, 2016; Navrotskaya and Oxenkrug, 2016), earlier studies had demonstrated physical absence of these organelles in the w mutants (Nolte, 1961; Shoup, 1966; Nickla, 1972). Could it be that all Drosophila mutants in the LRO-biogenesis pathway (Lloyd et al., 1998; Krämer, 2002; Dell'Angelica, 2009; Cheli et al., 2010; Harris et al., 2011), including w, lacked zinc storage? To test this idea, total body zinc content was determined in the corresponding mutants.
LRO-biogenesis mutants lack body zinc stores
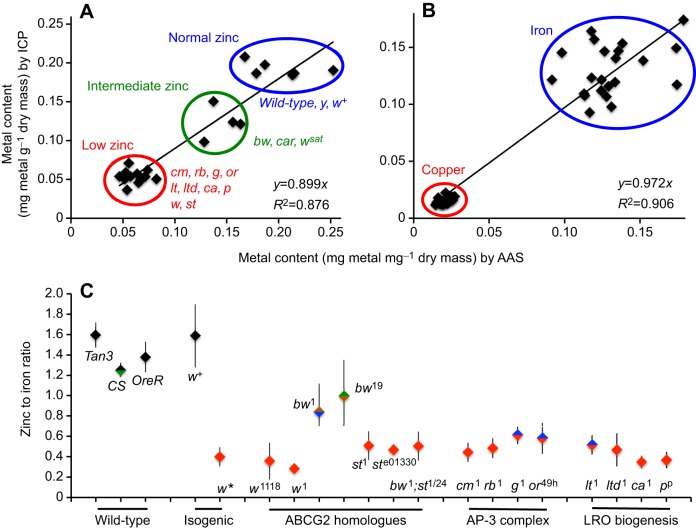
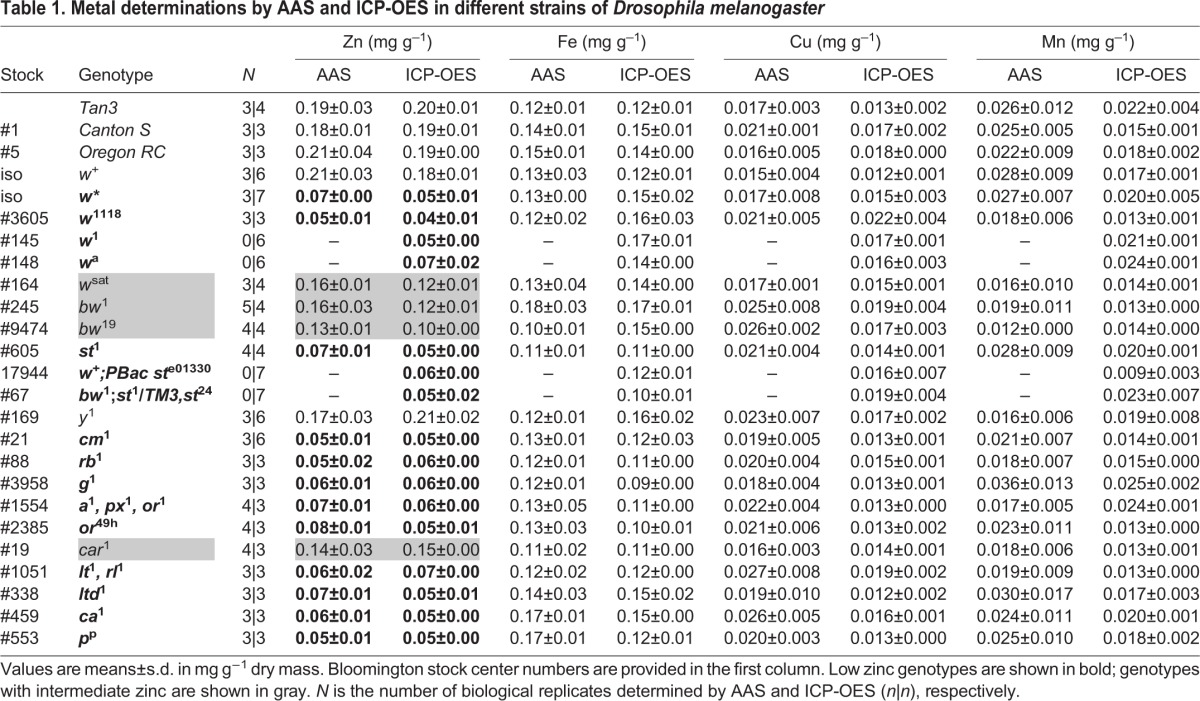
Metal measurements by AAS and ICP-OES produced a good correlation between the two sets of data (Table 1). The analysed genotypes readily separated into three groups according to their zinc content (Fig. 2A). Null mutants in the w gene were all low in zinc; the reduction in metal content was specific for zinc and not seen for iron, copper and manganese measured in parallel (Table 1 and Fig. 2B). In contrast, a w+ strain derived after 20 consecutive generations of single-pair backcrossing into w showed normal zinc accumulation.
Fig. 2.
Mutants in the LRO-biogenesis pathway have low body zinc content. (A) Linear regression between zinc determinations by AAS versus ICP-OES. The mean value determined for each genotype (Table 1), measured by both methods in biological replicates, is plotted. Stocks with normal, intermediate and low levels of zinc are readily identifiable. (B) Values for iron and copper are shown; here genotypes do not segregate. (C) Zinc (mg) to iron (mg) ratio was calculated for every independent measurement made (by either AAS or ICP-OES). Mean values and standard deviations are plotted; the different colors indicate statistically significant differences as revealed by Tukey’s post hoc test following one-way ANOVA.
We calculated the zinc-to-iron ratio from every measurement obtained per genotype irrespective of the technique used, and plotted the mean values and standard deviations from the mean (Fig. 2C). Given the 3-fold lower zinc-to-iron ratio in w mutants, we also tested bw1 and bw19, which showed a minor reduction in zinc accumulation, whereas st1 and ste01330 were low in zinc, similar to w and to the double mutant bw1;st1/24. These results implicated the white-scarlet dimer (Mackenzie et al., 2000) in Drosophila body zinc accumulation. Moreover, the AP-3 complex related mutants cm1, g1, rb1 and or49h also had low body zinc as was true for the other LRO-biogenesis mutants ltd1, ca1, pp and lt1.
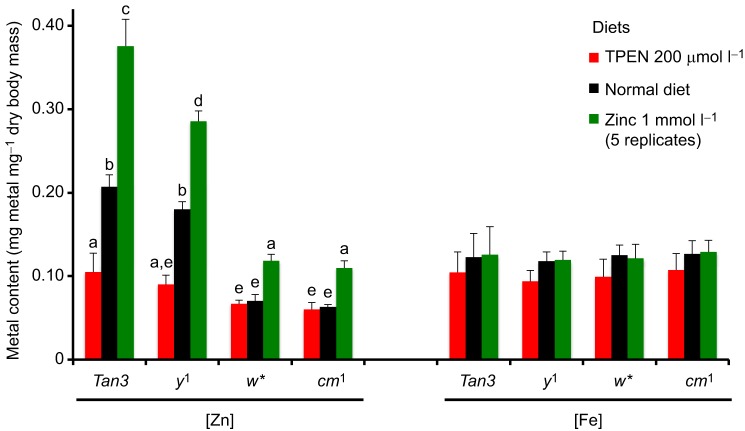
The response to dietary zinc chelation or supplementation was compared between Tan3 wild-type flies and y1 mutants (used as an additional laboratory strain control) and w* and cm1 mutants (Fig. 3). The control genotypes responded as expected to both treatments, reducing body zinc content when feeding on a diet supplemented with 200 µmol l−1 N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN; a zinc-specific chelator) and increasing body zinc content on a diet supplemented with 1 mmol l−1 zinc sulfate. In contrast, zinc chelation with TPEN had no effect on the body zinc content of w* and cm1 mutants, whereas zinc supplementation resulted in a small increase, barely reaching the body zinc content of Tan3 wild-type flies and y1 mutants fed on 200 µmol l−1 TPEN (Fig. 3). These results suggest that w and cm1 mutants are defective in zinc storage, lacking the part of wild-type zinc that is chelatable with dietary TPEN.
Fig. 3.
Zinc storage is affected in w and cm mutants. Two control strains (wild-type Tan3 and y1) and two low-zinc mutants (w* and cm1) were grown on media with the zinc chelator TPEN (red bars) or supplemented with zinc sulfate (green bars) prior to measuring zinc and iron by AAS in whole flies from these populations. The control strains respond to the zinc treatments by changing body zinc stores, whereas this response is impaired in low-zinc mutants. Iron was unaffected. Two-way ANOVA showed differences by diet and by genotype; groups not different from each other in a Tukey's post hoc analysis are marked by the same lower case letter.
Malpighian tubule LROs are a major site for physiological zinc storage
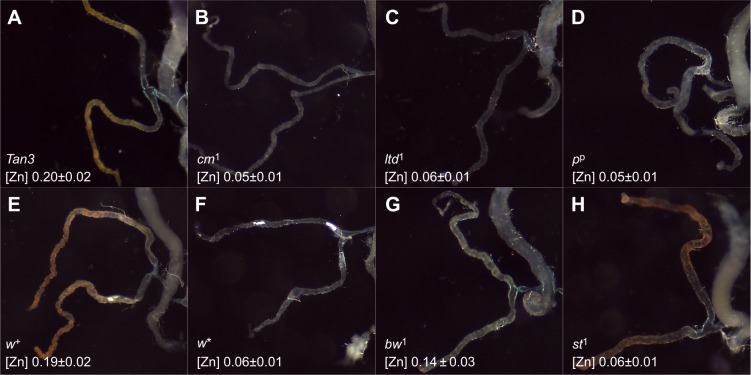
A common feature of all LRO-biogenesis mutants is a reduction of pigment granules in their eyes. Null mutants in the w gene completely lack these organelles (Nolte, 1961; Shoup, 1966). Eye pigment granules are commonly rescued with the mini-white transgene (Pirrotta et al., 1985), but the resulting stocks often remain low in body zinc (Bettedi et al., 2011; Gutiérrez et al., 2013). Thus eye pigment cells are an unlikely location for the LROs mediating body zinc storage. Previous reports have documented zinc storage granules in the Malpighian tubules of Musca domestica (Sohal et al., 1976), Drosophila hydei (Zierold and Wessing, 1990) and of Tumulitermes tumuli, a termite species (Stewart et al., 2011). Another common feature of all LRO-biogenesis mutants is a reduction of riboflavin-containing pigment granules in their Malpighian tubules (Beadle, 1937; Brehme and Demerec, 1942; Nickla, 1972; van Breugel, 1987; Yagi and Ogawa, 1996) and, moreover, using a penetrative ion microprobe technique, high zinc concentrations were detected in the Malpighian tubules of Drosophila melanogaster (Schofield et al., 1997). Generally, yellow Malpighian tubules correlated with normal zinc content, whereas loss of coloration correlated with low body zinc (Fig. 4). Note, however, that bw and st mutants did not follow this rule, an exception to which we shall return later. For the remaining mutants presented in this study, our hypothesis was that disruption of LROs in the Malpighian tubules (as evidenced by loss of riboflavin granules) resulted in loss of zinc storage granules.
Fig. 4.
Mutants in the LRO-biogenesis pathway fail to accumulate riboflavin in the Malpighian tubules. The Malpighian tubules of adult females are shown for the indicated genotypes along with the concentration of total body zinc in mg Zn g−1 dry mass. (A) Riboflavin in wild-type Malpighian tubules gives them their characteristic yellow-orange color (Nickla, 1972). (B–D) Three representative LRO-biogenesis mutants are all colorless and low in zinc. A comparison between isogenic (E) w+ and (F) w* suggests that the w gene is required for the accumulation of both riboflavin and zinc. (G) The bw mutants are severely reduced in their coloration and less so in their zinc content. (H) The st mutants show riboflavin coloration, but severely reduced zinc concentration.
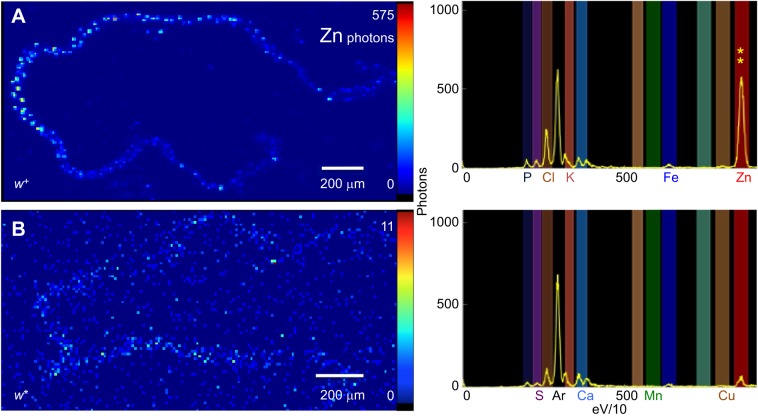
To directly visualize zinc, synchrotron X-ray fluorescence imaging was performed (Korbas et al., 2008; Popescu et al., 2009; Bourassa et al., 2014; Jones et al., 2015). Zinc was the major metal element detected in Malpighian tubules from w+ female flies (Fig. 5A). In contrast, only trace amounts of zinc were detectable in Malpighian tubules from w* flies (Fig. 5B). Variation in the spectral emissions from other elements was minimal between the two samples, suggesting once again that the w gene affects zinc accumulation specifically in this tissue.
Fig. 5.
Synchrotron X-ray fluorescence microscopy demonstrates the presence of zinc in Malpighian tubules from adult female flies. Pixel resolution was 10 µm2. (A) Representative heat-map image for the zinc signal is shown for w+ Malpighian tubules. The spectral maximum for zinc emission is 575 photons per 100 ms (also indicated by the two yellow asterisks along with the full spectrum in the right-hand panel). (B) Almost no zinc is detectable in Malpighian tubules from the w mutant.
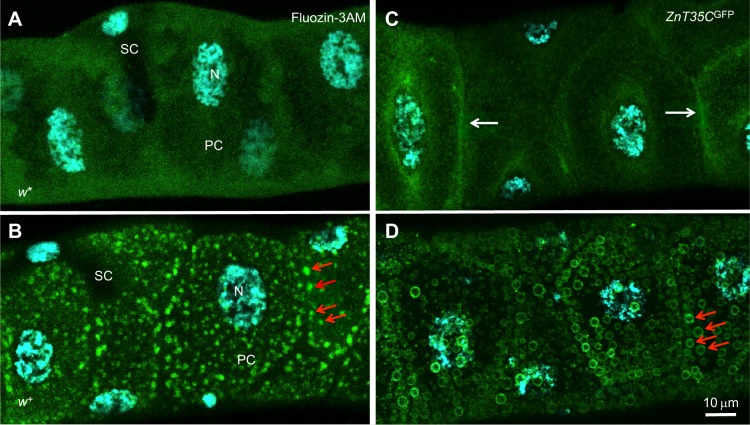
To visualize zinc storage granules, Malpighian tubules were incubated with the zinc indicator Fluozin-3AM and examined by confocal microscopy. Whereas the Malpighian tubules from adult female w* flies showed only a diffuse background signal (Fig. 6A), in Malpighian tubules from adult female w+ flies multiple, distinct accumulations of fluorescence with a diameter of approximately 1 µm were observed (Fig. 6B). These fluorescent structures, which we suggest are zinc storage granules, were present only in principal tubule cells and not in the supporting stellate cells (Halberg et al., 2015).
Fig. 6.
Zinc storage granules are present in the Malpighian tubules. Confocal images of Fluozin-3AM fluorescence, indicative of labile zinc in the Malpighian tubule of (A) a w* female adult or (B) an isogenic w+ female adult. Red arrows point to a subset of zinc storage granules. (C) The protein trap line y,w;ZnT35CGFP was used to monitor the subcellular localization of ZnT35C, confirming previous observations that it associates with the plasma membrane (white arrows). (D) The same reporter in the w+ background clearly marks the zinc storage granule membrane (red arrows). All flies were grown on a diet supplemented with 5 mmol l−1 zinc sulfate. SC, stellate cell; PC, principal cell; N, nucleus.
Zinc accumulation in vesicles normally depends on specialized transporters. ZnT8, for example, is responsible for zinc entry into pancreatic insulin-granules (Pound et al., 2009), while ZnT3 takes over this function in glutamatergic vesicles of the mossy fibers (Cole et al., 1999; McAllister and Dyck, 2017). We hypothesized that the best candidate Drosophila zinc transporter to mediate this function was ZnT35C, which is phylogenetically related to human ZnT3 and ZnT8 (Lye et al., 2012) and highly expressed in the Malpighian tubules (Yepiskoposyan et al., 2006; Chi et al., 2015; Yin et al., 2017). We used a strain that inserts GFP into the endogenous ZnT35C open reading frame (Nagarkar-Jaiswal et al., 2015) and observed the subcellular localization of the tagged transporter in Malpighian tubules of adult female flies grown on a zinc-supplemented diet. In the w mutant that lacks LROs, ZnT35C was localized in the proximity of the plasma membrane (Fig. 6C), consistent with previous observations in Malpighian tubules from the larvae (Yepiskoposyan et al., 2006; Yin et al., 2017). In contrast, ZnT35CGFP clearly marked a subset of LROs in w+ flies, which correspond to zinc storage granules (Fig. 6D).
Lastly, to determine if the Malpighian tubules are the main tissue where zinc accumulates and to directly assess if zinc accumulation varies in a sex-dependent manner, we used ICP-OES in samples prepared from females, males and dissected body parts, including the Malpighian tubules, intestines, ovaries, testes and heads (Table 2 and Fig. 7). Malpighian tubules from both sexes accumulate zinc in w+ individuals. Zinc was also present at high concentrations in three out of nine w+ testes from this genotype. These preliminary observations require further study given that riboflavin granules are also present in the epithelial sheath of the testes (Nickla, 1972; van Breugel, 1987).
Table 2.
Metal determinations by ICP-OES in different tissues of Drosophila melanogaster
Fig. 7.
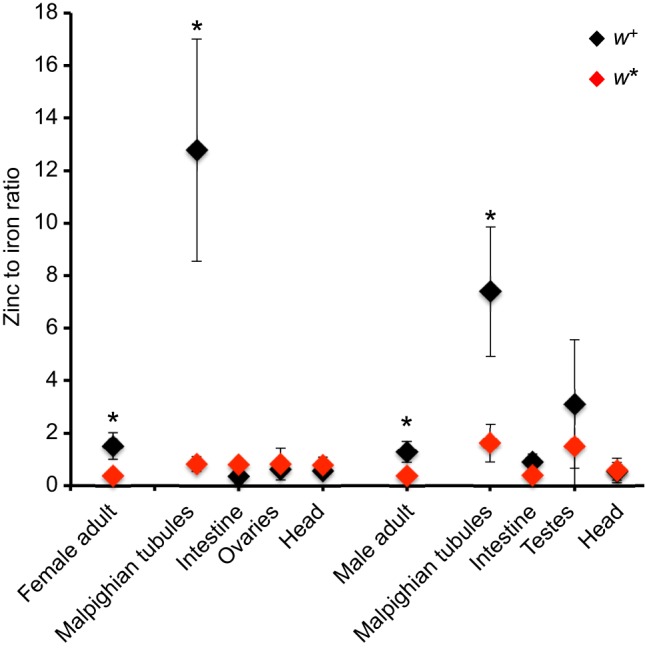
Zinc accumulates in the Malpighian tubules of both sexes and in testes. Zinc (mg) to iron (mg) ratio was calculated for every independent measurement made by ICP-OES. Mean values and standard deviations are plotted (w+ in black and w* in red). Two-way ANOVA indicated statistically significant differences both by tissue and by genotype; asterisks denote difference between genotypes for any given tissue from a Tukey’s post hoc analysis.
DISCUSSION
Zinc storage in D. melanogaster and other animals
We propose that the zinc storage granules in principal cells of the Drosophila Malpighian tubules (Fig. 6) have a similar function to ferritin-containing Golgi-related vesicles in iron cells of the middle midgut (Locke and Leung, 1984; Missirlis et al., 2007). The latter serve for iron storage as a physiological parallel of liver ferritin (Mehta et al., 2009), and the former could do the same for zinc storage. As there is no generally accepted site for body zinc storage in humans or other mammals, it is worth investigating if zinc-containing granules such as those present in the pancreas (Scott and Fisher, 1938; Timm and Neth, 1958; Kawanishi, 1966; Rutter et al., 2016; Maret, 2017) or in intestinal paneth cells (Okamoto, 1942; Giblin et al., 2006) serve as a reservoir for this metal, as may be the case, alternatively or additionally, for bone depositions of zinc (Berg and Kollmer, 1988; Huang et al., 2007). The only animal where a zinc storage site has been proposed is the nematode Caenorhabditis elegans (Roh et al., 2012; Warnhoff et al., 2017). Upon feeding on a diet with high content of zinc, C. elegans generates new granules within intestinal cells to store the excess metal. Thus zinc storage granules, or zincosomes as they have been also called (Beyersmann and Haase, 2001; Colvin et al., 2016), appear to be a conserved LRO in animal biology.
Insect zinc storage granules were first described in M. domestica (Sohal et al., 1976), D. hydei (Zierold and Wessing, 1990) and have also been observed in termites (Stewart et al., 2011). Twenty-four species of flies (from the Drosophilidae and the Tephritidae families) have similar zinc content, suggesting that the function of Malpighian tubules in zinc storage is evolutionarily conserved (Sadraie and Missirlis, 2011; Rempoulakis et al., 2014). Nevertheless, the findings we report show that zinc storage granules are not required for the viability of flies, raising the question of what physiological function(s) they might serve. As the Malpighian tubules are typically involved in excretion (Chi et al., 2015; Halberg et al., 2015; Yin et al., 2017), one intriguing possibility is that zinc storage granules provide a source of zinc for the intestinal lumen. In this view, the Malpighian tubule principal cells would have a similar physiological purpose as the intestinal paneth cells in mammals, where the secreted zinc has been implicated in the maintenance of the stem cell niche and epithelial integrity (Geiser et al., 2012; Ohashi et al., 2016; Podany et al., 2016; Sunuwar et al., 2016, 2017a, 2017b). Another possibility is that luminal zinc may function as a regulator for intestinal microbiota and endosymbionts, or for the control of a commonly occurring natural pathogen (Brownlie et al., 2009; Bonfini et al., 2016; Mistry et al., 2016; Martino et al., 2017; Martinson et al., 2017; Clark and Walker, 2018). Finally, it will be of interest to investigate if secreted zinc is reabsorbed by the hindgut, as is the case for the alkali metal ions (O'Donnell and Maddrell, 1995).
Different types of LROs defined by bw and st
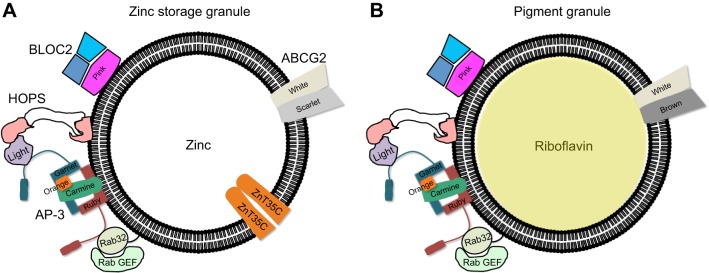
The Malpighian tubules are also known to store riboflavin into LROs (Nickla, 1972; van Breugel, 1987). Our results suggest that the brown-white dimer is primarily required for the formation of the riboflavin LRO (Fig. 4G), a finding also true in the silkworm Bombyx mori (Zhang et al., 2018), whereas the scarlet-white dimer is required for the zinc storage granule (Fig. 4H). All other genes tested and known to be involved in the biogenesis of LROs affected both riboflavin accumulation and zinc storage (Fig. 4). This poses an interesting cell biology question, as the function of the Rab32, AP3, HOPS and BLOC complexes is understood as enabling the trafficking (segregation) of transporters such as ZnT35C to the LRO, defining in this way the identity of the organelle (Lloyd et al., 1998; Dell'Angelica et al., 2000; Mackenzie et al., 2000; Bultema et al., 2012; Gerondopoulos et al., 2012; Bonifacino and Neefjes, 2017). Our description of two types of LROs in the Malpighian tubule principal cells (Fig. 8) requires an explanation of how the brown-white dimer is segregated away from the scarlet-white dimer to give rise to different types of LROs.
Fig. 8.
Schematic representation of a zinc storage granule and a riboflavin pigment granule, in principal cells of the Malpighian tubules. (A) Zinc storage granule. (B) Riboflavin pigment granule. On the left of each vesicle known players in protein trafficking are shown, common for the biogenesis of both types of LROs. Transporters are on the right-hand side. It is unclear how cells differentially give rise to the two LROs.
On the function of the ABCG2 transporters
The human ABCG2 has been studied extensively, because of early reports associating ABCG2 over-expression with the development of cancer resistance to drugs inhibiting the cell cycle by intercalating into DNA (Chen et al., 1990). The consensus for the mechanism of action of ABCG2 is its direct activity in exporting drugs from cells, but the apparent lack of specificity in the transported molecules is puzzling: more than 200 substrates have been described for ABCG2 (Goler-Baron and Assaraf, 2011; Horsey et al., 2016; Taylor et al., 2017). Amongst these substrates, one is riboflavin (van Herwaarden et al., 2007), another is cGMP (de Wolf et al., 2007; Evans et al., 2008). Does the function of ABCG2 relate more to the failure of formation of a LRO (Lloyd et al., 2002; Goler-Baron et al., 2012) in the cells or animal models where it has been silenced, and less to the specific transport of the various substrates it has been claimed to move across membranes? At least in Drosophila, the w gene is required for the process of pigment granule formation per se (Yagi and Ogawa, 1996). How the white protein functions in the biogenesis of the LROs remains unclear. Based on the results described here, we cannot formally exclude a direct implication of the white-scarlet dimer in zinc transport. Transporters of the ZnT family have been proposed to function through a Zn2+/H+ mechanism (Ohana et al., 2009; Shusterman et al., 2014). If the proton gradient sustained by V-ATPase (Bouché et al., 2016; Overend et al., 2016; Tognon et al., 2016) does not provide a thermodynamic explanation how ZnT35C accumulates zinc in storage granules as would be the current thinking in the field (Jeong and Eide, 2013; Kambe et al., 2017; Lee et al., 2017b), the alternative hypothesis would be that the ATPase activity of the ABCG2 homologues white and scarlet are implicated in zinc transport, perhaps working in a similar way to KATP channels (Lee et al., 2017a), i.e. by forming a pump in complex with ZnT35C. No evidence for an association between ZnTs and ABCG2 exists to date. Further experiments are required to distinguish between the above possibilities.
On the use of the w mutant as a control in Drosophila experiments
Many authors warn against the possible alterations of normal cell physiology in the w mutant and consider implications of using it as the major control strain in experiments (Campbell and Nash, 2001; Borycz et al., 2008; Chetverina et al., 2008; Krstic et al., 2013; Chan et al., 2014; Xiao and Robertson, 2016; Ferreiro et al., 2018). Direct implications for the field of Drosophila zinc biology, almost entirely based on transgenes carrying the mini-white marker, have been raised before (Afshar et al., 2013; Richards and Burke, 2016).
A potential role for zinc in human Hermansky–Pudlak syndrome
Mutations in nine different genes can cause Hermansky–Pudlak syndrome (HPS) in humans. HPS is characterized by oculocutaneous albinism, a platelet storage pool deficiency and lysosomal accumulation of ceroid lipofuscin (Seward and Gahl, 2013). Patients with the genotypes HPS-1, HPS-2 or HPS-4 are predisposed to interstitial lung disease and may develop granulomatous colitis. Hypopigmentation is the prominent feature of HPS, attributable to the disrupted biogenesis of LROs (Wei et al., 2013). Is zinc homeostasis altered in human patients diagnosed with HPS? To our knowledge this question has not been addressed. We conclude by pointing out that fly mutants lacking zinc storage granules correspond to known genetic alterations in HPS patients. Besides the identification of p as the homologue of the HPS type 5 gene (Falcón-Pérez et al., 2007; Syrzycka et al., 2007), rb encodes for the type 2 syndrome protein (Gochuico et al., 2012), and a second neuronal-specific human homologue of the β-subunit of AP-3 was recently related to an early-onset epileptic encephalopathy with optic atrophy (Assoum et al., 2016), and g encodes for the δ-subunit of the AP-3 complex, recently shown to define a new type of the HPS (Ammann et al., 2016). Likewise, the corresponding mouse mutants are known to lack zinc granules (Kantheti et al., 1998). Thus the regulation of zinc homeostasis and trafficking in HPS patients deserves further investigation.
Acknowledgements
The authors thank the Bloomington Drosophila Stock Center for the flies used in this study, Refugio Rodríguez Vázquez for providing access to the atomic absorption spectrometer, Alma Isabel Santos Díaz for participating in experiments as part of her social service training in the laboratory, and Marcos Nahmad, Irene Miguel-Aliaga, John F. Allen and two anonymous reviewers for critical comments on the manuscript. The authors thank Nicholas P. Edwards and Courtney M. Krest for excellent beam line support and José Mustre de León for approval of travel expenses to visit the Synchrotron instrument at Stanford University.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: C.T.-G., A.R.-A., B.O., F.M.; Methodology: C.T.-G., A.R.-A., T.K., S.M.W., M.B.-A., B.O., F.M.; Software: S.M.W.; Validation: C.T.-G., A.R.-A., M.B., B.O., F.M.; Formal analysis: C.T.-G., F.M.; Investigation: C.T.-G., A.R.-A., B.O., F.M.; Resources: T.K., F.M.; Data curation: C.T.-G., T.K., F.M.; Writing - original draft: F.M.; Writing - review & editing: C.T.-G., A.R.-A, T.K., S.M.W., M.B., B.O., F.M.; Visualization: A.R.-A, T.K., F.M.; Supervision: A.R.-A, T.K., S.M.W., B.O., F.M.; Project administration: T.K., B.O., F.M.; Funding acquisition: T.K., F.M.
Funding
Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences (contract no. DE-AC02-76SF00515). The SSRL Structural Molecular Biology Program is supported by the U.S. Department of Energy Office of Biological and Environmental Research and the National Institutes of Health, National Institute of General Medical Sciences (P41GM103393). The MARS6 microwave digestion system and the PerkinElmer Optima 8300 ICP-OES instrument were acquired with the Consejo Nacional de Ciencia y Tecnología (CONACYT) infrastructure grant (no. 268296). Consejo Nacional de Ciencia y Tecnología (CONACYT) also supported C.T.-G. and A.R.-A. with PhD (no. 299627) and postdoctoral (no. 189290) fellowships, respectively. Deposited in PMC for release after 12 months.
Data availability
Raw data for metal determinations and the synchrotron spectra are available from the corresponding author upon request. The same applies to fly strains not available from the Bloomington Drosophila Stock Center.
References
- Afshar N., Argunhan B., Bettedi L., Szular J. and Missirlis F. (2013). A recessive X-linked mutation causes a threefold reduction of total body zinc accumulation in Drosophila melanogaster laboratory strains. FEBS Open Bio 3, 302-304. 10.1016/j.fob.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann S., Schulz A., Krageloh-Mann I., Dieckmann N. M., Niethammer K., Fuchs S., Eckl K. M., Plank R., Werner R., Altmuller J. et al. (2016). Mutations in AP3D1 associated with immunodeficiency and seizures define a new type of Hermansky-Pudlak syndrome. Blood 127, 997-1006. 10.1182/blood-2015-09-671636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. R., Kirby K., Hilliker A. J. and Phillips J. P. (2005). RNAi-mediated suppression of the mitochondrial iron chaperone, frataxin, in Drosophila. Hum. Mol. Genet. 14, 3397-3405. 10.1093/hmg/ddi367 [DOI] [PubMed] [Google Scholar]
- Assoum M., Philippe C., Isidor B., Perrin L., Makrythanasis P., Sondheimer N., Paris C., Douglas J., Lesca G., Antonarakis S. et al. (2016). Autosomal-recessive mutations in AP3B2, adaptor-related protein complex 3 β2 subunit, cause an early-onset epileptic encephalopathy with optic atrophy. Am. J. Hum. Genet. 99, 1368-1376. 10.1016/j.ajhg.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanesyan L., Günther V., Celniker S. E., Georgiev O. and Schaffner W. (2011). Characterization of MtnE, the fifth metallothionein member in Drosophila. J. Biol. Inorg Chem. 16, 1047-1056. 10.1007/s00775-011-0825-4 [DOI] [PubMed] [Google Scholar]
- Babula P., Masarik M., Adam V., Eckschlager T., Stiborova M., Trnkova L., Skutkova H., Provaznik I., Hubalek J. and Kizek R. (2012). Mammalian metallothioneins: properties and functions. Metallomics 4, 739-750. 10.1039/c2mt20081c [DOI] [PubMed] [Google Scholar]
- Beadle G. W. (1937). Development of eye colors in Drosophila: fat bodies and Malpighian tubes in relation to diffusible substances. Genetics 22, 587-611. 10.1073/pnas.23.3.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. and Kollmer W. E. (1988). The influence of zinc deficiency on the storage of zinc in bone. In Trace Elements in Man and Animals, vol. 6 (ed. Hurley L. S., Keen C. L., Lönnerdal B. and Rucker R. B.), pp. 455-457. New York: Springer. [Google Scholar]
- Bettedi L., Aslam M. F., Szular J., Mandilaras K. and Missirlis F. (2011). Iron depletion in the intestines of Malvolio mutant flies does not occur in the absence of a multicopper oxidase. J. Exp. Biol. 214, 971-978. 10.1242/jeb.051664 [DOI] [PubMed] [Google Scholar]
- Beyersmann D. and Haase H. (2001). Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 14, 331-341. 10.1023/A:1012905406548 [DOI] [PubMed] [Google Scholar]
- Binks T., Lye J. C., Camakaris J. and Burke R. (2010). Tissue-specific interplay between copper uptake and efflux in Drosophila. J. Biol. Inorg Chem. 15, 621-628. 10.1007/s00775-010-0629-y [DOI] [PubMed] [Google Scholar]
- Bird A. J. (2015). Cellular sensing and transport of metal ions: implications in micronutrient homeostasis. J. Nutr. Biochem. 26, 1103-1115. 10.1016/j.jnutbio.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaart A. M. and Bernini L. F. (1981). The molybdoenzyme system of Drosophila-melanogaster. I. Sulfite oxidase: identification and properties. Expression of the enzyme in maroon-like (mal), low-xanthine dehydrogenase (lxd), and cinnamon (cin) flies. Biochem. Genet. 19, 929-946. 10.1007/BF00504258 [DOI] [PubMed] [Google Scholar]
- Bonfini A., Liu X. and Buchon N. (2016). From pathogens to microbiota: how Drosophila intestinal stem cells react to gut microbes. Dev. Comp. Immunol. 64, 22-38. 10.1016/j.dci.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S. and Neefjes J. (2017). Moving and positioning the endolysosomal system. Curr. Opin. Cell Biol. 47, 1-8. 10.1016/j.ceb.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borycz J., Borycz J. A., Kubow A., Lloyd V. and Meinertzhagen I. A. (2008). Drosophila ABC transporter mutants white, brown and scarlet have altered contents and distribution of biogenic amines in the brain. J. Exp. Biol. 211, 3454-3466. 10.1242/jeb.021162 [DOI] [PubMed] [Google Scholar]
- Bouché V., Espinosa A. P., Leone L., Sardiello M., Ballabio A. and Botas J. (2016). Drosophila Mitf regulates the V-ATPase and the lysosomal-autophagic pathway. Autophagy 12, 484-498. 10.1080/15548627.2015.1134081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa D., Gleber S.-C., Vogt S., Yi H., Will F., Richter H., Shin C. H. and Fahrni C. J. (2014). 3D imaging of transition metals in the zebrafish embryo by X-ray fluorescence microtomography. Metallomics 6, 1648-1655. 10.1039/C4MT00121D [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehme K. S. and Demerec M. (1942). A survey of Malpighian tube color in the eye color mutants of Drosophila melanogaster. Growth 6, 351-355. [Google Scholar]
- Brownlie J. C., Cass B. N., Riegler M., Witsenburg J. J., Iturbe-Ormaetxe I., McGraw E. A. and O'Neill S. L. (2009). Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog. 5, e1000368 10.1371/journal.ppat.1000368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultema J. J., Ambrosio A. L., Burek C. L. and Di Pietro S. M. (2012). BLOC-2, AP-3, and AP-1 proteins function in concert with Rab38 and Rab32 proteins to mediate protein trafficking to lysosome-related organelles. J. Biol. Chem. 287, 19550-19563. 10.1074/jbc.M112.351908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calap-Quintana P., González-Fernández J., Sebastiá-Ortega N., Llorens J. V. and Moltó M. D. (2017). Drosophila melanogaster models of metal-related human diseases and metal toxicity. Int. J. Mol. Sci. 18, 1456 10.3390/ijms18071456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camaschella C., Pagani A., Nai A. and Silvestri L. (2016). The mutual control of iron and erythropoiesis. Int. J. Lab. Hematol. 38, 20-26. 10.1111/ijlh.12505 [DOI] [PubMed] [Google Scholar]
- Campbell J. L. and Nash H. A. (2001). Volatile general anesthetics reveal a neurobiological role for the white and brown genes of Drosophila melanogaster. J. Neurobiol. 49, 339-349. 10.1002/neu.10009 [DOI] [PubMed] [Google Scholar]
- Chan R. F., Lewellyn L., DeLoyht J. M., Sennett K., Coffman S., Hewitt M., Bettinger J. C., Warrick J. M. and Grotewiel M. (2014). Contrasting influences of Drosophila white/mini-white on ethanol sensitivity in two different behavioral assays. Alcohol. Clin. Exp. Res. 38, 1582-1593. 10.1111/acer.12421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheli V. T., Daniels R. W., Godoy R., Hoyle D. J., Kandachar V., Starcevic M., Martinez-Agosto J. A., Poole S., DiAntonio A., Lloyd V. K. et al. (2010). Genetic modifiers of abnormal organelle biogenesis in a Drosophila model of BLOC-1 deficiency. Hum. Mol. Genet. 19, 861-878. 10.1093/hmg/ddp555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. N., Mickley L. A., Schwartz A. M., Acton E. M., Hwang J. L. and Fojo A. T. (1990). Characterization of adriamycin-resistant human breast cancer cells which display overexpression of a novel resistance-related membrane protein. J. Biol. Chem. 265, 10073-10080. [PubMed] [Google Scholar]
- Chetverina D., Savitskaya E., Maksimenko O., Melnikova L., Zaytseva O., Parshikov A., Galkin A. V. and Georgiev P. (2008). Red flag on the white reporter: a versatile insulator abuts the white gene in Drosophila and is omnipresent in mini-white constructs. Nucleic Acids Res. 36, 929-937. 10.1093/nar/gkm992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi T., Kim M. S., Lang S., Bose N., Kahn A., Flechner L., Blaschko S. D., Zee T., Muteliefu G., Bond N. et al. (2015). A Drosophila model identifies a critical role for zinc in mineralization for kidney stone disease. PLoS ONE 10, e0124150 10.1371/journal.pone.0124150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. I. and Walker D. W. (2018). Role of gut microbiota in aging-related health decline: insights from invertebrate models. Cell. Mol. Life Sci. 75, 93 10.1007/s00018-017-2671-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole T. B., Wenzel H. J., Kafer K. E., Schwartzkroin P. A. and Palmiter R. D. (1999). Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. USA 96, 1716-1721. 10.1073/pnas.96.4.1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin R. A., Jin Q., Lai B. and Kiedrowski L. (2016). Visualizing metal content and intracellular distribution in primary hippocampal neurons with synchrotron x-ray fluorescence. PLoS ONE 11, e0159582 10.1371/journal.pone.0159582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert M. S. and Philpott C. C. (2013). Regulation of cation balance in Saccharomyces cerevisiae. Genetics 193, 677-713. 10.1534/genetics.112.147207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wolf C. J. F., Yamaguchi H., van der Heijden I., Wielinga P. R., Hundscheid S. L., Ono N., Scheffer G. L., de Haas M., Schuetz J. D., Wijnholds J. et al. (2007). cGMP transport by vesicles from human and mouse erythrocytes. FEBS J. 274, 439-450. 10.1111/j.1742-4658.2006.05591.x [DOI] [PubMed] [Google Scholar]
- Dechen K., Richards C. D., Lye J. C., Hwang J. E. C. and Burke R. (2015). Compartmentalized zinc deficiency and toxicities caused by ZnT and Zip gene overexpression result in specific phenotypes in Drosophila. Int. J. Biochem. Cell Biol. 60, 23-33. 10.1016/j.biocel.2014.12.017 [DOI] [PubMed] [Google Scholar]
- Dell'Angelica E. C. (2009). AP-3-dependent trafficking and disease: the first decade. Curr. Opin. Cell Biol. 21, 552-559. 10.1016/j.ceb.2009.04.014 [DOI] [PubMed] [Google Scholar]
- Dell'Angelica E. C., Mullins C., Caplan S. and Bonifacino J. S. (2000). Lysosome-related organelles. FASEB J. 14, 1265-1278. 10.1096/fj.14.10.1265 [DOI] [PubMed] [Google Scholar]
- Dow J. A. (2017). The essential roles of metal ions in insect homeostasis and physiology. Curr. Opin. Insect Sci. 23, 43-50. 10.1016/j.cois.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Drakesmith H., Nemeth E. and Ganz T. (2015). Ironing out ferroportin. Cell Metab. 22, 777-787. 10.1016/j.cmet.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreesen T. D., Johnson D. H. and Henikoff S. (1988). The brown protein of Drosophila melanogaster is similar to the white protein and to components of active transport complexes. Mol. Cell. Biol. 8, 5206-5215. 10.1128/MCB.8.12.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duttaroy A., Parkes T., Emtage P., Kirby K., Boulianne G. L., Wang X. D., Hilliker A. J. and Phillips J. P. (1997). The manganese superoxide dismutase gene of Drosophila: structure, expression, and evidence for regulation by MAP kinase. DNA Cell Biol. 16, 391-399. 10.1089/dna.1997.16.391 [DOI] [PubMed] [Google Scholar]
- Egli D., Selvaraj A., Yepiskoposyan H., Zhang B., Hafen E., Georgiev O. and Schaffner W. (2003). Knockout of ‘metal-responsive transcription factor’ MTF-1 in Drosophila by homologous recombination reveals its central role in heavy metal homeostasis. EMBO J. 22, 100-108. 10.1093/emboj/cdg012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli D., Yepiskoposyan H., Selvaraj A., Balamurugan K., Rajaram R., Simons A., Multhaup G., Mettler S., Vardanyan A., Georgiev O. et al. (2006). A family knockout of all four Drosophila metallothioneins reveals a central role in copper homeostasis and detoxification. Mol. Cell. Biol. 26, 2286-2296. 10.1128/MCB.26.6.2286-2296.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G., Vos M., Vilain S., Swerts J., De Sousa Valadas J., Van Meensel S., Schaap O. and Verstreken P. (2013). Aconitase causes iron toxicity in Drosophila pink1 mutants. PLoS Genet. 9, e1003478 10.1371/journal.pgen.1003478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. M., Day J. P., Cabrero P., Dow J. A. T. and Davies S.-A. (2008). A new role for a classical gene: white transports cyclic GMP. J. Exp. Biol. 211, 890-899. 10.1242/jeb.014837 [DOI] [PubMed] [Google Scholar]
- Falcón-Pérez J. M., Romero-Calderón R., Brooks E. S., Krantz D. E. and Dell'Angelica E. C. (2007). The Drosophila pigmentation gene pink (p) encodes a homologue of human Hermansky-Pudlak syndrome 5 (HPS5). Traffic 8, 154-168. 10.1111/j.1600-0854.2006.00514.x [DOI] [PubMed] [Google Scholar]
- Ferreiro M. J., Perez C., Marchesano M., Ruiz S., Caputi A., Aguilera P., Barrio R. and Cantera R. (2018). Drosophila melanogaster white mutant w1118 undergo retinal degeneration. Front. Neurosci. 11, 732 10.3389/fnins.2017.00732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser J., Venken K. J. T., De Lisle R. C. and Andrews G. K. (2012). A mouse model of acrodermatitis enteropathica: loss of intestine zinc transporter ZIP4 (Slc39a4) disrupts the stem cell niche and intestine integrity. PLoS Genet. 8, e1002766 10.1371/journal.pgen.1002766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerondopoulos A., Langemeyer L., Liang J.-R., Linford A. and Barr F. A. (2012). BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr. Biol. 22, 2135-2139. 10.1016/j.cub.2012.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblin L. J., Chang C. J., Bentley A. F., Frederickson C., Lippard S. J. and Frederickson C. J. (2006). Zinc-secreting Paneth cells studied by ZP fluorescence. J. Histochem. Cytochem. 54, 311-316. 10.1369/jhc.5A6724.2005 [DOI] [PubMed] [Google Scholar]
- Gochuico B. R., Huizing M., Golas G. A., Scher C. D., Tsokos M., Denver S. D., Frei-Jones M. J. and Gahl W. A. (2012). Interstitial lung disease and pulmonary fibrosis in Hermansky-Pudlak syndrome type 2, an adaptor protein-3 complex disease. Mol. Med. 18, 56-64. 10.2119/molmed.2011.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goler-Baron V. and Assaraf Y. G. (2011). Structure and function of ABCG2-rich extracellular vesicles mediating multidrug resistance. PLoS ONE 6, e16007 10.1371/journal.pone.0016007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goler-Baron V., Sladkevich I. and Assaraf Y. G. (2012). Inhibition of the PI3K-Akt signaling pathway disrupts ABCG2-rich extracellular vesicles and overcomes multidrug resistance in breast cancer cells. Biochem. Pharmacol. 83, 1340-1348. 10.1016/j.bcp.2012.01.033 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Morales N., Mendoza-Ortiz M. A., Blowes L. M., Missirlis F. and Riesgo-Escovar J. R. (2015). Ferritin is required in multiple tissues during Drosophila melanogaster development. PLoS ONE 10, e0133499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E. W., Campesan S., Breda C., Sathyasaikumar K. V., Muchowski P. J., Schwarcz R., Kyriacou C. P. and Giorgini F. (2012). Drosophila eye color mutants as therapeutic tools for Huntington disease. Fly (Austin) 6, 117-120. 10.4161/fly.19999 [DOI] [PubMed] [Google Scholar]
- Groth C., Sasamura T., Khanna M. R., Whitley M. and Fortini M. E. (2013). Protein trafficking abnormalities in Drosophila tissues with impaired activity of the ZIP7 zinc transporter Catsup. Development 140, 3018-3027. 10.1242/dev.088336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther V., Lindert U. and Schaffner W. (2012). The taste of heavy metals: gene regulation by MTF-1. Biochim. Biophys. Acta 1823, 1416-1425. 10.1016/j.bbamcr.2012.01.005 [DOI] [PubMed] [Google Scholar]
- Gutiérrez L., Sabaratnam N., Aktar R., Bettedi L., Mandilaras K. and Missirlis F. (2010). Zinc accumulation in heterozygous mutants of fumble, the pantothenate kinase homologue of Drosophila. FEBS Lett. 584, 2942-2946. 10.1016/j.febslet.2010.05.029 [DOI] [PubMed] [Google Scholar]
- Gutiérrez L., Zubow K., Nield J., Gambis A., Mollereau B., Lázaro F. J. and Missirlis F. (2013). Biophysical and genetic analysis of iron partitioning and ferritin function in Drosophila melanogaster. Metallomics 5, 997-1005. 10.1039/c3mt00118k [DOI] [PubMed] [Google Scholar]
- Halberg K. A., Terhzaz S., Cabrero P., Davies S. A. and Dow J. A. T. (2015). Tracing the evolutionary origins of insect renal function. Nat. Commun. 6, 6800 10.1038/ncomms7800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. A., Kim K., Nakahara K., Vásquez-Doorman C. and Carthew R. W. (2011). Cargo sorting to lysosome-related organelles regulates siRNA-mediated gene silencing. J. Cell Biol. 194, 77-87. 10.1083/jcb.201102021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Muckenthaler M. U. and Andrews N. C. (2004). Balancing acts: molecular control of mammalian iron metabolism. Cell 117, 285-297. 10.1016/S0092-8674(04)00343-5 [DOI] [PubMed] [Google Scholar]
- Hersh B. M. (2016). More than meets the eye: a primer for ‘timing of locomotor recovery from anoxia modulated by the white gene in Drosophila melanogaster’. Genetics 204, 1369-1375. 10.1534/genetics.116.196519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsey A. J., Cox M. H., Sarwat S. and Kerr I. D. (2016). The multidrug transporter ABCG2: still more questions than answers. Biochem. Soc. Trans. 44, 824-830. 10.1042/BST20160014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Yu Y. Y., Kirschke C. P., Gertz E. R. and Lloyd K. K. C. (2007). Znt7 (Slc30a7)-deficient mice display reduced body zinc status and body fat accumulation. J. Biol. Chem. 282, 37053-37063. 10.1074/jbc.M706631200 [DOI] [PubMed] [Google Scholar]
- Jeong J. and Eide D. J. (2013). The SLC39 family of zinc transporters. Mol. Aspects Med. 34, 612-619. 10.1016/j.mam.2012.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. W. M., de Jonge M. D., James S. A. and Burke R. (2015). Elemental mapping of the entire intact Drosophila gastrointestinal tract. J. Biol. Inorg Chem. 20, 979-987. 10.1007/s00775-015-1281-3 [DOI] [PubMed] [Google Scholar]
- Kambe T., Matsunaga M. and Takeda T. A. (2017). Understanding the contribution of zinc transporters in the function of the early secretory pathway. Int. J. Mol. Sci. 18, e2179 10.3390/ijms18102179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantheti P., Qiao X., Diaz M. E., Peden A. A., Meyer G. E., Carskadon S. L., Kapfhamer D., Sufalko D., Robinson M. S., Noebels J. L. et al. (1998). Mutation in AP-3 delta in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron 21, 111-122. 10.1016/S0896-6273(00)80519-X [DOI] [PubMed] [Google Scholar]
- Kawanishi H. (1966). Electron microscopic studies on secretory mechanism of pancreatic islet cells with particular reference to beta cells. 2. Secretion of beta granules in islets of Langerhans particularly in association with intracellular reactive zinc under normal conditions during prolonged starvation and after administration of dithizone in rabbits. Endocrinol. Jpn. 13, 384-408. [DOI] [PubMed] [Google Scholar]
- Kim H., Kim K. and Yim J. (2013). Biosynthesis of drosopterins, the red eye pigments of Drosophila melanogaster. IUBMB Life 65, 334-340. 10.1002/iub.1145 [DOI] [PubMed] [Google Scholar]
- Kimura T. and Kambe T. (2016). The functions of metallothionein and ZIP and ZnT transporters: an overview and perspective. Int. J. Mol. Sci. 17, 336 10.3390/ijms17030336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby K., Jensen L. T., Binnington J., Hilliker A. J., Ulloa J., Culotta V. C. and Phillips J. P. (2008). Instability of superoxide dismutase 1 of Drosophila in mutants deficient for its cognate copper chaperone. J. Biol. Chem. 283, 35393-35401. 10.1074/jbc.M807131200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbas M., Blechinger S. R., Krone P. H., Pickering I. J. and George G. N. (2008). Localizing organomercury uptake and accumulation in zebrafish larvae at the tissue and cellular level. Proc. Natl. Acad. Sci. USA 105, 12108-12112. 10.1073/pnas.0803147105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer H. (2002). Sorting out signals in fly endosomes. Traffic 3, 87-91. 10.1034/j.1600-0854.2002.030201.x [DOI] [PubMed] [Google Scholar]
- Kretzschmar D., Poeck B., Roth H., Ernst R., Keller A., Porsch M., Strauss R. and Pflugfelder G. O. (2000). Defective pigment granule biogenesis and aberrant behavior caused by mutations in the Drosophila AP-3β adaptin gene ruby. Genetics 155, 213-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll T., Hadt R. G., Wilson S. A., Lundberg M., Yan J. J., Weng T.-C., Sokaras D., Alonso-Mori R., Casa D., Upton M. H. et al. (2014). Resonant inelastic X-ray scattering on ferrous and ferric bis-imidazole porphyrin and cytochrome c: nature and role of the axial methionine-Fe bond. J. Am. Chem. Soc. 136, 18087-18099. 10.1021/ja5100367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krstic D., Boll W. and Noll M. (2013). Influence of the White locus on the courtship behavior of Drosophila males. PLoS ONE 8, e77904 10.1371/journal.pone.0077904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn L. C. (2015). Iron regulatory proteins and their role in controlling iron metabolism. Metallomics 7, 232-243. 10.1039/C4MT00164H [DOI] [PubMed] [Google Scholar]
- Lee K., Chen J. and MacKinnon R. (2017a). Molecular structure of human KATP in complex with ATP and ADP. eLife 6, e32481 10.7554/eLife.32481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Rivera O. C. and Kelleher S. L. (2017b). Zinc transporter 2 interacts with vacuolar ATPase and is required for polarization, vesicle acidification and secretion in mammary epithelial cells. J. Biol. Chem. 292, 21598-21613. 10.1074/jbc.M117.794461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. (2010). Identification of iron-loaded ferritin as an essential mitogen for cell proliferation and postembryonic development in Drosophila. Cell Res. 20, 1148-1157. 10.1038/cr.2010.102 [DOI] [PubMed] [Google Scholar]
- Llano E., Pendas A. M., Aza-Blanc P., Kornberg T. B. and Lopez-Otin C. (2000). Dm1-MMP, a matrix metalloproteinase from Drosophila with a potential role in extracellular matrix remodeling during neural development. J. Biol. Chem. 275, 35978-35985. 10.1074/jbc.M006045200 [DOI] [PubMed] [Google Scholar]
- Llorens J. V., Metzendorf C., Missirlis F. and Lind M. I. (2015). Mitochondrial iron supply is required for the developmental pulse of ecdysone biosynthesis that initiates metamorphosis in Drosophila melanogaster. J. Biol. Inorg Chem. 20, 1229-1238. 10.1007/s00775-015-1302-2 [DOI] [PubMed] [Google Scholar]
- Lloyd V., Ramaswami M. and Krämer H. (1998). Not just pretty eyes: Drosophila eye-colour mutations and lysosomal delivery. Trends Cell Biol. 8, 257-259. 10.1016/S0962-8924(98)01270-7 [DOI] [PubMed] [Google Scholar]
- Lloyd V. K., Sinclair D. A. R., Alperyn M. and Grigliatti T. A. (2002). Enhancer of garnet/δAP-3 is a cryptic allele of the white gene and identifies the intracellular transport system for the white protein. Genome 45, 296-312. 10.1139/g01-139 [DOI] [PubMed] [Google Scholar]
- Locke M. and Leung H. (1984). The induction and distribution of an insect ferritin – a new function for the endoplasmic reticulum. Tissue Cell 16, 739-766. 10.1016/0040-8166(84)90007-7 [DOI] [PubMed] [Google Scholar]
- Lye J. C., Richards C. D., Dechen K., Paterson D., de Jonge M. D., Howard D. L., Warr C. G. and Burke R. (2012). Systematic functional characterization of putative zinc transport genes and identification of zinc toxicosis phenotypes in Drosophila melanogaster. J. Exp. Biol. 215, 3254-3265. 10.1242/jeb.069260 [DOI] [PubMed] [Google Scholar]
- Lye J. C., Richards C. D., Dechen K., Warr C. G. and Burke R. (2013). In vivo zinc toxicity phenotypes provide a sensitized background that suggests zinc transport activities for most of the Drosophila Zip and ZnT genes. J. Biol. Inorg Chem. 18, 323-332. 10.1007/s00775-013-0976-6 [DOI] [PubMed] [Google Scholar]
- Ma J., Plesken H., Treisman J. E., Edelman-Novemsky I. and Ren M. (2004). Lightoid and Claret: a rab GTPase and its putative guanine nucleotide exchange factor in biogenesis of Drosophila eye pigment granules. Proc. Natl. Acad. Sci. USA 101, 11652-11657. 10.1073/pnas.0401926101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie S. M., Howells A. J., Cox G. B. and Ewart G. D. (2000). Sub-cellular localisation of the white/scarlet ABC transporter to pigment granule membranes within the compound eye of Drosophila melanogaster. Genetica 108, 239-252. 10.1023/A:1004115718597 [DOI] [PubMed] [Google Scholar]
- Mandilaras K. and Missirlis F. (2012). Genes for iron metabolism influence circadian rhythms in Drosophila melanogaster. Metallomics 4, 928-936. 10.1039/c2mt20065a [DOI] [PubMed] [Google Scholar]
- Mandilaras K., Pathmanathan T. and Missirlis F. (2013). Iron absorption in Drosophila melanogaster. Nutrients 5, 1622-1647. 10.3390/nu5051622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marelja Z., Dambowsky M., Bolis M., Georgiou M. L., Garattini E., Missirlis F. and Leimkuhler S. (2014). The four aldehyde oxidases of Drosophila melanogaster have different gene expression patterns and enzyme substrate specificities. J. Exp. Biol. 217, 2201-2211. 10.1242/jeb.102129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret W. (2017). Zinc in pancreatic islet biology, insulin sensitivity, and diabetes. Prev. Nutr. Food Sci. 22, 1-8. 10.3746/pnf.2017.22.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino M. E., Ma D. and Leulier F. (2017). Microbial influence on Drosophila biology. Curr. Opin. Microbiol. 38, 165-170. 10.1016/j.mib.2017.06.004 [DOI] [PubMed] [Google Scholar]
- Martinson V. G., Carpinteyro-Ponce J., Moran N. A. and Markow T. A. (2017). A distinctive and host-restricted gut microbiota in populations of a cactophilic Drosophila species. Appl. Environ. Microbiol. 83, e01551-17 10.1128/AEM.01551-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister B. B. and Dyck R. H. (2017). Zinc transporter 3 (ZnT3) and vesicular zinc in central nervous system function. Neurosci. Biobehav. Rev. 80, 329-350. 10.1016/j.neubiorev.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Mehta A., Deshpande A., Bettedi L. and Missirlis F. (2009). Ferritin accumulation under iron scarcity in Drosophila iron cells. Biochimie 91, 1331-1334. 10.1016/j.biochi.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Mercer S. W., Wang J. and Burke R. (2017). In vivo modeling of the pathogenic effect of copper transporter mutations that cause Menkes and Wilson diseases, motor neuropathy, and susceptibility to Alzheimer's disease. J. Biol. Chem. 292, 4113-4122. 10.1074/jbc.M116.756163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrit T. J. S. and Bewick A. J. (2017). Genetic diversity in insect metal tolerance. Front Genet. 8, 172 10.3389/fgene.2017.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missirlis F., Kosmidis S., Brody T., Mavrakis M., Holmberg S., Odenwald W. F., Skoulakis E. M. C. and Rouault T. A. (2007). Homeostatic mechanisms for iron storage revealed by genetic manipulations and live imaging of Drosophila ferritin. Genetics 177, 89-100. 10.1534/genetics.107.075150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry R., Kounatidis I. and Ligoxygakis P. (2016). Exploring interactions between pathogens and the Drosophila gut. Dev. Comp. Immunol. 64, 3-10. 10.1016/j.dci.2016.01.016 [DOI] [PubMed] [Google Scholar]
- Mohr S. E., Rudd K., Hu Y., Song W. R., Gilly Q., Buckner M., Housden B. E., Kelley C., Zirin J., Tao R. et al. (2017). Zinc detoxification: a functional genomics and transcriptomics analysis in Drosophila melanogaster cultured cells. G3 (Bethesda) 8, 631- 641 10.1534/g3.117.300447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan T. H. (1910). Sex limited inheritance in Drosophila. Science 32, 120-122. 10.1126/science.32.812.120 [DOI] [PubMed] [Google Scholar]
- Muckenthaler M. U., Rivella S., Hentze M. W. and Galy B. (2017). A red carpet for iron metabolism. Cell 168, 344-361. 10.1016/j.cell.2016.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins C., Hartnell L. M., Wassarman D. A. and Bonifacino J. S. (1999). Defective expression of the µ3 subunit of the AP-3 adaptor complex in the Drosophila pigmentation mutant carmine. Mol. Gen. Genet. 262, 401-412. 10.1007/s004380051099 [DOI] [PubMed] [Google Scholar]
- Mullins C., Hartnell L. M. and Bonifacino J. S. (2000). Distinct requirements for the AP-3 adaptor complex in pigment granule and synaptic vesicle biogenesis in Drosophila melanogaster. Mol. Gen. Genet. 263, 1003-1014. 10.1007/PL00008688 [DOI] [PubMed] [Google Scholar]
- Nagarkar-Jaiswal S., DeLuca S. Z., Lee P. T., Lin W. W., Pan H., Zuo Z., Lv J., Spradling A. C. and Bellen H. J. (2015). A genetic toolkit for tagging intronic MiMIC containing genes. eLife 4, e08469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro, J. A. and Schneuwly S. (2017). Copper and zinc homeostasis: lessons from Drosophila melanogaster. Front Genet. 8, 223 10.3389/fgene.2017.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navrotskaya V. and Oxenkrug G. (2016). Effect of kynurenic acid on development and aging in wild type and vermilion mutants of Drosophila melanogaster. Pharmacol Drug Dev. Ther. 1, 2-3. 10.15761/PDDT.1000104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla H. (1972). Interaction between pteridine synthesis and riboflavin accumulation in Drosophila melanogaster. Can. J. Genet. Cytol. 14, 105-111. 10.1139/g72-013 [DOI] [PubMed] [Google Scholar]
- Nolte D. J. (1961). The pigment granules in compound eyes of Drosophila. Heredity 16, 25-38. 10.1038/hdy.1961.2 [DOI] [Google Scholar]
- O'Donnell M. J. and Maddrell S. H. (1995). Fluid reabsorption and ion transport by the lower Malpighian tubules of adult female Drosophila. J. Exp. Biol. 198, 1647-1653. [DOI] [PubMed] [Google Scholar]
- Ohana E., Hoch E., Keasar C., Kambe T., Yifrach O., Hershfinkel M. and Sekler I. (2009). Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J. Biol. Chem. 284, 17677-17686. 10.1074/jbc.M109.007203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi W., Kimura S., Iwanaga T., Furusawa Y., Irié T., Izumi H., Watanabe T., Hijikata A., Hara T., Ohara O. et al. (2016). Zinc transporter SLC39A7/ZIP7 promotes intestinal epithelial self-renewal by resolving ER stress. PLoS Genet. 12, e1006349 10.1371/journal.pgen.1006349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K. (1942). Biologische untersuchungen der Metalle. Trans. Soc. Path. Jap. 32, 99-105. [Google Scholar]
- Ooi C. E., Moreira J. E., Dell'Angelica E. C., Poy G., Wassarman D. A. and Bonifacino J. S. (1997). Altered expression of a novel adaptin leads to defective pigment granule biogenesis in the Drosophila eye color mutant garnet. EMBO J. 16, 4508-4518. 10.1093/emboj/16.15.4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgad S., Nelson H., Segal D. and Nelson N. (1998). Metal ions suppress the abnormal taste behavior of the Drosophila mutant malvolio. J. Exp. Biol. 201, 115-120. [DOI] [PubMed] [Google Scholar]
- Ott S., Dziadulewicz N. and Crowther D. C. (2015). Iron is a specific cofactor for distinct oxidation- and aggregation-dependent Aβ toxicity mechanisms in a Drosophila model. Dis. Model. Mech. 8, 657-667. 10.1242/dmm.019042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overend G., Luo Y., Henderson L., Douglas A. E., Davies S. A. and Dow J. A. (2016). Molecular mechanism and functional significance of acid generation in the Drosophila midgut. Sci. Rep. 6, 27242 10.1038/srep27242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palandri A., L'hôte D., Cohen-Tannoudji J., Tricoire H. and Monnier V. (2015). Frataxin inactivation leads to steroid deficiency in flies and human ovarian cells. Hum. Mol. Genet. 24, 2615-2626. 10.1093/hmg/ddv024 [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Steller H. and Bozzetti M. P. (1985). Multiple upstream regulatory elements control the expression of the Drosophila white gene. EMBO J. 4, 3501-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L. M., Rink L. and Haase H. (2010). The essential toxin: impact of zinc on human health. Int. J. Environ. Res. Public Health 7, 1342-1365. 10.3390/ijerph7041342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podany A. B., Wright J., Lamendella R., Soybel D. I. and Kelleher S. L. (2016). ZnT2-mediated zinc import into Paneth cell granules is necessary for coordinated secretion and Paneth cell function in mice. Cell. Mol. Gastroenterol. Hepatol. 2, 369-383. 10.1016/j.jcmgh.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu B. F., Robinson C. A., Chapman L. D. and Nichol H. (2009). Synchrotron X-ray fluorescence reveals abnormal metal distributions in brain and spinal cord in spinocerebellar ataxia: a case report. Cerebellum 8, 340-351. 10.1007/s12311-009-0102-z [DOI] [PubMed] [Google Scholar]
- Pound L. D., Sarkar S. A., Benninger R. K. P., Wang Y., Suwanichkul A., Shadoan M. K., Printz R. L., Oeser J. K., Lee C. E., Piston D. W. et al. (2009). Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem. J. 421, 371-376. 10.1042/BJ20090530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang W., Huang Y., Wan Z. and Zhou B. (2017). Metal-metal interaction mediates the iron induction of Drosophila MtnB. Biochem. Biophys. Res. Commun. 487, 646-652. 10.1016/j.bbrc.2017.04.109 [DOI] [PubMed] [Google Scholar]
- Qin Q. H., Wang X. X. and Zhou B. (2013). Functional studies of Drosophila zinc transporters reveal the mechanism for dietary zinc absorption and regulation. BMC Biol. 11, 101 10.1186/1741-7007-11-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redemann N., Gaul U. and Jäckle H. (1988). Disruption of a putative Cys zinc interaction eliminates the biological activity of the Kruppel finger protein. Nature 332, 90-92. 10.1038/332090a0 [DOI] [PubMed] [Google Scholar]
- Rempoulakis P., Afshar N., Osorio B., Barajas-Aceves M., Szular J., Ahmad S., Dammalage T., Tomas U. S., Nemny-Lavy E., Salomon M. et al. (2014). Conserved metallomics in two insect families evolving separately for a hundred million years. Biometals 27, 1323-1335. 10.1007/s10534-014-9793-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. D. and Burke R. (2016). A fly's eye view of zinc homeostasis: novel insights into the genetic control of zinc metabolism from Drosophila. Arch. Biochem. Biophys. 611, 142-149. 10.1016/j.abb.2016.07.015 [DOI] [PubMed] [Google Scholar]
- Richards C. D., Warr C. G. and Burke R. (2015). A role for dZIP89B in Drosophila dietary zinc uptake reveals additional complexity in the zinc absorption process. Int. J. Biochem. Cell Biol. 69, 11-19. 10.1016/j.biocel.2015.10.004 [DOI] [PubMed] [Google Scholar]
- Richards C. D., Warr C. G. and Burke R. (2017). A role for the Drosophila zinc transporter Zip88E in protecting against dietary zinc toxicity. PLoS ONE 12, e0181237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fernandez I. A. and Dell'Angelica E. C. (2015). Identification of Atg2 and ArfGAP1 as candidate genetic modifiers of the eye pigmentation phenotype of Adaptor Protein-3 (AP-3) mutants in Drosophila melanogaster. PLoS ONE 10, e0143026 10.1371/journal.pone.0143026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh H. C., Collier S., Guthrie J., Robertson J. D. and Kornfeld K. (2012). Lysosome-related organelles in intestinal cells are a zinc storage site in C. elegans. Cell Metab. 15, 88-99. 10.1016/j.cmet.2011.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Arellano A., Vásquez-Procopio J., Gambis A., Blowes L. M., Steller H., Mollereau B. and Missirlis F. (2016). Ferritin assembly in enterocytes of Drosophila melanogaster. Int. J. Mol. Sci. 17, 27 10.3390/ijms17020027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter G. A., Chabosseau P., Bellomo E. A., Maret W., Mitchell R. K., Hodson D. J., Solomou A. and Hu M. (2016). Intracellular zinc in insulin secretion and action: a determinant of diabetes risk? Proc. Nutr. Soc. 75, 61-72. 10.1017/S0029665115003237 [DOI] [PubMed] [Google Scholar]
- Sadraie M. and Missirlis F. (2011). Evidence for evolutionary constraints in Drosophila metal biology. Biometals 24, 679-686. 10.1007/s10534-011-9420-y [DOI] [PubMed] [Google Scholar]
- Salazar G., Love R., Werner E., Doucette M. M., Cheng S., Levey A. and Faundez V. (2004). The zinc transporter ZnT3 interacts with AP-3 and it is preferentially targeted to a distinct synaptic vesicle subpopulation. Mol. Biol. Cell 15, 575-587. 10.1091/mbc.E03-06-0401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M.-L. (2000). Drosophila arginase is produced from a nonvital gene that contains the elav locus within its third intron. J. Biol. Chem. 275, 31107-31114. 10.1074/jbc.M001346200 [DOI] [PubMed] [Google Scholar]
- Schofield R. M., Postlethwait J. H. and Lefevre H. W. (1997). MeV-ion microprobe analyses of whole Drosophila suggest that zinc and copper accumulation is regulated storage not deposit excretion. J. Exp. Biol. 200, 3235-3243. [DOI] [PubMed] [Google Scholar]
- Schuh R., Aicher W., Gaul U., Côte S., Preiss A., Maier D., Seifert E., Nauber U., Schröder C., Kemler R. et al. (1986). A conserved family of nuclear proteins containing structural elements of the finger protein encoded by Kruppel, a Drosophila segmentation gene. Cell 47, 1025-1032. 10.1016/0092-8674(86)90817-2 [DOI] [PubMed] [Google Scholar]
- Scott D. A. and Fisher A. M. (1938). The insulin and the zinc content of normal and diabetic pancreas. J. Clin. Invest. 17, 725-728. 10.1172/JCI101000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seward S. L. Jr. and Gahl W. A. (2013). Hermansky-Pudlak syndrome: health care throughout life. Pediatrics 132, 153-160. 10.1542/peds.2012-4003 [DOI] [PubMed] [Google Scholar]
- Shoup J. R. (1966). The development of pigment granules in the eyes of wild type and mutant Drosophila melanogaster. J. Cell Biol. 29, 223-249. 10.1083/jcb.29.2.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusterman E., Beharier O., Shiri L., Zarivach R., Etzion Y., Campbell C. R., Lee I.-H., Okabayashi K., Dinudom A., Cook D. I. et al. (2014). ZnT-1 extrudes zinc from mammalian cells functioning as a Zn2+/H+ exchanger. Metallomics 6, 1656-1663. 10.1039/C4MT00108G [DOI] [PubMed] [Google Scholar]
- Sims H. I., Chirn G.-W. and Marr M. T. II (2012). Single nucleotide in the MTF-1 binding site can determine metal-specific transcription activation. Proc. Natl. Acad. Sci. USA 109, 16516-16521. 10.1073/pnas.1207737109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal R. S., Peters P. D. and Hall T. A. (1976). Fine structure and X-ray microanalysis of mineralized concretions in the Malpighian tubules of the housefly, Musca domestica. Tissue Cell 8, 447-458. 10.1016/0040-8166(76)90005-7 [DOI] [PubMed] [Google Scholar]
- Southon A., Burke R. and Camakaris J. (2013). What can flies tell us about copper homeostasis? Metallomics 5, 1346-1356. 10.1039/c3mt00105a [DOI] [PubMed] [Google Scholar]
- Stewart A. D., Anand R. R., Laird J. S., Verrall M., Ryan C. G., de Jonge M. D., Paterson D. and Howard D. L. (2011). Distribution of metals in the termite Tumulitermes tumuli (Froggatt): two types of Malpighian tubule concretion host Zn and Ca mutually exclusively. PLoS ONE 6, e27578 10.1371/journal.pone.0027578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. T. and Sullivan M. C. (1975). Transport defects as the physiological basis for eye color mutants of Drosophila melanogaster. Biochem. Genet. 13, 603-613. 10.1007/BF00484918 [DOI] [PubMed] [Google Scholar]
- Sullivan D. T., Bell L. A., Paton D. R. and Sullivan M. C. (1979). Purine transport by Malpighian tubules of pteridine-deficient eye color mutants of Drosophila melanogaster. Biochem. Genet. 17, 565-573. 10.1007/BF00498891 [DOI] [PubMed] [Google Scholar]
- Sunuwar L., Asraf H., Donowitz M., Sekler I. and Hershfinkel M. (2017a). The Zn2+-sensing receptor, ZnR/GPR39, upregulates colonocytic Cl− absorption, via basolateral KCC1, and reduces fluid loss. Biochim. Biophys. Acta 1863, 947-960. 10.1016/j.bbadis.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunuwar L., Gilad D. and Hershfinkel M. (2017b). The zinc sensing receptor, ZnR/GPR39, in health and disease. Front. Biosci. 22, 1469-1492. 10.2741/4554 [DOI] [PubMed] [Google Scholar]
- Sunuwar L., Medini M., Cohen L., Sekler I. and Hershfinkel M. (2016). The zinc sensing receptor, ZnR/GPR39, triggers metabotropic calcium signalling in colonocytes and regulates occludin recovery in experimental colitis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrzycka M., McEachern L. A., Kinneard J., Prabhu K., Fitzpatrick K., Schulze S., Rawls J. M., Lloyd V. K., Sinclair D. A. R. and Honda B. M. (2007). The pink gene encodes the Drosophila orthologue of the human Hermansky-Pudlak syndrome 5 (HPS5) gene. Genome 50, 548-556. 10.1139/G07-032 [DOI] [PubMed] [Google Scholar]
- Tang X. and Zhou B. (2013). Iron homeostasis in insects: insights from Drosophila studies. IUBMB Life 65, 863-872. 10.1002/iub.1211 [DOI] [PubMed] [Google Scholar]
- Taylor N. M. I., Manolaridis I., Jackson S. M., Kowal J., Stahlberg H. and Locher K. P. (2017). Structure of the human multidrug transporter ABCG2. Nature 546, 504-509. 10.1038/nature22345 [DOI] [PubMed] [Google Scholar]
- Thibault S. T., Singer M. A., Miyazaki W. Y., Milash B., Dompe N. A., Singh C. M., Buchholz R., Demsky M., Fawcett R., Francis-Lang H. L. et al. (2004). A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat. Genet. 36, 283-287. 10.1038/ng1314 [DOI] [PubMed] [Google Scholar]
- Tiklová K., Senti K.-A., Wang S., Gräslund A. and Samakovlis C. (2010). Epithelial septate junction assembly relies on melanotransferrin iron binding and endocytosis in Drosophila. Nat. Cell Biol. 12, 1071-1077. 10.1038/ncb2111 [DOI] [PubMed] [Google Scholar]
- Timm F. and Neth R. (1958). [Histochemistry of islets of Langerhans]. Z. Naturforsch. B. 13B, 538-542. [PubMed] [Google Scholar]
- Tognon E., Kobia F., Busi I., Fumagalli A., De Masi F. and Vaccari T. (2016). Control of lysosomal biogenesis and Notch-dependent tissue patterning by components of the TFEB-V-ATPase axis in Drosophila melanogaster. Autophagy 12, 499-514. 10.1080/15548627.2015.1134080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breugel F. M. A. (1987). Differential riboflavin deposition in white and variegated white mutants of Drosophila hydei. Dev. Genet. 8, 45-58. 10.1002/dvg.1020080107 [DOI] [PubMed] [Google Scholar]
- van Herwaarden A. E., Wagenaar E., Merino G., Jonker J. W., Rosing H., Beijnen J. H. and Schinkel A. H. (2007). Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol. Cell. Biol. 27, 1247-1253. 10.1128/MCB.01621-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villee C. A. (1948). Studies in biochemical genetics in Drosophila. J. Gen. Physiol. 31, 337-345. 10.1085/jgp.31.4.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wu Y. and Zhou B. (2009). Dietary zinc absorption is mediated by ZnT1 in Drosophila melanogaster. FASEB J. 23, 2650-2661. 10.1096/fj.08-126649 [DOI] [PubMed] [Google Scholar]
- Warner C. K. and Finnerty V. (1981). Molybdenum hydroxylases in Drosophila. II. Molybdenum cofactor in xanthine dehydrogenase, aldehyde oxidase and pyridoxal oxidase. Molecular & General Genetics 184, 92-96. [DOI] [PubMed] [Google Scholar]
- Warner T. S., Sinclair D. A. R., Fitzpatrick K. A., Singh M., Devlin R. H. and Honda B. M. (1998). The light gene of Drosophila melanogaster encodes a homologue of VPS41, a yeast gene involved in cellular-protein trafficking. Genome 41, 236-243. 10.1139/g98-017 [DOI] [PubMed] [Google Scholar]
- Warnhoff K., Roh H. C., Kocsisova Z., Tan C.-H., Morrison A., Croswell D., Schneider D. L. and Kornfeld K. (2017). The nuclear receptor HIZR-1 uses zinc as a ligand to mediate homeostasis in response to high zinc. PLoS Biol. 15, e2000094 10.1371/journal.pbio.2000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S. M. (2011). The microanalysis toolkit: X-ray fluorescence image processing software. 10th International Conference on X-Ray Microscopy 1365, 196-199. [Google Scholar]
- Wei A.-H., He X. and Li W. (2013). Hypopigmentation in Hermansky-Pudlak syndrome. J. Dermatol. 40, 325-329. 10.1111/1346-8138.12025 [DOI] [PubMed] [Google Scholar]
- Wessing A., Zierold K. and Bertram G. (1997). Carbonic anhydrase supports electrolyte transport in Drosophila Malpighian tubules. Evidence by X-ray microanalysis of cryosections. J. Insect Physiol. 43, 17-28. [DOI] [PubMed] [Google Scholar]
- Wiley K. and Forrest H. S. (1981). Terminal synthesis of xanthommatin in Drosophila melanogaster. IV. Enzymatic and non-enzymatic catalysis. Biochem. Genet. 19, 1211-1221. [DOI] [PubMed] [Google Scholar]
- Xiao G., Fan Q., Wang X. and Zhou B. (2013). Huntington disease arises from a combinatory toxicity of polyglutamine and copper binding. Proc. Natl. Acad. Sci. USA 110, 14995-15000. 10.1073/pnas.1308535110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C. and Robertson R. M. (2016). Timing of locomotor recovery from anoxia modulated by the white gene in Drosophila. Genetics 203, 787-797. 10.1534/genetics.115.185066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G. R., Wan Z. H., Fan Q. W., Tang X. N. and Zhou B. (2014). The metal transporter ZIP13 supplies iron into the secretory pathway in Drosophila melanogaster. Elife 3, e03191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G. and Zhou B. (2016). What can flies tell us about zinc homeostasis? Arch. Biochem. Biophys. 611, 134-141. 10.1016/j.abb.2016.04.016 [DOI] [PubMed] [Google Scholar]
- Yagi S. and Ogawa H. (1996). Effect of tryptophan metabolites on fluorescent granules in the Malpighian tubules of eye color mutants of Drosophila melanogaster. Zoolog. Sci. 13, 97-104. 10.2108/zsj.13.97 [DOI] [PubMed] [Google Scholar]
- Yepiskoposyan H., Egli D., Fergestad T., Selvaraj A., Treiber C., Multhaup G., Georgiev O. and Schaffner W. (2006). Transcriptome response to heavy metal stress in Drosophila reveals a new zinc transporter that confers resistance to zinc. Nucleic Acids Res. 34, 4866-4877. 10.1093/nar/gkl606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S., Qin Q. and Zhou B. (2017). Functional studies of Drosophila zinc transporters reveal the mechanism for zinc excretion in Malpighian tubules. BMC Biol. 15, 12 10.1186/s12915-017-0355-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. L., Ghosh M. C. and Rouault T. A. (2014). The physiological functions of iron regulatory proteins in iron homeostasis – an update. Front. Pharmacol. 5, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Kiuchi T., Hirayama C., Katsuma S. and Shimada T. (2018). Bombyx ortholog of the Drosophila eye color gene brown controls riboflavin transport in Malpighian tubules. Insect Biochem. Mol. Biol. 92, 65-72. 10.1016/j.ibmb.2017.11.012 [DOI] [PubMed] [Google Scholar]
- Zhu Z. J., Wu K. C., Qian Z. M., Yung W. H. and Ke Y. (2014). Drosophila models for studying iron-related neurodegenerative diseases. Sheng Li Xue Bao 66, 47-54. [PubMed] [Google Scholar]
- Zierold K. and Wessing A. (1990). Mass dense vacuoles in Drosophila Malpighian tubules contain zinc, not sodium. A reinvestigation by X-ray microanalysis of cryosections. Eur. J. Cell Biol. 53, 222-226. [PubMed] [Google Scholar]