ABSTRACT
Elevated oxidized stress contributes to lens cataracts, and gap junctions play important roles in maintaining lens transparency. As well as forming gap junctions, connexin (Cx) proteins also form hemichannels. Here, we report a new mechanism whereby hemichannels mediate transport of reductant glutathione into lens fiber cells and protect cells against oxidative stress. We found that Cx50 (also known as GJA8) hemichannels opened in response to H2O2 in lens fiber cells but that transport through the channels was inhibited by two dominant-negative mutants in Cx50, Cx50P88S, which inhibits transport through both gap junctions and hemichannels, and Cx50H156N, which only inhibits transport through hemichannels and not gap junctions. Treatment with H2O2 increased the number of fiber cells undergoing apoptosis, and this increase was augmented with dominant-negative mutants that disrupted both hemichannels formed from Cx46 (also known as GJA3) and Cx50, while Cx50E48K, which only impairs gap junctions, did not have such an effect. Moreover, hemichannels mediate uptake of glutathione, and this uptake protected lens fiber cells against oxidative stress, while hemichannels with impaired transport had less protective benefit from glutathione. Taken together, these results show that oxidative stress activates connexin hemichannels in the lens fiber cells and that hemichannels likely protect lens cell against oxidative damage through transporting extracellular reductants.
KEY WORDS: Connexin, Hemichannel, Lens fiber cell, Oxidative stress, Glutathione
Summary: Connexin hemichannels activated by oxidative stress in lens fiber cells mediate a cell protective mechanism through the uptake of reductants, maintaining lens transparency and homeostasis.
INTRODUCTION
Gap junctions, which are formed by connexin (Cx) molecules, are transmembrane channels that allow the exchange of small molecules (molecular mass≤1 kDa), such as metabolites, ions and second messengers, between contacting cells. This type of cell–cell communication is critical in maintaining normal cell and tissue functions (Goodenough et al., 1996). There are 20 different members of the Cx family in humans (Willecke et al., 2002). In addition to forming gap junctions, connexins form hemichannels, unopposed halves of gap junction channels, which mediate the passage of biological molecules. Connexin hemichannels are permissive to the passage of small molecules, such as ATP, NAD+ and prostaglandin E2 (PGE2), and play roles that are different from those of gap junctions in the cell (Cherian et al., 2005; Fruscione et al., 2011; Lohman and Isakson, 2014).
Three connexins have been identified in the mammalian lens, Cx43, Cx46 and Cx50 (also known as GJA1, GJA3 and GJA8, respectively), of which Cx46 and Cx50 are highly enriched in lens fibers. Previous studies have shown that mice lacking genes encoding either Cx46 or Cx50 develop lens cataracts (Gong et al., 1997; Rong et al., 2002; White et al., 1998). Moreover, mutations of Cx46 and Cx50 genes are major causes of congenital cataracts (Pichi et al., 2016; White and Paul, 1999). Among connexin mutations, we primarily focused on three Cx50 mutations: E48K, P88S and H156N. Cx50E48K in the first extracellular loop was first mapped from linkage analysis in a family of Pakistani origin with three generations of autosomal dominant cataracts mutation (Berry et al., 1999). Hemichannels formed from Cx50E48K have normal activity but the mutation acts as a dominant negative for transport through gap junctions (Banks et al., 2009; Berry et al., 1999). Cx50P88S, in the second transmembrane domain, has a dominant-negative function on both transport through gap junctions and hemichannels (Banks et al., 2007; Pal et al., 1999; Shiels et al., 1998). Cx50H156N located in third transmembrane domain only inhibits transport through hemichannels, and not gap junctions, as determined in a Xenopus oocyte expression system (Beahm and Hall, 2002). Cx50E48K and Cx50P88S mutations are associated with human autosomal dominant-negative cataracts (Berry et al., 1999; Shiels et al., 1998; Pal et al., 1999). These dominant-negative mutants provide means to selectively distinguish the functions of gap junctions and hemichannels, which are both formed by connexins.
The eye lenses are constantly subject to oxidative stress from UV, radiation and other sources. The generation of reactive oxygen species (ROS), such as superoxide and H2O2 can cause DNA damage, protein modification, denaturation and aggregation (Nagaraj et al., 2012). Clinical and morphological features of cataractogenesis in the OXYS strain of rats, which generate excess ROS, have been described (Marsili et al., 2004). Substantial evidence has accumulated to support the conclusion that ROS and resulting oxidative damage are the major factors contributing to the development of various types of cataracts (Berthoud and Beyer, 2009; Thiagarajan and Manikandan, 2013).
There are extensive prior studies regarding the roles of gap junction channels in the lens; however, the physiological importance of connexin hemichannels remains largely unknown. In this study, by using various mutants in Cx50 that impair transport through the gap junction or hemichannels, we discovered that connexin hemichannels mediate a new cell protective mechanism against oxidative insults in lens fiber cells.
RESULTS
Connexin hemichannels open upon H2O2 treatment but this is inhibited in channels composed of dominant-negative Cx50 mutants
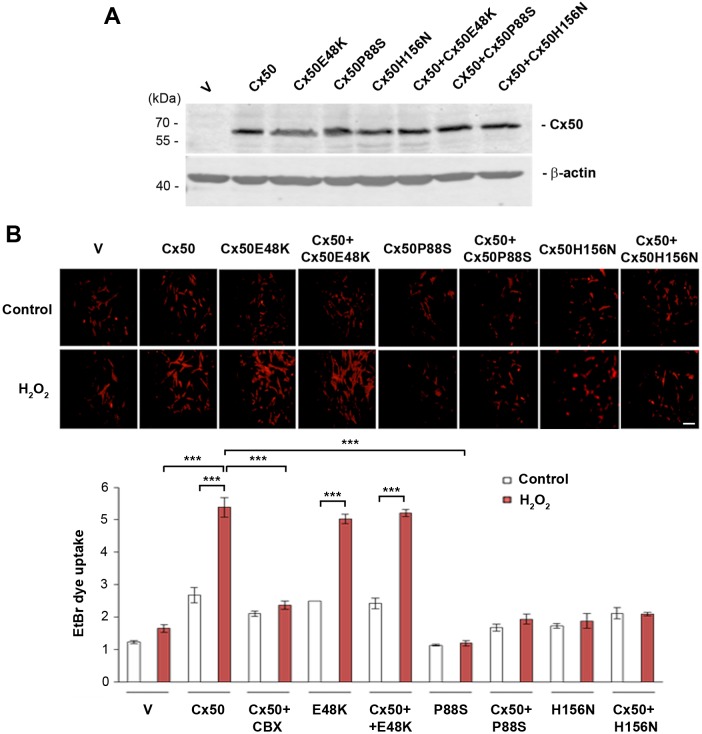
Connexin hemichannels are inactive under normal physiological conditions, and are activated in response to certain stimuli and cell stress (Kar et al., 2013; Schulz et al., 2015). To elucidate the effect of oxidative stress on lens connexin hemichannel activity, we infected chick embryo fibroblast (CEF) cells with recombinant RCAS(A) retrovirus containing FLAG-tagged wild-type Cx50 and/or Cx50 mutants (E48K, P88S and H156N), and treated the cells with H2O2. As we reported previously, we have not detected expression of other possible connexin subtypes or the activities of connexin channels in these cells (Banks et al., 2007; Hu et al., 2017). With retroviral infection, almost all CEF cells express exogenous connexins (Gu et al., 2003; Jiang, 2001). We have shown in our previous studies that Cx50 and mutants are expressed at a similar level on the cell surface (Banks et al., 2007). Here, comparable levels of wild-type, mutant or combinations of Cx50 proteins were detected by western blotting (Fig. 1A). To determine hemichannel activity, a cellular dye uptake assay with ethidium bromide (EtBr) was performed with or without H2O2 treatment. We detected the uptake of EtBr in cells expressing Cx50, Cx50E48K mutant and both Cx50 and Cx50E48K (Fig. 1B). Interestingly, the treatment of H2O2 significantly increased EtBr uptake in Cx50-expressing cells compared to what was seen in cells treated with vehicle (V) control, and this increase was completely inhibited by a potent chemical blocker carbenoxolone (CBX). The cells expressing the Cx50E48K mutant showed increased dye uptake, at a similar level to cells expressing Cx50, while cells expressing Cx50P88S and Cx50H156N both had a low uptake, suggesting these two mutants formed hemichannels that only allowed impaired transport. Moreover, expression of either Cx50P88S or Cx50H156N suppressed the ability of wild-type Cx50 to form functional hemichannels, confirming these two mutants inhibit Cx50 hemichannels in a dominant-negative manner.
Fig. 1.
Cx50 hemichannels are opened by H2O2 and inhibited by Cx50 mutants in a dominant-negative manner. CEF cells were infected with high-titer RCAS(A) retroviral vehicle (V) or recombinant RCAS(A) retroviruses containing WT Cx50, Cx50 mutants, E48K, P88S or H156N, or Cx50 WT and mutants in combination. (A) Whole-cell lysate extracts were prepared and then immunoblotted with anti-FLAG or β-actin antibody. (B) Cells were cultured at low density then treated with 0.3 mM H2O2 for 2 h, and 0.05 mM cabenoxolone for 20 min, followed by incubating with 0.1 mM EtBr for 5 min. At least four microphotographs of fluorescence fields were taken under a 20× microscope (Olympus) with a Rhodamine filter. The average pixel density of 30 random cells was measured by using ImageJ software. The data are presented as the mean±s.e.m. (n=3). ***P<0.001. Scale bar: 100 μm.
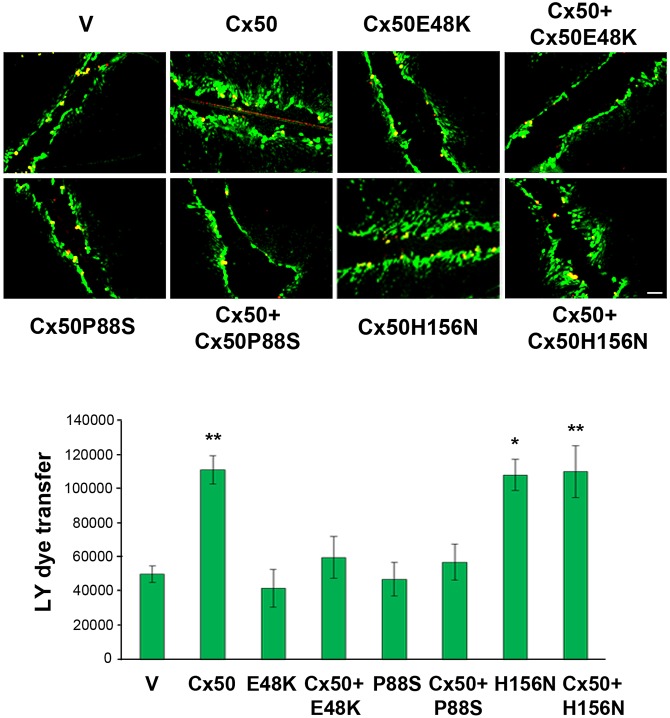
Both Cx50E48K and Cx50P88S mutations inhibit gap junction coupling and function towards wild-type Cx50 in a dominant-negative manner (Banks et al., 2009, 2007; Xu and Ebihara, 1999). The human Cx50 H161N mutant (chick Cx50H156N) does not form detectable hemichannels, but forms gap junctions that are indistinguishable from wild-type Cx50 channels in Xenopus oocytes expressing exogenous Cx50 (Beahm and Hall, 2002). To determine whether Cx50H156N forms functional gap junction channels in cells, wild-type and mutant Cx50 were expressed in CEF cells through retroviral expression. We used a scrape loading dye transfer assay to assess the intercellular coupling; here, Lucifer Yellow (LY) signals from the scrape line to the cell front where dye transfer stops were measured, with Rhodamine-conjugated dextran (RD) serving as a non-transferring control, as we have previously reported (Banks et al., 2007). LY transfer between Cx50-expressing CEF cells was around twofold greater than between cells treated with vehicle (V) control, or between cells expressing Cx50E48K, Cx50P88S or either of the mutants in combination with Cx50 (Fig. 2). Moreover, there was no significant difference between cells expressing Cx50 and Cx50H156N, suggesting that the Cx50H156N mutant has normal gap junctional function. Thus, we took an advantage of these three Cx50 mutants to dissect the specific function of hemichannels and gap junction channels in lens cells.
Fig. 2.
Cx50 mutants E48K and P88S inhibit Cx50 gap junction channels in a dominant-negative manner. CEF cells were infected with high-titer RCAS(A) retroviral vehicle (V) or recombinant RCAS(A) retroviruses containing Cx50, Cx50 mutants E48K, P88S or H156N, or Cx50 WT and mutants in combination. Cells were grown to confluence to maximize cell–cell contact. The scrape-loading dye transfer assay was performed with RD as a tracer dye and LY as a transferring dye. The extent of dye transfer was measured as the area of LY-stained cells minus that of RD-labeled cells. The data are presented as the mean±s.e.m. (n=3). *P<0.05; **P<0.01 [compared to vehicle (V) control]. Scale bar: 100 μm.
The Cx50 mutants P88S and H156N not only function as dominant negatives for Cx50, but also for Cx46
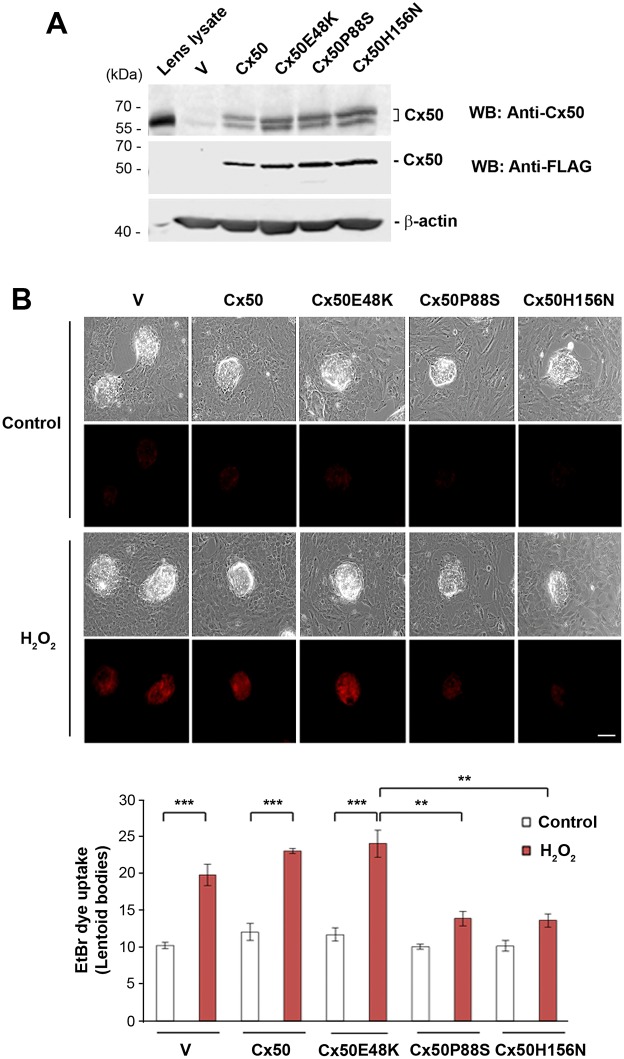
We examined the responses of hemichannels to oxidative stress in primary lens cell culture. A high efficiency of exogenous expression of FLAG-tagged Cx50 and mutants was achieved by recombinant retroviral infection. Comparable levels of exogenous and total levels of wild-type and mutant proteins were detected with anti-FLAG tag antibody and anti-Cx50 antibody, respectively (Fig. 3A). Endogenously expressed Cx50 protein was also detected in vehicle (V) control (Fig. 3A, lower band in upper panel). After 10–11 days of culturing, when primary lens cultures were fully differentiated and show the formation of lentoid structures with characteristics of lens fiber cells, an EtBr dye uptake assay was performed to determine hemichannel activity in response to H2O2. The treatment with H2O2 increased dye uptake in lentoid bodies, suggesting an increase in hemichannel activity. Expression of the dominant-negative Cx50 mutants P88S and H156N, but not E48K, reduced the level of dye uptake in response to H2O2 in lentoid bodies (Fig. 3B), demonstrating that these two mutants functioned in a dominant-negative manner in lens fiber cells, in accordance with what we observed in CEF cells.
Fig. 3.
Hemichannels in lens fiber cells are opened by H2O2 and this opening is inhibited by dominant-negative Cx50 mutants. Primary chick lens cell cultures were infected with high-titer RCAS(A) retroviral vehicle (V) or recombinant RCAS(A) retroviruses containing Cx50, or Cx50 mutants E48K, P88S or H156N. (A) Whole-cell lysate extracts were prepared and then immunoblotted with anti-Cx50, anti-FLAG-tag or β-actin antibody. (B) After primary cultures reached maturation at ∼10–11 days of cell culturing, they were treated with or without 0.3 mM H2O2 for 20 min, and then an EtBr dye uptake assay was performed. The EtBr uptake and corresponding phase-contrast images of lens fiber cells (lentoids bodies) were taken at the same magnification by fluorescence microscopy. The average pixel density of 30 lentoid bodies was measured using ImageJ software. The data are presented as the mean±s.e.m. (n=3). **P<0.01; ***P<0.001. Scale bar: 100 μm.
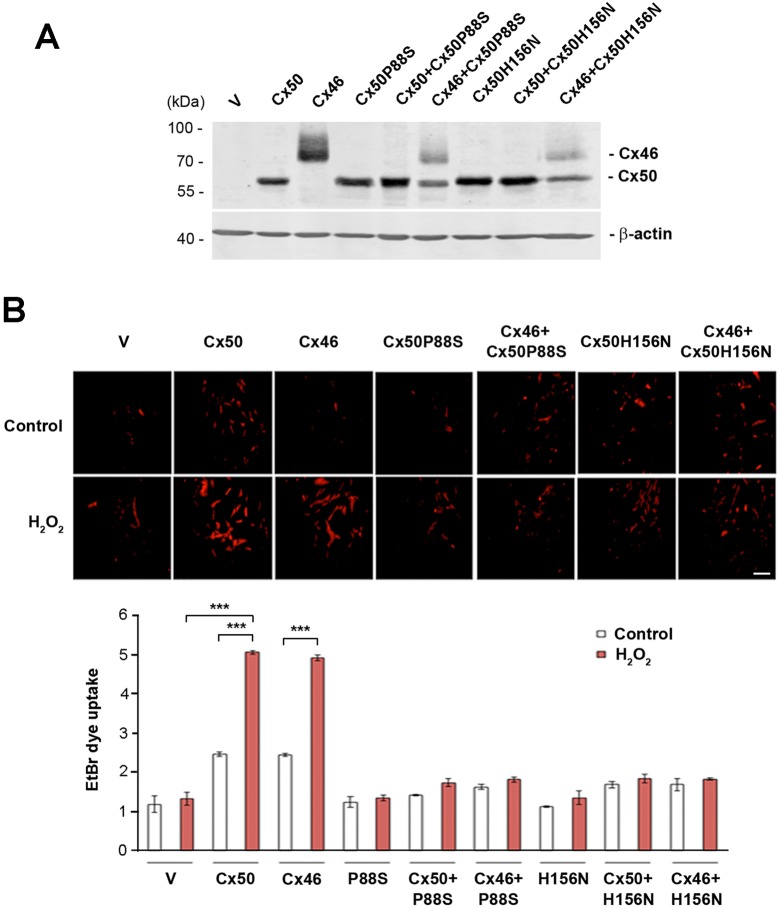
Cx46 primarily expressed in lens fiber cells has been previously reported to form hemichannels in isolated lens fibers (Ebihara et al., 2011). We showed above that Cx50 P88S and H156N are dominant-negative mutants for Cx50 hemichannels. In lens fiber cells, there are two subtypes of connexins, Cx46 and Cx50 and these two connexins are known to form heteromeric connexons (hemichannels) (Jiang and Goodenough, 1996). It is therefore possible that the expression of the dominant-negative mutants of Cx50 in lens fiber cells will also have inhibitory effects on endogenous Cx46 hemichannels. To test this possibility, we conducted dye uptake assay in CEF cells co-expressing either FLAG-tagged Cx50 P88S or H156N, and Cx46. The expression of Cx50, Cx46 and the Cx50 mutants (P88S or H156N) in combination or with vehicle control was detected by using an anti-FLAG tag antibody (Fig. 4A). The dye uptake in cells expressing Cx46 induced by H2O2 was attenuated in cells co-expressing Cx50P88S or Cx50H156N to a comparable level to that in cells co-expressing Cx50 (Fig. 4B). These data suggest that these two Cx50 mutants also have a dominant-negative action on Cx46 hemichannels, supporting the observation that P88S and H156N completely inhibit the activity of hemichannels formed by Cx50 and Cx46 in lens fiber cells.
Fig. 4.
Dominant-negative mutants of Cx50 also inhibit Cx46 hemichannel opening induced by H2O2 in a dominant-negative manner. CEF cells were infected with high-titer RCAS(A) retroviral vehicle (V) or recombinant RCAS(A) retroviruses containing Cx50, Cx46 and Cx50 mutants, P88S or H156N, or Cx46 WT and mutants in combination. (A) Whole-cell lysate extracts were prepared and then immunoblotted with anti-FLAG or β-actin antibody. (B) Cells cultured at low density were treated with H2O2 and EtBr dye uptake assays were performed as described above. At least four microphotographs of fluorescence fields were taken under a 20× microscope (Olympus) with a Rhodamine filter. The average pixel density of 30 random cells was measured using ImageJ software. The data are presented as the mean±s.e.m. (n=3). ***P<0.001. Scale bar: 100 μm.
Activation of hemichannels protects lens fiber cells against oxidative stress
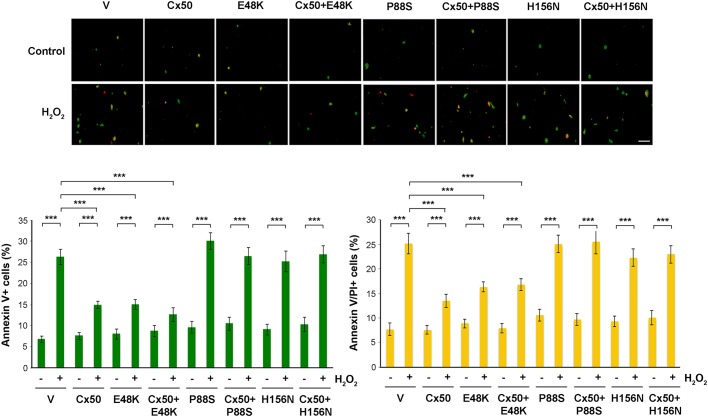
Oxidative damage results in lens cells apoptosis and cell death in the ocular lens (Berthoud and Beyer, 2009; Brennan et al., 2012). Here, we determined the activation of connexin hemichannels in response to oxidative stress. An Annexin V (green) and propidium iodide (PI; red) staining assay was used to measure the extent of cells undergoing apoptosis and necrosis. In overlay images, the green cells labeled with Annexin V and yellow cells labeled with both Annexin V and PI represent cells in the early and late stages of apoptosis, respectively. Treatment with H2O2 increased the level of apoptosis in CEF cells while expression of Cx50 and its E48K mutant significantly reduced it (Fig. 5). Cx50 P88S and H156N, which inhibit hemichannels, not only had minimal effect on apoptosis as in the control group, but also attenuated the inhibitory effect on apoptosis mediated by Cx50 in co-expressing cells. This result suggests that functional Cx50 hemichannels protect cells from oxidative stress. Given that both mutants have impaired hemichannels, H156N forms functional gap junctions, and P88S has impaired gap junction permeability, this result suggests that hemichannels, not gap junctions are responsible for protecting cells against oxidative stress.
Fig. 5.
Treatment with H2O2 increased the numbers of apoptotic cells and this increase was augmented in cells expressing dominant-negative mutants that impair hemichannels. CEF cells were infected with high-titer RCAS(A) retroviral vehicle (V) or recombinant RCAS(A) retroviruses containing Cx50, and Cx50 mutants E48K, P88S or H156N, or Cx50 WT and mutants in in combination. Cells were treated with 1 mM H2O2 for 10 h, and analyzed by using the Dead Cell Apoptosis Kit with FITC–Annexin V and PI (Molecular Probes). At least five microphotographs of fluorescence fields were taken under a 10× microscope (Olympus) under phase-contrast or with FITC or Rhodamine filters. Positive cells were quantified by counting and are shown as a percentage. The data are presented as the mean±s.e.m. (n=3). ***P<0.001. Scale bar: 50 μm.
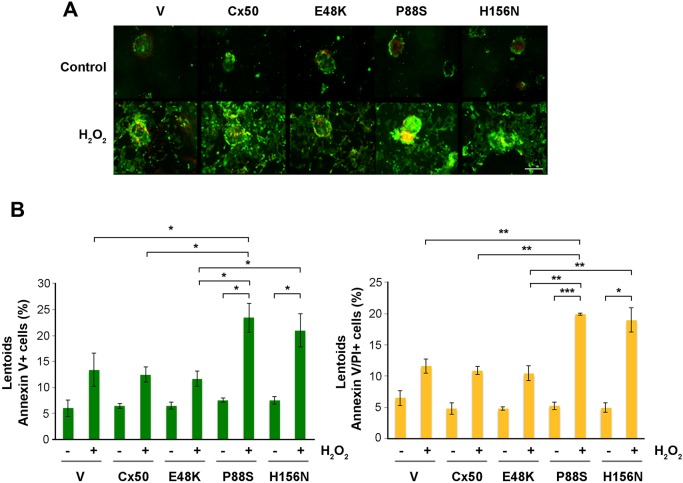
We similarly treated lens primary culture after 10–11 days of culturing with H2O2 and determined the proportion of lens fiber cells undergoing apoptosis by performing the Annexin V and PI assay (Fig. 6). Expression of Cx50 and its E48K mutant reduced the level of apoptosis in lentoid bodies, but P88S and H156N had no such effect (Fig. 6A,B). Interestingly, there ∼5% of cells showed Annexin-V-positive signals in untreated controls. It is possible that lentoid-like cortical fiber cells may slightly activate an apoptosis-like mechanism. The increased Annexin V signals in lentoids was also observed with functional hemichannels when exposed to H2O2, although the increase was not significant, which suggests a minor activation of a hemichannel-independent pathway in response to H2O2. Taken together, this data suggest that hemichannels formed by Cx50 and Cx46 in lens fibers protect lens fiber cells against oxidative damage.
Fig. 6.
Treatment with H2O2 increased apoptosis and cell death of lens fiber cells, and this increase was augmented in lens fiber cells with impairment of Cx50 and Cx46 hemichannels. (A) Primary chick lens cell cultures were infected with high-titer RCAS(A) retroviral vehicle (V) or recombinant RCAS(A) retroviruses containing Cx50, and Cx50 mutants E48K, P88S or H156N. After 10–11 days of cell culturing, cells were treated with 0.2 mM H2O2 for 4 h and analyzed by using the FITC–Annexin V Apoptosis Detection Kit with PI (BioLegend). At least five microphotographs of fluorescence fields were taken under a 20× microscope (Olympus) under phase-contrast or with FITC or Rhodamine filters. Scale bar: 100 μm. (B) Positive cell areas were quantified by means of ImageJ software and are shown as a percentage. The data are presented as the mean±s.e.m. (n=3). *P<0.05; **P<0.01; ***P<0.001.
Hemichannels mediate cellular uptake of glutathione and protect cells against oxidative stress
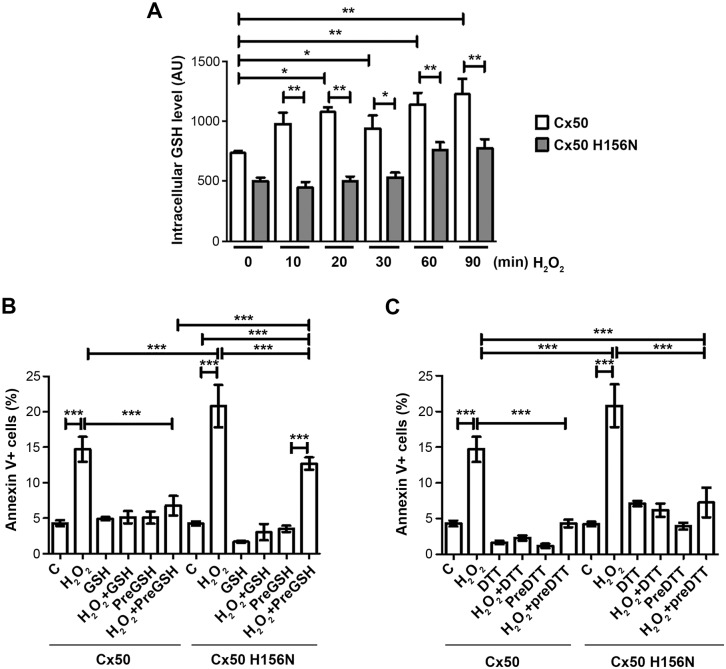
Previous studies have shown that connexin hemichannels are permeable to glutathione (GSH) (Slavi et al., 2014; Stridh et al., 2008; Tong et al., 2015). Given that the GSH concentration in vitreous humor is unusually high and hemichannels transport molecules with sizes no more than 1 kDa, we tested the hypothesis that opening of hemichannels in response to oxidative stress facilitates entry of GSH into the cell and protects cells against oxidative damage. We first determined GSH uptake in CEF cells expressing WT Cx50 and its H156N mutant by performing a ThiolTracker assay. In the presence of extracellular GSH, the intracellular GSH level was significantly increased at only 10 min after H2O2 treatment in cells expressing WT Cx50, while this increase was significantly diminished in H156N-expressing cells (Fig. 7A). The increase in apoptotic cells by H2O2 was reduced by GSH pretreatment in cells expressing WT Cx50, but not the H156N mutant (Fig. 7B). In WT Cx50-expressing cells, a protective effect was shown even with the removal of pretreated GSH (preGSH) before application of H2O2; however, this effect was not observed in H156N mutant-expressing cells. Additionally, we added both H2O2 and GST reagents at the same time (H2O2+GSH) to the cell. GSH, a membrane-impermeable reagent reduces H2O2 extracellularly before H2O2 has a chance to exert its oxidizing action on cells. In this case, cells were protected by extracellular GSH regardless of the presence of functional hemichannels with low levels of Annexin V-positive signals in both WT- and mutant Cx50-expressing cells. This result suggests the protection against H2O2-induced apoptosis is a result of the entry of extracellular GSH mediated by hemichannels. To further substantiate this result, we treated cells with the membrane permeable reductant dithiothreitol (DTT). Like GSH, DTT treatment protected cells, as shown by a reduction in apoptosis in response to H2O2, and protection was observed in both WT Cx50 and H156N-expressing cells (Fig. 7C), suggesting that passage of membrane permeable DTT, unlike membrane impermeable GSH, does not require hemichannels. Taken together, this data suggests that transport of extracellular GSH by hemichannels offers a cell protective mechanism against apoptosis resulting from oxidative stress.
Fig. 7.
Cx50 hemichannels mediate uptake of GSH and protect cells against oxidative stress induced by H2O2. CEF cells were infected with high-titer recombinant RCAS(A) retroviruses containing Cx50 or the Cx50 H156N mutant. (A) Cells were treated with 1 mM GSH for various periods of time then rinsed and labeled with fluorescent Thioltracker™. The intracellular GSH level was quantified by determining the fluorescence intensity. (B,C) Cells were pretreated with 1 mM GSH (B) or 1 mM DTT (C) for 20 min, and in some groups GSH or DTT was removed (preGSH or preDTT) before the treatment with 1 mM H2O2. Cells under apoptosis were analyzed by using the Dead Cell Apoptosis Kit with FITC–Annexin V and PI (BioLegend, San Diego, CA). At least five microphotographs of fluorescence fields were taken under a 10× microscope (Olympus) with under phase-contrast or with FITC or Rhodamine filters. Positive cells were quantified by counting and are shown as a percentage. The data are presented as the mean±s.e.m. (n=3). *P<0.05; **P<0.01; ***P<0.001.
DISCUSSION
During aging, accumulation of oxidized components and decreased efficiency of repair mechanisms are involved in the development of lens opacities or cataracts (Berthoud and Beyer, 2009; Brennan et al., 2012). Gap junction channels that communicate between adjacent cells contribute to lens homeostasis and the maintenance of transparency. In this study, we demonstrated a new mechanism by which connexin hemichannels, unopposed halves of gap junctions, activated by oxidative stress play a critical role in protecting lens fiber cells against oxidative stress. This protective mechanism is likely to be mediated by intracellular delivery of extracellular reductants through connexin hemichannels.
Previous studies have shown that cigarette smoke extract and H2O2 induce connexin hemichannel opening (Ramachandran et al., 2007). Additionally, we have previously shown that H2O2 can open Cx43 hemichannels in bone cells and protect osteocytes from cell death (Kar et al., 2013). Until recently, there were few studies revealing the role of lens connexin hemichannels under oxidative stress in the lens fiber cells, which are highly vulnerable to oxidative insults and the only cell type in the entire body that last for the entire lifespan. We carried out this study in order to explore how lens connexin channels respond to oxidative stress and if active hemichannels have any impact on cells survival. We used H2O2, a membrane-permeable and fast-acting oxidizing reagent. Here, we showed that H2O2 induced hemichannel opening in lens fiber cells. Our earlier study shows that Cx46 and Cx50 form hexameric hemichannels (connexins) in lens fiber cells (Jiang and Goodenough, 1996). The evidence of the existence of functional heteromeric hemichannels is supported by our data here showing that dominant-negative Cx50 mutants prevent hemichannel function in lens fiber cells and also prevent transport through hemichannels formed by Cx46.
In this study, we took advantage of three dominant-negative Cx50 mutants, Cx50E48K, Cx50P88S and Cx50H156N to dissect the specific function of hemichannels and gap junctions in the lens. Two of the mutants, E48K and P88S, are reported to cause congenital cataracts in humans (Berry et al., 1999; Shiels et al., 1998). These two sites are highly conserved across animal species. We have confirmed dominant-negative effects of these two mutants in transfected chick cells and chick lens primary cultures (Banks et al., 2009). Cx50H156N is a unique mutation at a conserved residue that only has a dominant-negative effect on hemichannels, but not gap junctions, when tested in Xenopus oocytes (Beahm and Hall, 2002). In this study, we verified the dominant-negative function of this mutant in chick cells and primary chick lens culture. We used retroviral expression systems developed in our laboratory (Jiang and Goodenough, 1998) and, with this approach, we are able to exogenously express these proteins in literally all cells in lens primary cultures. Our data show that these three mutants are effective tools to dissect specific function of connexin channels, that is, gap junctions versus hemichannels. We showed that functional hemichannel activity protected cells against apoptosis, as seen with WT and dominant-negative mutants that only effect gap junctions. In contrast, mutants that impede hemichannels but can form functional gap junctions, lead to cell death. These experimental data reveal that active hemichannels play an important role in the protection against cell apoptosis in both CEF cells and lens primary fiber cells.
Mature fiber cells in the core region of lens never turn over throughout the entire animal lifespan; therefore, they possess unique cell protective mechanisms against accumulated oxidative and other insults. In the lens, a unique microcirculation system delivers antioxidants (e.g. GSH) and nutrients (e.g. glucose) to lens fibers (Donaldson et al., 2001). Reduced GSH at an unusually high concentration is the most important antioxidant molecule of the lens (Giblin, 2000). However, how these molecules are delivered into fiber cells remains obscure. The existence and function of GSH transporters are still controversial (Bachhawat et al., 2013). A GSH transporter was reported in lens epithelial cells (Kannan et al., 1996); however, no GSH transporter has been identified in lens fibers. A study by Slavi et al. showed that gap junction channels formed by Cx46 are involved in the delivery of GSH to the lens nucleus (Slavi et al., 2014). However, how extracellular GSH is transported into lens fiber cells remains largely unknown. We showed that GSH uptake and the cell protective function of GSH only occurred in cells expressing functional Cx50 hemichannels, not in cells with mutants that exert a dominant-negative function on hemichannels. These data support hemichannels as a likely portal for the entry of GSH into lens fiber cells, protecting cells against oxidative stress-induced cell death. Taken together, the results of this study suggest that connexin hemichannels protect lens fiber cells against oxidative damage and that this action is likely to be fulfilled through the uptake of reductants, such as GSH, that are enriched in vitreous humor.
MATERIALS AND METHODS
Materials
Fertilized unincubated white leghorn chicken eggs were obtained from Texas A&M University Agriculture & Poultry Science (College Station, TX) and incubated for 11 days in a humidified 37°C incubator. Anti-FLAG monoclonal antibody was from Sigma (F1804, St Louis, MO), anti-β-actin was from Sigma (A-5441) and anti-Cx50 (chicken) was generated and affinity-purified as previously reported (Jiang et al., 1994). FITC–dextran was from Sigma; Lucifer yellow (LY) and Rhodamine–dextran were from ThermoFisher (Waltham, MA). The Dead Cell Apoptosis Kit with FITC–Annexin V and PI was from BioLegend (San Diego, CA). Paraformaldehyde (PFA, 16% stock solution) was from Electron Microscope Sciences (Fort Washington, PA). Dulbecco's modified Eagle's medium (DMEM), 0.25% Trypsin-EDTA solution and penicillin-streptomycin were purchased from ThermoFisher (Waltham, MA). Fetal bovine serum (FBS) was from Hyclone Laboratories (Logan, UT). All other chemicals were obtained from either Sigma or Fisher Scientific (Pittsburgh, PA).
Site-directed mutagenesis, and preparation of recombinant retrovirus containing Cx50, Cx50P88S, Cx50E48K or Cx50H156N
Retroviral constructs and high-titer retroviruses were prepared based on our previously described protocol (Jiang, 2001; Jiang and Goodenough, 1998). Briefly, a cDNA fragment containing wild-type Cx50 and FLAG tag linked at Cx50 C-terminus was made by PCR and was constructed into the retroviral vector RCAS(A). We have shown that compared to untagged connexin, the FLAG tag has a minimal effect on membrane distribution and activities of connexin channels (Jiang and Goodenough, 1998). With the wild-type RCAS(A)-Cx50 DNA construct as a template, retroviral constructs of Cx50 mutants containing point mutations were generated with the QuikChange™ site-directed mutagenesis kit (Agilent, Santa Clara, CA) according to the manufacturer's instructions with the following pairs of primers: Cx50H156N sense 5′-CCTGAGAACCTACATCCTCAACATCATTTTCAAAACTCTC-3′, and anti-sense 5′-GAGAGTTTTGAAAATGATGTTGAGGATGTAGGTTCTCAGG-3′. Cx50P88S and Cx50E48K mutant constructs were generated as described previously (Banks et al., 2009, 2007). High-titer recombinant retroviruses were generated (1×108–5×108 colony-forming units per ml) through transfection of these DNA constructs into CEF cells using Lipofectamine® according to manufacturer's instruction (ThermoFisher, Waltham, MA). Crude membrane extracts of transfected CEF cells were prepared and immunoblotted with rabbit anti-FLAG antibody to examine the expression of connexins. Conditioned media were collected and concentrated to make high-titer retrovirus.
Cell culture
CEF cells were cultured in DMEM plus 10% fetal calf serum and 2% chick serum. CEF cells were infected with high-titer retroviruses at the second day of culturing. After reaching confluence, CEF cells were digested with 0.05% trypsin and passaged.
We consulted the ARRIVE guideline to ensure proper reporting of in vivo experiments. The protocol of using chicken embryos was reviewed and approved by our Institutional Animal Care and Use Committee (IACUC). Primary lens cell cultures were prepared by a modified method described previously (Jiang et al., 1993; Menko et al., 1984). Lenses from 11-day-old chick embryos were dissected, washed with TD buffer (140 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, 5 mM glucose and 25 mM Tris-HCl pH 7.4), and digested with 0.1% trypsin in TD buffer at 37°C, and then broken up in M199 medium plus 10% FBS. Cells were collected and resuspended in M199 medium. Living cells were then counted and seeded at 4×105 cells per well in 12-well culture plates. On the second day of cell culturing, Cx50 WT or mutant-type retroviruses were added to primary lens cultures. The cultures were incubated at 37°C with 5% CO2 and were fed every day. In the beginning of culturing, monolayer lens epithelial cells, but not fiber cells proliferated on the culture plates. After 4–5 days, lens epithelial cells became confluent and began to differentiate and form fiber-like lentoid structures.
Preparation of cell lysate extracts and western blotting
Confluent CEF or primary lens culture cells were collected in ice-cold PBS buffer (PH 7.4), homogenized and centrifuged for 2 min at 10,000 g and 4°C, and pellets were then resuspended in PBS buffer (pH 7.4). Whole-cell lysate extracts were boiled in 0.6% SDS and analyzed on 10% SDS-PAGE gel. Western blots were performed by probing with anti-FLAG (1:1000 dilution), anti-Cx50 (1:300 dilution) or anti-β-actin (1:5000 dilution) antibody. We verified specificity of these antibodies in the current study. Primary antibodies were then detected with IRDye 800CW goat anti-rabbit IgG (1:15,000 dilution) or IRDye 680RD goat anti-mouse IgG (1:15,000 dilution) by using a Licor Odyssey system (Lincoln, NE).
Dye uptake assay
CEF cells or primary lens cell culture were infected with recombinant RCAS(A) containing Cx50, Cx46 or Cx50 mutants E48K, P88S or H156N in combination, or with vehicle control. CEF cells were grown at a low-cell density to ensure that the majority of the cells were not physically in contact and primary lens culture cells were grown up to 11 or 12 days until the maturation of lentoid structures. Ethidium bromide (EtBr) was used as a tracer for hemichannel activity, and fluorescein dextran (10 kDa), which is too large to pass through hemichannels, but is taken up by dying cells, was used as a control. Different types of cells may have different responses to H2O2 treatment. We tried several conditions, including various dosages and time durations of H2O2 for CEF and primary lens cultures, respectively, and determined the treatment regimen as follows: 0.3 mM H2O2 for 2 h for CEF cells and 20 min for primary lens cell culture. After treatment, dye-uptake experiments were conducted in the presence of 0.1 mM EtBr and 1 mg/ml fluorescein dextran for 5 min, and the cells were rinsed three times with HBSS and then fixed with 2% PFA for 30 min. At least four microphotographs of fluorescence fields were taken with a 20× dry objective in an inverted microscope (Olympus) with a Rhodamine filter. For CEF cells, the average pixel density of 30 random cells was measured with ImageJ software (Bethesda, MD). For lentoids in lens primary culture, we quantified the integrated pixel intensity of lentoid areas as determined by examining phase-contrast images and then divided by the total areas of lentoids to obtain a mean intensity value.
Scrape-loading dye transfer assay
CEF cells were grown to confluence to maximize cell–cell contact. The CEF cell morphology makes it difficult to visualize dye transfer through individual cells; therefore, a total area of dye transfer from after scrape loading was used. The scrape-loading dye transfer assay was performed based on a modified procedure (El-Fouly et al., 1987). Briefly, cells were scratched in the presence of two fluorescent dyes, Lucifer Yellow (LY; 457 Da), which can pass through gap junction channels, plus Rhodamine–dextran (RD; 10 kDa), which is too large to pass through gap junction channels. Therefore, the presence of LY indicates cells participating in gap junctional coupling and RD serves as a tracer dye for cells originally receiving the dyes. Cells were washed three times with HBSS plus 1% bovine serum albumin (BSA) for 5 min each, and then a mix containing 1% LY and 1% RD in PBS was applied, after which plates were scraped lightly with a 26.5-gauge needle. After 15 min incubation, cells were washed with HBSS three times, twice with PBS and then fixed in fresh 2% PFA for 30 min. Dye-transfer results were examined by using an IX70 fluorescence microscope (Olympus, Tokyo, Japan) in which LY and RD could be detected with the using fluorescein and RD filters, respectively. Using RD staining as the reference for original dye-loaded cells, the extent of dye transfer was measured as the area of LY-labeled cells minus that of the RD-labeled cells. At least four images per condition tested were used to assess the extent of dye transfer.
Determination of the cells under apoptosis and cell death
CEF cells and the primary lens cell culture were grown to 90% confluence. After exposure to 1 mM H2O2 for 10 h (CEF cells) or 0.2 mM H2O2 for 4 h (primary lens cell culture), cells were trypsinized and collected. Annexin V–FITC and PI staining were then examined in cell suspension by using the Dead Cell Apoptosis Kit (BioLegend, San Diego, CA) according to manufacturer's instructions. At least three random fields were examined under a fluorescence microscope for each condition treated. For CEF cells, the percentage of positive staining cells was then analyzed by counting. For primary culture, positive staining areas with lentoid structures were quantified with ImageJ software.
The protective role of Cx50 hemichannels against oxidative stress as mediated by GSH was measured by determining the apoptosis levels by using the FITC–Annexin V Apoptosis Detection Kit (BioLegend, San Diego, CA) as described above. Briefly, CEF cells infected with RCAS(A) containing Cx50 or Cx50 H156N were seeded in six-well plates each with 2.5 ml medium. After cells reached 60% confluency, fresh medium was added. After 72 h of culturing, half of the medium volume was removed and kept. Then some plates were incubated in the absence or presence of 1 mM GSH or DTT for 20 min, the medium was then removed, and cells were rinsed three times with PBS (SH pre-loaded). Afterwards, the cells were incubated with another half of medium that was kept in absence or presence of 1 mM H2O2 for 10 h with or without 1 mM GSH or DTT.
GSH uptake assay
We determined GSH transport by performing a dynamic uptake measurement with ThiolTracker™ (ThermoFisher, Waltham, MA) in live CEF cells. Briefly, infected CEF cells were incubated with 1 mM GSH for different times. Then cells were rinsed with HBBS and incubated with 10 µM ThiolTracker for 30 min. Cells were rinsed three times with PBS and fixed with 2% PFA. The conjugation of ThiolTracker and GSH forms a fluorescent molecule that was measured and quantified by determining the fluorescence intensity by using NIH ImageJ software.
Statistical analysis
All data were analyzed with GraphPad Prism 5 software (GraphPad Software, La Jolla, CA). Two group comparisons were performed with a paired design t-test. Multiple group comparisons were conducted by means of a one-way ANOVA and Newmann-Keul's multiple comparison test. The data are presented as the mean±s.e.m. of at least three measurements. P<0.05 was designated as a statistically significant difference. Asterisks in all figures indicate the degree of significant differences compared to controls: *P<0.05; **P<0.01; ***P<0.001.
Acknowledgements
The authors thank Hongyun Cheng for technical assistance and Daniel Shropshire for critical reading of the paper.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: W.S., M.A.R., S.G., J.X.J.; Methodology: W.S., S.G., J.X.J.; Validation: M.A.R., S.G.; Investigation: W.S., M.A.R., S.G., J.X.J.; Resources: J.X.J.; Data curation: W.A., J.X.J.; Writing - original draft: W.S., J.X.J.; Writing - review & editing: M.A.R., S.G., J.XJ.; Supervision: S.G., J.X.J.; Project administration: J.X.J.; Funding acquisition: J.X.J.
Funding
The work was supported by the National Eye Institute (grant EY012085) and the Welch Foundation (grant AQ-1507) to J.X.J. Deposited in PMC for release after 12 months.
References
- Bachhawat A. K., Thakur A., Kaur J. and Zulkifli M. (2013). Glutathione transporters. Biochim. Biophys. Acta 1830, 3154-3164. 10.1016/j.bbagen.2012.11.018 [DOI] [PubMed] [Google Scholar]
- Banks E. A., Yu X. S., Shi Q. and Jiang J. X. (2007). Promotion of lens epithelial-fiber differentiation by the C-terminus of connexin 45.6 a role independent of gap junction communication. J. Cell Sci. 120, 3602-3612. 10.1242/jcs.000935 [DOI] [PubMed] [Google Scholar]
- Banks E. A., Toloue M. M., Shi Q., Zhou Z. J., Liu J., Nicholson B. J. and Jiang J. X. (2009). Connexin mutation that causes dominant congenital cataracts inhibits gap junctions, but not hemichannels, in a dominant negative manner. J. Cell Sci. 122, 378-388. 10.1242/jcs.034124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beahm D. L. and Hall J. E. (2002). Hemichannel and junctional properties of connexin 50. Biophys. J. 82, 2016-2031. 10.1016/S0006-3495(02)75550-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry V., Mackay D., Khaliq S., Francis P. J., Hameed A., Anwar K., Mehdi S. Q., Newbold R. J., Ionides A., Shiels A. et al. (1999). Connexin 50 mutation in a family with congenital “zonular nuclear” pulverulent cataract of Pakistani origin. Hum. Genet. 105, 168-170. 10.1007/s004399900094 [DOI] [PubMed] [Google Scholar]
- Berthoud V. M. and Beyer E. C. (2009). Oxidative stress, lens gap junctions, and cataracts. Antioxid. Redox. Signal. 11, 339-353. 10.1089/ars.2008.2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan L. A., McGreal R. S. and Kantorow M. (2012). Oxidative stress defense and repair systems of the ocular lens. Front. Biosci. 4, 141-155. 10.2741/e365 [DOI] [PubMed] [Google Scholar]
- Cherian P. P., Siller-Jackson A. J., Burra S., Gu S., Bonewald L. F., Sprague E. and Jiang J. X. (2005). Potential role of α5 integrin as a mechanosensor in the opening of hemichannels for the release of prostaglandin in response to mechanical stress. J. Bone Min. Res. 20 Suppl. 1, S25. [Google Scholar]
- Donaldson P., Kistler J. and Mathias R. T. (2001). Molecular solutions to mammalian lens transparency. News Physiol. 16, 118-123. 10.1152/physiologyonline.2001.16.3.118 [DOI] [PubMed] [Google Scholar]
- Ebihara L., Tong J.-J., Vertel B., White T. W. and Chen T.-L. (2011). Properties of connexin 46 hemichannels in dissociated lens fiber cells. Invest. Opthalmol. Vis. Sci 52, 882-889. 10.1167/iovs.10-6200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Fouly M. H., Trosko J. E. and Chang C. C. (1987). Scrape-loading and dye transfer. A rapid and simple technique to study gap junctional intercellular communication. Exp. Cell Res. 168, 422-430. 10.1016/0014-4827(87)90014-0 [DOI] [PubMed] [Google Scholar]
- Fruscione F., Scarfì S., Ferraris C., Bruzzone S., Benvenuto F., Guida L., Uccelli A., Salis A., Usai C., Jacchetti E. et al. (2011). Regulation of human mesenchymal stem cell functions by an autocrine loop involving NAD(+) release and P2Y11-mediated signaling. Stem Cells Dev. 20, 1183-1198. 10.1089/scd.2010.0295 [DOI] [PubMed] [Google Scholar]
- Giblin F. J. (2000). Glutathione: a vital lens antioxidant. J. Ocul. Pharmacol. Ther. 16, 121-135. 10.1089/jop.2000.16.121 [DOI] [PubMed] [Google Scholar]
- Gong X., Li E., Klier G., Huang Q., Wu Y., Lei H., Kumar N. M., Horwitz J. and Gilula N. B. (1997). Disruption of α3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell 91, 833-843. 10.1016/S0092-8674(00)80471-7 [DOI] [PubMed] [Google Scholar]
- Goodenough D. A., Goliger J. A. and Paul D. L. (1996). Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 65, 475-502. 10.1146/annurev.bi.65.070196.002355 [DOI] [PubMed] [Google Scholar]
- Gu S., Yu X. S., Yin X. and Jiang J. X. (2003). Stimulation of lens cell differentiation by gap junction protein connexin 45.6. Invest. Ophthalmol. Vis. Sci. 44, 2103-2111. 10.1167/iovs.02-1045 [DOI] [PubMed] [Google Scholar]
- Hu Z., Shi W., Riquelme M. A., Shi Q., Biswas S., Lo W.-K., White T. W., Gu S. and Jiang J. X. (2017). Connexin 50 functions as an adhesive molecule and promotes lens cell differentiation. Sci. Rep. 7, 5298 10.1038/s41598-017-05647-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. X., Paul D. L. and Goodenough D. A. (1993). Posttranslational phosphorylation of lens fiber connexin46: a slow occurrence. Invest Ophthalmol Vis. Sci. 34, 3558-3565. [PubMed] [Google Scholar]
- Jiang J. X. and Goodenough D. A. (1996). Heteromeric connexons in lens gap junction channels. Proc. Natl. Acad. Sci. USA 93, 1287-1291. 10.1073/pnas.93.3.1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. X. and Goodenough D. A. (1998). Retroviral expression of connexins in embryonic chick lens. Invest. Ophthalmol. Vis. Sci. 39, 537-543. [PubMed] [Google Scholar]
- Jiang J. X., White T. W., Goodenough D. A. and Paul D. L. (1994). Molecular cloning and functional characterization of chick lens fiber connexin 45.6. Mol. Biol. Cell 5, 363-373. 10.1091/mbc.5.3.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. X. (2001). Use of retroviruses to express connexins. In Connexin Methods and Protocols (ed. Bruzzone R. and Giaume C.), pp. 159-174. Totowa: Humana Press. [DOI] [PubMed] [Google Scholar]
- Kannan R., Yi J. R., Tang D., Zlokovic B. V. and Kaplowitz N. (1996). Identification of a novel, sodium-dependent, reduced glutathione transporter in the rat lens epithelium. Invest. Ophthalmol. Vis. Sci. 37, 2269-2275. [PubMed] [Google Scholar]
- Kar R., Riquelme M. A., Werner S. and Jiang J. X. (2013). Connexin 43 channels protect osteocytes against oxidative stress-induced cell death. J. Bone Miner. Res. 28, 1611-1621. 10.1002/jbmr.1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman A. W. and Isakson B. E. (2014). Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett. 588, 1379-1388. 10.1016/j.febslet.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsili S., Salganik R. I., Albright C. D., Freel C. D., Johnsen S., Peiffer R. L. and Costello M. J. (2004). Cataract formation in a strain of rats selected for high oxidative stress. Exp. Eye Res. 79, 595-612. 10.1016/j.exer.2004.06.008 [DOI] [PubMed] [Google Scholar]
- Menko A. S., Klukas K. A. and Johnson R. G. (1984). Chicken embryo lens cultures mimic differentiation in the lens. Dev. Biol. 103, 129-141. 10.1016/0012-1606(84)90014-9 [DOI] [PubMed] [Google Scholar]
- Nagaraj RH, Panda AK, Shanthakumar S, Santhoshkumar P, Pasupuleti N, Wang B, et al. (2012). Hydroimidazolone modification of the conserved Arg12 in small heat shock proteins: Studies on the structure and chaperone function using mutant mimics. PLoS One 7, e30257 10.1371/journal.pone.0030257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal J. D., Berthoud V. M., Beyer E. C., Mackay D., Shiels A. and Ebihara L. (1999). Molecular mechanism underlying a Cx50-linked congenital cataract. Am. J. Physiol. 276, C1443-C1446. 10.1152/ajpcell.1999.276.6.C1443 [DOI] [PubMed] [Google Scholar]
- Pichi F., Lembo A., Serafino M. and Nucci P. (2016). Genetics of congenital cataract. Dev. Ophthalmol. 57, 1-14. 10.1159/000442495 [DOI] [PubMed] [Google Scholar]
- Ramachandran S., Xie L.-H., John S. A., Subramaniam S. and Lal R. (2007). A novel role for connexin hemichannel in oxidative stress and smoking-induced cell injury. PLoS ONE 2, e712 10.1371/journal.pone.0000712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong P., Wang X., Niesman I., Wu Y., Benedetti L. E., Dunia I., Levy E. and Gong X. (2002). Disruption of Gja8 (α8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development 129, 167-174. [DOI] [PubMed] [Google Scholar]
- Schulz R., Görge P. M., Görbe A., Ferdinandy P., Lampe P. D. and Leybaert L. (2015). Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol. Ther. 153, 90-106. 10.1016/j.pharmthera.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A., Mackay D., Ionides A., Berry V., Moore A. and Bhattacharya S. (1998). A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am. J. Hum. Genet. 62, 526-532. 10.1086/301762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavi N., Rubinos C., Li L., Sellitto C., White T. W., Mathias R. and Srinivas M. (2014). Connexin 46 (cx46) gap junctions provide a pathway for the delivery of glutathione to the lens nucleus. J. Biol. Chem. 289, 32694-32702. 10.1074/jbc.M114.597898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stridh M. H., Tranberg M., Weber S. G., Blomstrand F. and Sandberg M. (2008). Stimulated efflux of amino acids and glutathione from cultured hippocampal slices by omission of extracellular calcium: likely involvement of connexin hemichannels. J. Biol. Chem 283, 10347-10356. 10.1074/jbc.M704153200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan R. and Manikandan R. (2013). Antioxidants and cataract. Free Radic Res. 47, 337-345. 10.3109/10715762.2013.777155 [DOI] [PubMed] [Google Scholar]
- Tong X., Lopez W., Ramachandran J., Ayad W. A., Liu Y., Lopez-Rodriguez A., Harris A. L. and Contreras J. E. (2015). Glutathione release through connexin hemichannels: implications for chemical modification of pores permeable to large molecules. J. Gen. Physiol. 146, 245-254. 10.1085/jgp.201511375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. W., Goodenough D. A. and Paul D. L. (1998). Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J. Cell Biol. 143, 815-825. 10.1083/jcb.143.3.815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.W. and Paul D.L. (1999). Genetic diseases and gene knockouts reveal diverse connexin functions. Annu. Rev. Physiol. 61, 283-310. 10.1146/annurev.physiol.61.1.283 [DOI] [PubMed] [Google Scholar]
- Willecke K., Eiberger J., Degen J., Eckardt D., Romualdi A., Güldenagel M., Deutsch U. and Söhl G. (2002). Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 383, 725-737. 10.1515/BC.2002.076 [DOI] [PubMed] [Google Scholar]
- Xu X. and Ebihara L. (1999). Characterization of a mouse Cx50 mutation associated with the No2 mouse cataract. Invest. Ophthalmol. Vis. Sci. 40, 1844-1850. [PubMed] [Google Scholar]