Abstract
The green alga Chlamydomonas reinhardtii can grow photoautotrophically utilizing CO2, heterotrophically utilizing acetate, and mixotrophically utilizing both carbon sources. Growth of cells in increasing concentrations of acetate plus 5% CO2 in liquid culture progressively reduced photosynthetic CO2 fixation and net O2 evolution without effects on respiration, photosystem II efficiency (as measured by chlorophyll fluorescence), or growth. Using the technique of on-line oxygen isotope ratio mass spectrometry, we found that mixotrophic growth in acetate is not associated with activation of the cyanide-insensitive alternative oxidase pathway. The fraction of carbon biomass resulting from photosynthesis, determined by stable carbon isotope ratio mass spectrometry, declined dramatically (about 50%) in cells grown in acetate with saturating light and CO2. Under these conditions, photosynthetic CO2 fixation and O2 evolution were also reduced by about 50%. Some growth conditions (e.g. limiting light, high acetate, solid medium in air) virtually abolished photosynthetic carbon gain. These effects of acetate were exacerbated in mutants with slowed electron transfer through the D1 reaction center protein of photosystem II or impaired chloroplast protein synthesis. Therefore, in mixotrophically grown cells of C. reinhardtii, interpretations of the effects of environmental or genetic manipulations of photosynthesis are likely to be confounded by acetate in the medium.
The green alga Chlamydomonas reinhardtii is a facultative “acetate flagellate” capable of growing heterotrophically on acetate, but not on Glc or other related carbon sources (Harris, 1989). Many studies of light stress and light regulation of photosynthetic gene expression have been carried out with acetate-grown cells (e.g. Ohad et al., 1990; Danon and Mayfield, 1991; Drapier et al., 1992). Therefore, we were interested in the potential effects acetate may have on photosynthesis and related processes. Acetate is metabolized to triose following ATP-dependent entry into the glyoxylate cycle, whereas inorganic carbon is reduced to triose during photosynthesis. Acetate metabolism may exert opposing influences on utilization of inorganic carbon. Previous studies have reported that acetate transiently inhibits photosynthesis (Endo and Asada, 1996) and stimulates respiration in light-grown cells of C. reinhardtii bubbled with air (Fett and Coleman, 1994; Endo and Asada, 1996), possibly via increased alternative oxidase activity (Weger et al., 1990a, 1990b). The ability of acetate to induce isocitrate lyase, the key glyoxylate cycle enzyme necessary for its utilization, is attenuated in the presence of light and inorganic carbon (Martinez-Rívas and Vega, 1993). Conversely, acetate represses expression of nuclear-encoded chloroplast proteins involved in light harvesting and inorganic carbon fixation (Goldschmidt-Clermont, 1986; Kindle, 1987). Thus, mixotrophically grown cells of C. reinhardtii may respond differently to light stress than photoautotrophically grown cells, potentially confounding interpretation of responses to genetic and environmental manipulations.
A particularly appropriate tool for studying biomass partitioning is stable isotope ratio mass spectrometry. Estep and Hoering (1980, 1981) determined the fraction of reduced biomass resulting from photosynthesis during mixotrophic growth of Chlorella sorokiniana on Glc or acetate medium in the presence of 1% CO2 using stable hydrogen isotope analysis. Differences in δ13C have also been used to determine the time of onset of autotrophy in developing seedlings (Deléens et al., 1984; Maillard et al., 1994a, 1994b). Dual isotope methods have been applied to assess carbon and nitrogen allocation during maize stem elongation (Cliquet et al., 1990) and biomass derived from translocated Suc and photosynthesis in the partially photosynthetic hypsophylls (husks) of maize (Yakir et al., 1991) and in vitro-grown potato plantlets (Wolf et al., 1998). Additionally, the effect of acetate on respiratory pathway partitioning can be assessed by on-line analysis of stable 18O2 discrimination (Weger et al., 1990a, 1990b; Ribas-Carbo et al., 1995).
The proportions of biomass attributable to photosynthetic CO2 assimilation and to heterotrophic respiration of a reduced carbon source in mixotrophically cultured algal cells can be estimated from stable isotope determinations using the following equation (modified after Cliquet et al., 1990), provided the isotopic signatures of the two sources of carbon are sufficiently different:
Photosynthetic fraction of carbon biomass=
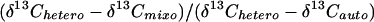 |
This quantitative relationship prevails because: (a) uptake and respiration of reduced carbon substrates result in comparatively little discrimination (about 1‰) relative to the source (DeNiro and Epstein, 1976), and (b) photosynthetic CO2 fixation is an irreversible process and therefore subsequent biochemical events have only small effects on the δ13C value (O'Leary, 1988). Results presented in this paper show that the presence of acetate during growth in saturating light and CO2 inhibits photosynthesis and autotrophic carbon assimilation in wild-type C. reinhardtii. This effect was exacerbated in wild-type C. reinhardtii grown under low irradiance or in air, and by site-specific chloroplast mutations that predispose C. reinhardtii to photoinhibition.
MATERIALS AND METHODS
Strains
Cultures of wild-type (CC-125, 137C mt+), a non-photosynthetic psbA deletion mutant (CC-744, ac-u-β mt+), and a respiration-deficient mutant of Chlamydomonas reinhardtii lacking cytochrome oxidase activity (CC-314, dk-97 mt−) described by Harris (1989) were obtained from the Chlamydomonas Genetics Center (Duke University, Durham, NC). The 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU)-resistant transformant dr (CC-2827) originated from biolistic bombardment of CC-125 with a cloned 10-kb BamHI-BglII fragment of chloroplast DNA containing the psbA gene from the herbicide-resistant DCMU-4 mutant (Erickson et al., 1984) bearing a Ser-264 to Ala change in the D1 protein of photosystem II (PSII). The spr/sr strain (CC-2811) is impaired in chloroplast protein synthesis as a consequence of two single antibiotic-resistance point mutations (A474-> C, A1123-> G) in the chloroplast-encoded 16S rRNA gene (Harris et al., 1989; Heifetz et al., 1997). This strain was obtained by biolistic transformation of CC-125 with a cloned 7.0-kb BamHI chloroplast DNA fragment containing the mutant 16S rRNA gene and most of the 23S rRNA gene proximal to the intron near the 3′ end of this gene.
Growth Conditions
Cells were grown in liquid cultures shaken and bubbled with 5% (v/v) CO2-enriched air at 25°C under continuous illumination from cool-white fluorescent lamps under low (<25 μmol m−2 s−1), moderate (350 μmol m−2 s−1), or high (600 μmol m−2 s−1) photosynthetically active radiation (400–700 nm). High-salt minimal medium (HS) was used for photoautotrophic experiments, whereas mixotrophic and heterotrophic growth were carried out in either high-salt acetate medium (HSHA) containing 29.4 mm sodium acetate or in Tris-acetate phosphate (TAP) buffer containing 17.5 mm acetate (Harris, 1989). Liquid cultures were maintained in the early- to mid-exponential growth phase by periodic dilution for several days to ensure acclimation to the growth environments. Aliquots of these cultures were used to inoculate 250- to 300-mL liquid cultures into 500-mL baffled shake flasks (Bellco, Vineland, NJ) at 2 × 105 cells mL−1, or were spread onto 1.5% agar plates of the same medium for analysis. The pH of liquid cultures in HS, HSHA, and TAP medium bubbled with 5% CO2 remained within the range 6.6 to 7.4. Cultures on agar plates supplemented with 5% CO2 were placed inside a closed plexiglass chamber and supplied with mixed gas at a flow rate of approximately 500 cm3 min−1, while those at ambient CO2 levels (in air) were maintained on lighted shelves at 25°C.
Measurement of Photosynthesis, Respiration, and Growth Rates
Cells for photosynthesis measurements were grown under high light and bubbled with 5% CO2 in cultures of HS supplemented with 0, 3.7. 7.4, 14.7, and 29.4 mm sodium acetate to the early exponential phase (A750 = approximately 0.1), gently pelleted, and resuspended (A750 = 0.175) in fresh growth medium with 10 mm NaHCO3. Respiration, maximum rate of net photosynthetic O2 evolution, and chlorophyll fluorescence quenching were measured at growth temperature under 300 and 600 μmol m−2 s−1 red actinic light, as described by Heifetz et al. (1997).
The incorporation of 14CO2 into acid-stable products was measured under high light in 1.5-mL aliquots of cells in 40-mL centrifuge tubes (Corex, Corning, NY) containing 0.5 mL of a bicarbonate reaction mixture (0.2 m Tris, pH 8.0, 40 mm NaHCO3, 4 μCi NaH14CO3 [6.6 Ci/mol, NEN Life Science Products, Boston]) and a 1-cm magnetic stir bar. Cells were stirred continuously, and 0.5-mL aliquots were removed after 6, 12, and 18 min and placed in scintillation vials with 500 μL of 1 n HCl to drive off the unincorporated 14C. Duplicate 100-μL aliquots from each sample were counted in 10 mL of EcoLume (ICN, Costa Mesa, CA) scintillation fluid. Rates of 14C incorporation into acid-stable products were linear for all samples over the 18-min assay period.
Chlorophyll content and exponential growth rates (cell/biomass doubling times) were calculated as described previously (Lers et al., 1992; Förster et al., 1997).
[13C]Acetate Labeling
The δ13C of acetate samples from eight different suppliers ranged from −44.1 to −19.5‰. Laboratory compressed air (δ13C approximately −8‰) was mixed with bottled CO2 from various sources to produce 5% CO2 in which the δ13C varied from −44‰ to +4‰ between different experiments. The dynamic range of the isotope discrimination assay for TAP-grown cells was expanded by supplementing the naturally available 13C from acetic acid with 2 mg L−1 1,2 [13C]acetate (Sigma-Aldrich, St. Louis, catalog no. 28,201–4). Thus, the span of δ13C of wild-type cultured in the dark or CC-744 cultured in dim light on TAP medium ranged from −21‰ (photoautotrophic growth on CO2) to >110‰ (heterotrophic growth on 13C-TAP), permitting a very accurate estimation of the photosynthetic fraction of carbon assimilated under mixotrophic conditions.
Sample Preparation for Carbon Isotope Mass Spectrometry and δ13C Determinations
Cells were harvested from liquid cultures in the mid-exponential phase (approximately 3 × 106 cells mL−1) by centrifugation at room temperature, washed three times in double-deionized H2O, pelleted in 1.5-mL microcentrifuge tubes at 4°C, and stored at −70°C until lyophilization. Cells grown for 5 to 10 d on agar plates were transferred directly to microcentrifuge tubes with sterile inoculating loops, avoiding transfer of agar substrate (δ13C = −17‰ to −19‰). Lyophilized samples were ground finely, and aliquots (200–2,000 μg) were weighed into tin capsules and combusted in an automated elemental analyzer (NA1500, Carlo Erba, Milan) for determination of 13C/12C ratios using a stable isotope ratio mass spectrometer (VG Isogas SIRA II, Middlewich, UK) (Yakir et al., 1991). Values are reported as means ± se of duplicate or triplicate samples as indicated in the figures and tables.
On-Line Respiratory 18O Fractionation
The on-line sample trapping and preparation system used for liquid-phase 18O2 discrimination during respiration was that described by Ribas-Carbo et al. (1995). Aliquots of exponentially growing liquid cultures (0.04 to 0.1 A750 = 0.5 to 2 × 106 cells mL−1) were transferred to a 30-mL capacity cylindrical plexiglass chamber connected to a vacuum trapping line and fitted with an O-ring-sealed plunger to facilitate sampling without the introduction of air bubbles. Dissolved gases were sparged from 5-mL aliquots of the cell suspension sampled at 8- to 20-min intervals by bubbling with He, and the O2 was trapped at 77°K on a molecular sieve after removal of CO2 and H2O in a vacuum line. Cells treated with 10 μm DCMU to inhibit photosynthetic O2 evolution gave the same results as experiments using a darkened chamber (data not shown). Oxygen isotope discrimination was calculated as described in Ribas-Carbo et al. (1995) using an Ar/N2 ratio of 0.0388 to account for the aqueous diffusivities of these gases. Slope ses were adjusted for sample size (Weger et al., 1990b) and F tests of significance were used for pairwise comparisons of regression slopes.
RESULTS
Effect of Acetate Concentration on Photosynthesis and Growth Rate in Saturating Light and CO2
Maximum rates of net O2 evolution and CO2 incorporation into acid stable products by wild-type cells of C. reinhardtii declined with increasing acetate concentration in the mixotrophic growth medium under high (saturating) light and CO2 conditions (Table I). HSHA, which contains 29.4 mm acetate, effected a 48% reduction in the maximum rate of O2 evolution and a 56% reduction in CO2 fixation rate. The lowest acetate concentration tested (3.7 mm) reduced O2 evolution and CO2 fixation by 26% and 34%, respectively. In contrast, the growth rate, respiration, PSII efficiency, and chlorophyll content were not affected by acetate concentration.
Table I.
Effect of acetate concentration on growth rate and photosynthesis of wild-type C. reinhardtii in the early log phase when grown and analyzed under saturating light (600 mmol m−2 s−1) in the presence of 5% CO2
| Acetate | Growth Ratea | Respirationb
|
Net Photosynthesisb
|
Fv/Fm | Chlorophyllc | |
|---|---|---|---|---|---|---|
| O2 uptake | O2 evolution | 14CO fixation | ||||
| mm | μmol mg−1 Chl h−1 | mg/A750 | ||||
| 0 | 149 ± 0.005 | −79 | 345 (100) | 465 (100) | 0.71 | 28 ± 3 |
| 3.7 | 0.162 ± 0.005 | −93 | 256 (74) | 308 (66) | 0.66 | 28 ± 3 |
| 7.4 | 0.153 ± 0.005 | −94 | 226 (66) | 360 (78) | 0.69 | 28 ± 2 |
| 14.7 | 0.153 ± 0.005 | −77 | 206 (60) | 257 (55) | 0.70 | 29 ± 3 |
| 29.4 | 0.148 ± 0.005 | −87 | 180 (52) | 207 (44) | 0.70 | 28 ± 2 |
Photosynthesis values are expressed in absolute units and as a percentage of the control HS culture without acetate.
Mean ± se from five independent experiments.
Data from single representative experiments measuring O2 consumption, O2 evolution, or 14CO2 fixation. Replicate experiments show the same relative reductions in both parameters with increasing acetate concentration in the growth medium.
Mean ± se from three independent experiments.
Evaluation of Isotopic Fractionation during Heterotrophic and Photoautotrophic Growth of C. reinhardtii
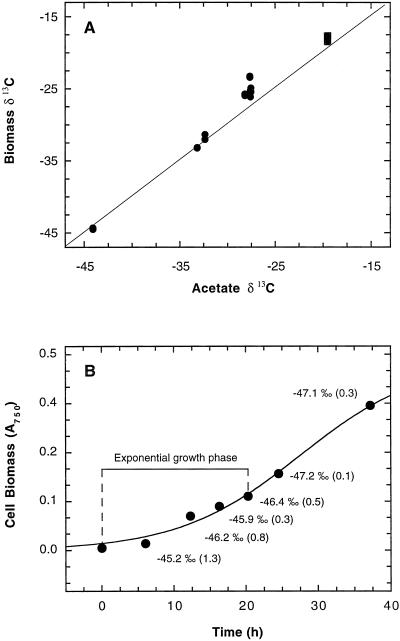
The assessment of the relative contributions of photosynthetic CO2 fixation and respiration of acetate to cell metabolism during mixotrophic growth first requires baseline isotopic signatures of cells grown heterotrophically and photoautotrophically. The δ13C value of heterotrophically grown wild-type cells (data not shown) and cells of a nonphotosynthetic psbA deletion mutant (CC-744) grown in dim light on HSHA (Fig. 1A) was strongly correlated with the δ13C of the acetate present in the growth medium. These results demonstrate that heterotrophic metabolism of acetate by C. reinhardtii results in little or no carbon isotope discrimination. The δ13C values of wild-type C. reinhardtii biomass grown photoautotrophically under saturating light and CO2 remained relatively constant throughout the exponential portion of the growth curve and increased only slightly in the early stationary phase (Fig. 1B). Growth of wild-type C. reinhardtii in HS liquid cultures supplemented with 5% CO2 at three irradiance levels (200, 350, and 600 μmol m−2 s−1) resulted in an average isotopic discrimination relative to the source (Δ) of 24.6‰ ± 0.3‰, which in agreement with earlier data (Sharkey and Berry, 1985). The much more negative biomass δ13C values in Figure 1B are due to the use of CO2 sources with different isotopic compositions. The Δ with respect to source CO2 in cells grown autotrophically on agar plates with 5% CO2 was 21.9‰ (19.9‰ to 22.6‰ in four separate experiments with different genotypes). In cells of wild-type grown autotrophically on agar plates in air, an even lower discrimination was observed (Δ = 6.3‰ to 10.7‰). Evidently, CO2 limitations in the wet cell mass on the agar surface and/or the CO2 concentrating mechanism were responsible for the smaller discriminations. As there was no detectable change in discrimination during heterotrophic growth on HSHA plates or liquid HSHA medium (Fig. 1A), acetate diffusion problems on the agar plates can be ruled out.
Figure 1.
Carbon isotope composition (δ13C) of cell biomass during photoautotrophic and heterotrophic growth of C. reinhardtii. A, Heterotrophic growth of C. reinhardtii on HSHA containing 29.4 mm acetate of varying δ13C. Cells of the non-photosynthetic mutant strain CC-744 were grown in dim light (5–25 μmol m−2 s−1) on HSHA plates (●) or 250-mL HSHA shake flask cultures (▪) formulated with sodium acetates differing in natural abundance of 13C. The stable carbon isotope compositions of the source acetates and lyophilized biomass were determined as described in the text. B, Photoautotrophic growth of wild-type C. reinhardtii in 250-mL liquid cultures bubbled with 5% CO2 under moderate light (350 μmol m−2 s−1) at 25°C. Samples were harvested at the indicated times for hemocytometer counts, spectrophotometric determination of biomass concentration (A750), and biomass δ13C measurement. Each value and data point are the means ± se (in parentheses) of two independent measurements. Data points were fitted to a logistic growth equation. Cell concentration at the beginning of the experiments was 2 × 105 cells mL−1 (A750 = 0.01).
Estimation of Carbon Acquisition during Mixotrophic Growth of Wild-Type C. reinhardtii and Mutants with Impaired PSII Function
Isotopic composition of mixotrophically grown wild-type C. reinhardtii was determined using HSHA (29.4 mm acetate, δ13C = −27‰ to −44‰) in liquid cultures bubbled with 5% CO2 (δ13C = −19.5‰) or on agar plates exposed to ambient CO2 in air (δ13C = −8‰). The photosynthetic fraction of carbon biomass (see equation) was calculated from these values, and the baseline heterotrophic and photoautotrophic isotopic composition. Consistent with the inhibitory effects of acetate on photosynthesis (Table I), marked reductions were observed in the fraction of biomass carbon assimilated photosynthetically (Table II). In HSHA liquid medium under saturating light and CO2, the photosynthetic fraction did not exceed 55%. On plates exposed to air in high light, this fraction was only 23%. Strikingly, wild-type cells grown on HSHA plates exposed to air in moderate or low light showed little or no detectable autotrophic carbon assimilation.
Table II.
Comparison of the photosynthetic fraction of carbon biomass in wild-type C. reinhardtii grown mixotrophically in HSHA (29.4 mM acetate) liquid medium bubbled with 5% CO2 and on agar plates exposed to air
| Growth Medium | CO2 Level | Irradiance | Photosynthetic Fraction of Carbon Biomass |
|---|---|---|---|
| μmol m−2 s−1 | % | ||
| HSHA liquid | 5% | 600 | 54 |
| HSHA plates | Air | 600 | 23 |
| 350 | 9 | ||
| <25 | 0→3 |
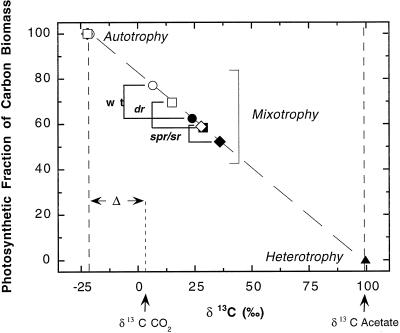
TAP medium (17.5 mm acetate) is commonly used to grow wild-type and mutant strains of C. reinhardtii for photosynthetic and molecular analysis (Rochaix et al., 1998). The relative photosynthetic fraction of carbon metabolism in the wild type and in mutations affecting chloroplast protein synthesis and PSII function was determined using TAP medium supplemented with 13C to make the δ13C acetate much more positive than air or the 5% CO2 source. The biomass of wild-type cells grown mixotrophically in liquid cultures of TAP medium (δ13C = approximately +95‰ to +99‰) bubbled with 5% CO2 (δ13C = +2.9‰) showed a photosynthetic fraction of only 78% under saturating irradiance and this declined to 62% at subsaturating irradiance. Thus, even under the optimal light and CO2 conditions, nearly one quarter of the carbon in the wild type was derived heterotrophically when the cells were provided 17.5 mm acetate and 5% CO2 as alternative carbon sources. As expected, δ13C values for the dr mutant, with slower PSII electron transfer, showed a lower photosynthetic fraction compared with wild type grown mixotrophically under identical moderate and high light conditions (Fig. 2). The spr/sr mutant, which has defects in chloroplast protein synthesis, was even more dependent on heterotrophically assimilated carbon during mixotrophic growth. Reductions in autotrophic competence of the two mutants under high light compared with the wild type correlate well with their impaired light-saturated photosynthetic rates and growth rates (Heifetz et al., 1992, 1997).
Figure 2.
Photosynthetic fraction of carbon biomass from wild-type and mutant C. reinhardtii cells grown on 13C-TAP (17.5 mm acetate) in liquid culture. Cells of wild type (circles), spr/sr (diamonds), and dr (squares) were grown autotrophically to the early-/mid-exponential phase in HS bubbled with 5% CO2 (δ13C = +2.9‰), heterotrophically in the dark on TAP supplemented with 13C (δ13C = +99‰; ▴), and mixotrophically on 13C TAP bubbled with 5% CO2 at two irradiance levels, 350 μmol m−2 s−1 (closed symbols) and 600 μmol m−2 s−1 (open symbols). The fraction of carbon biomass resulting from photosynthetic carbon reduction was calculated as described in the text from the average photoautotrophic, heterotrophic, and mixotrophic biomass δ13C values.
Role of the Alternative Oxidase during Mixotrophic and Autotrophic Growth
We established baseline isotopic signatures for respiratory O2 exchange via the cytochrome oxidase and alternative (cyanide insensitive oxidase) pathways during mixotrophic and photoautotrophic growth of wild-type cells to determine if partitioning between the two respiratory pathways is influenced by acetate. For end point determinations of discrimination due to only the alternative or cytochrome oxidases, wild-type cells were pretreated for 15 min with KCN or the alternative oxidase inhibitor propyl gallate. Alternatively, photoautotrophically grown cells of the dk-97 mutant lacking cytochrome oxidase activity (Wiseman et al., 1977; Husic and Tolbert, 1987) were used to assess discrimination due to the alternative oxidase pathway. The oxygen isotope discrimination in photoautotrophically grown wild-type cells in minimal medium (Δ = 18.8‰) reveals little engagement of the alternative pathway (Table III), which is in agreement with previous work (Weger et al., 1990b). Respiratory discrimination of wild-type cells grown in the presence of acetate and 5% CO2 (20.8‰) was not significantly affected by propyl gallate treatment (Table III). This demonstrates that the alternative oxidase was not engaged in the presence or absence of acetate under these conditions. These results, together with the lack of increased dark respiration in mixotrophically grown cells, indicate that the effects of acetate are on photosynthesis rather than on respiration.
Table III.
Discrimination against 18O2 during dark respiration by wild-type C. reinhardtii cells grown to the mid-exponential phase at 600 μmol m−2 s−1 irradiance in liquid cultures bubbled with 5% CO2
| Genotype | Medium | Inhibitor | Discrimination | n |
|---|---|---|---|---|
| % ± se | ||||
| dk-97 | HS | None | 24.3 ± 0.5 | 3 |
| Wild type | HS | None | 18.8 ± 0.4 | 10 |
| Wild type | HS | 1 mm KCN | 24.2 ± 1.2 | 5 |
| Wild type | TAP | None | 20.8 ± 0.8 | 6 |
| Wild type | TAP | l mm KCN | 24.2 ± 1.9 | 5 |
| Wild type | TAP | 500 μm Propyl gallate | 21.1 ± 0.9 | 4 |
KCN results in discrimination due solely to O2 consumption via the alternative oxidase pathway. Propyl gallate results in discrimination due solely to O2 consumption via the cytochrome oxidase pathway.
DISCUSSION
Our results demonstrate that growth of wild-type C. reinhardtii in the presence of 3.7 to 29.4 mm acetate in saturating light and CO2 inhibits photosynthesis, as measured by the maximum rates of net O2 evolution and 14C fixation. However, neither dark respiration nor engagement of the alternative oxidase pathway were affected. Fett and Coleman (1994) reported that acetate stimulated respiration in cells grown mixotrophically in air, and Endo and Asada (1996) demonstrated a similar response immediately upon addition of acetate to autotrophically grown cells. Growth rates in our experiments were unaffected by the large reduction in photosynthesis in the presence of acetate (Table I). Moreover, analysis of stable carbon isotope composition of biomass from mixotrophically grown cells revealed a marked shift from autotrophic to heterotrophic carbon metabolism in response to both environmental and genetic manipulation of C. reinhardtii. The stable isotope data (Table II) indicate that carbon derived from acetate in the light can substitute for up to 50% of photoautotrophically acquired carbon in liquid cultures under the saturating light and CO2 conditions optimal for photosynthetic growth of C. reinhardtii (Heifetz et al., 1997). At subsaturating irradiance and CO2 levels in the presence of specific mutations reducing photosynthetic performance, further decreases in the contribution of photosynthetic carbon assimilation were observed under mixotrophic growth conditions.
Although one might expect that the addition of a reduced carbon source would lower the proportion of biomass carbon derived from photosynthesis, the notion that acetate metabolism in saturating light and CO2 can quantitatively substitute for photosynthetic carbon assimilation to drive growth in C. reinhardtii is probably overly simplistic. There is undoubtedly a dynamic relationship between acetate metabolism and photosynthesis that involves both mitochondria and chloroplasts. Consistent with other treatments that deplete cell ATP, Gans and Rebéillé (1990) found that the addition of acetate to autotrophically grown C. reinhardtii decreased PSII fluorescence and promoted a transition from state I to state II, presumably with attendant adjustment of the antenna architecture of the photosynthetic apparatus (Bulté et al., 1990). These observations were confirmed and extended by Endo and Asada (1996), who showed that the addition of acetate produced transient non-photochemical quenching in the light, which was sustained in the dark and associated with a reduction in PSII efficiency. Whether these primary events, thought to be mediated by chlororespiration (Bennoun, 1998), account for the long-term decline in photosynthetic O2 evolution and carbon assimilation observed here remains to be assessed. Greater inhibition of photosynthesis by acetate at lower light intensities (Table II; Fig. 2) would be consistent with such a reduction in PSII efficiency, but this was not reflected in our dark-adapted measurements of Fv/Fm (Table I).
The first step in acetate utilization is the ATP-dependent production of acetyl coenzyme A. Therefore, in mixotrophic growth under limiting light, ATP demand for acetate assimilation may itself limit photosynthetic carbon reduction. These effects may be exacerbated if CO2 is limited due to the induction of the carbon concentrating mechanism (Spalding, 1998). Acetate may also exert inhibitory effects on metabolism, as concentrations above 6.7 mm were reported to inhibit heterotrophic growth of wild-type C. reinhardtii (Chen and Johns, 1994). In the absence of acetate, reduced photosynthesis in several C. reinhardtii mutants with impaired D1 function (Fšrster et al., 1997; Lardans et al., 1998) or resistance to very high light (Förster et al., 1999) did not directly affect growth rate. These observations suggest that metabolic variables other than photosynthetic CO2 fixation may sometimes limit growth.
The mechanisms underlying the effects of acetate on photosynthesis in our long-term growth experiments may also involve changes in gene expression. In plants and algae, carbon metabolites (including acetate) are known to down-regulate the expression of nuclear genes encoding chloroplast proteins involved in photosynthesis and in non-photosynthetic carbon metabolism (Kindle, 1987; Sheen, 1990, 1994). At the transcriptional level, acetate is a potent repressor of synthesis of enzymes involved in photosynthetic carbon reduction, as well as an inducer of the glyoxylate cycle-specific enzymes malate synthetase (Neilson and Lewin, 1974) and isocitrate lyase (Martinez-Rívas and Vega, 1993). Thus, transcriptional and translational regulation of both nuclear and chloroplast genes encoding photosynthetic components and enzymes involved in acetate metabolism would be expected to respond dynamically to the presence of acetate. These molecular processes, as well as the physiological events they influence, should therefore be compared in both mixotrophically and photoautotrophically grown cells.
In summary, we show that both photosynthetic incorporation of inorganic carbon and the maximum rate of O2 evolution in C. reinhardtii can be significantly diminished by growth in the presence of acetate. Under some circumstances (limiting light, high acetate concentrations, growth on solid medium in air) photosynthetic carbon gain is virtually abolished. In studies involving mutants of C. reinhardtii with partial photosynthetic defects that do not cause obligate heterotrophy, very different interpretations of their metabolic consequences might be obtained depending on the presence or absence of acetate. Consequently, interpretation of the effects of environmental or other manipulations may be confounded by acetate-induced impairment of photosynthetic performance.
ACKNOWLEDGMENTS
We acknowledge the support of Dr. Nicholas W. Gillham throughout this project. Dr. Amnon Lers provided the chloroplast transformants used in these experiments, and Drs. Joseph A. Berry and Miquel Ribas-Carbo gave invaluable assistance with the on-line determination of oxygen isotope discrimination.
Footnotes
This work was supported by the U.S. Department of Energy (grant DE–FG05–89ER14005).
LITERATURE CITED
- Bennoun P. Chlororespiration, sixteen years later. In: Rochaix J-D, Goldschmidt-Clermont M, Merchant S, editors. The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Advances in Photosynthesis. Vol. 7. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 675–683. [Google Scholar]
- Bulté L, Gans P, Rebéillé F, Wollman F-A. ATP control on state transitions in vivo in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1990;1020:72–80. [Google Scholar]
- Chen F, Johns MR. Substrate inhibition of Chlamydomonas reinhardtii by acetate in heterotrophic culture. Proc Biochem. 1994;29:245–252. [Google Scholar]
- Cliquet J-B, Deléens E, Bousser A, Martin M, Lescure J-C, Prioul L, Mariotti A, Morot-Gaudry J-F. Estimation of carbon and nitrogen allocation during stalk elongation by 13C and 15N tracing in Zea mays L. Plant Physiol. 1990;92:79–87. doi: 10.1104/pp.92.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A, Mayfield SPY. Light regulated translational activators: identification of chloroplast gene specific mRNA binding proteins. EMBO J. 1991;10:3993–4001. doi: 10.1002/j.1460-2075.1991.tb04974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deléens E, Gregory N, Bourdu R. Transition between seed reserve use and photosynthetic supply during development of maize seedlings. Plant Sci Lett. 1984;37:35–39. [Google Scholar]
- DeNiro MJ, Epstein S. You are what you eat (plus a few ‰): the carbon isotope cycle in food chains. Geol Soc Am Abs Prog. 1976;8:834–835. [Google Scholar]
- Drapier D, Girard-Bascou J, Wollman F-A. Evidence for nuclear control of the expression of the atpA and atpB chloroplast genes in Chlamydomonas. Plant Cell. 1992;4:283–295. doi: 10.1105/tpc.4.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Asada K. Dark induction of the non-photochemical quenching of chlorophyll fluorescence by acetate in Chlamydomonas reinhardtii. Plant Cell Physiol. 1996;37:551–555. [Google Scholar]
- Erickson JM, Rahire M, Bennoun P, Delepelaire P, Diner B, Rochaix J. Herbicide resistance in Chlamydomonas reinhardtii results from a mutation in the chloroplast gene for the 32–kilodalton protein of photosystem II. Proc Natl Acad Sci USA. 1984;81:3617–3621. doi: 10.1073/pnas.81.12.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estep MF, Hoering TC. Biogeochemistry of the stable hydrogen isotopes. Geochim Cosmochim Acta. 1980;44:1197–1206. [Google Scholar]
- Estep MF, Hoering TC. Stable hydrogen isotope fractionation during autotrophic and mixotrophic growth of microalgae. Plant Physiol. 1981;67:474–477. doi: 10.1104/pp.67.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett JP, Coleman JR. Regulation of periplasmic carbonic anhydrase expression in Chlamydomonas reinhardtii by acetate and pH. Plant Physiol. 1994;106:103–108. doi: 10.1104/pp.106.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster B, Heifetz PB, Boynton JE, Gillham NW. Herbicide resistance and growth of D1 Ala251 mutants in Chlamydomonas. Z Naturforsch. 1997;52c:654–664. [Google Scholar]
- Förster B, Osmond CB, Boynton JE, Gillham NW. Mutants of Chlamydomonas reinhardtii resistant to very high light. J Photochem Photobiol B Biol. 1999;48:127–135. [Google Scholar]
- Gans P, Rebéillé F. Control in the dark of plasoquinone redox state by mitochondrial activity in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1990;72:72–80. [Google Scholar]
- Goldschmidt-Clermont M. The two genes for the small subunit of RuBp carboxylase/oxygenase are closely linked in Chlamydomonas reinhardtii. Plant Mol Biol. 1986;6:13–21. doi: 10.1007/BF00021302. [DOI] [PubMed] [Google Scholar]
- Harris EH. The Chlamydomonas Sourcebook. San Diego: Academic Press; 1989. [Google Scholar]
- Harris EH, Burkhart BD, Gillham NW, Boynton JE. Antibiotic resistance mutations in the chloroplast 16S and 23S rRNA genes of Chlamydomonas reinhardtii: correlation of genetic and physical maps of the chloroplast genome. Genetics. 1989;123:281–292. doi: 10.1093/genetics/123.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz PB, Lers A, Turpin DH, Gillham NW, Osmond CB. dr and spr/sr mutations of Chlamydomonas reinhardtii affecting D1 protein function and synthesis define two independent steps leading to chronic photoinhibition and confer differential fitness. Plant Cell Environ. 1997;20:1145–1157. [Google Scholar]
- Heifetz PB, Lers AL, Boynton JE, Gillham NW, Osmond CB. Photosynthetic consequences of specific chloroplast gene mutations affecting synthesis and function of the photosystem II D1 protein. In: Murata N, editor. Current Research in Photosynthesis. III. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 417–420. [Google Scholar]
- Husic DW, Tolbert NE. Inhibition of glycolate and d-lactate metabolism in a Chlamydomonas reinhardtii mutant deficient in mitochondrial respiration. Proc Natl Acad Sci USA. 1987;84:1555–1559. doi: 10.1073/pnas.84.6.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle KL. Expression of a gene for a light-harvesting chlorophyll a/b-binding protein in Chlamydomonas reinhardtii: effect of light and acetate. Plant Mol Biol. 1987;9:547–563. doi: 10.1007/BF00020532. [DOI] [PubMed] [Google Scholar]
- Lardans A, Förster B, Prásil O, Falkowski PG, Sobolev V, Edelman M, Osmond CB, Gillham NW, Boynton JE. Biophysical, biochemical and physiological characterization of Chlamydomonas reinhardtii mutants with amino acid substitutions at the Ala251 residue in the D1 protein that result in varying levels of photosynthetic competence. J Biol Chem. 1998;273:11082–11091. doi: 10.1074/jbc.273.18.11082. [DOI] [PubMed] [Google Scholar]
- Lers A, Heifetz PB, Boynton JE, Gillham NW, Osmond CB. The carboxyl-terminal extension of the D1 protein of photosystem II is not required for optimal photosynthetic performance under CO2- and light-saturated growth conditions. J Biol Chem. 1992;267:17494–17497. [PubMed] [Google Scholar]
- Maillard P, Deléens E, Daudet FA, Lacointe A, Frossard JS. Carbon and nitrogen partitioning in walnut seedlings during the acquisition of autotrophy through simultaneous 13CO2 and 15NO3 long-term labelling. J Exp Bot. 1994a;45:203–210. [Google Scholar]
- Maillard P, Deléens E, Daudet FA, Lacointe A, Frossard JS. Carbon economy in walnut seedlings during the acquisition of autotrophy studied by long-term labelling with 13CO2. Physiol Plant. 1994b;91:359–368. [Google Scholar]
- Martinez-Rívas JM, Vega JM. Effect of culture conditions on the isocitrate dehydrogenase and isocitrate lyase activities in Chlamydomonas reinhardtii. Physiol Plant. 1993;88:599–603. doi: 10.1111/j.1399-3054.1993.tb01377.x. [DOI] [PubMed] [Google Scholar]
- Neilson AH, Lewin RA. The uptake and utilization of organic carbon by algae: an essay in comparative biochemistry. Phycologia. 1974;13:227–264. [Google Scholar]
- Ohad I, Adir N, Koike H, Kyle DJ, Inoue Y. Mechanism of photoinhibition in vivo: a reversible light-induced conformational change of reaction center II is related to an irreversible modification of the D1 protein. J Biol Chem. 1990;265:1972–1979. [PubMed] [Google Scholar]
- O'Leary MH. Carbon isotopes in photosynthesis. Bioscience. 1988;38:328–336. [Google Scholar]
- Ribas-Carbo M, Berry JA, Yakir D, Giles L, Robinson SA, Lennon AM, Siedow JN. Electron partitioning between the cytochrome and alternative pathways in plant mitochondria. Plant Physiol. 1995;109:829–837. doi: 10.1104/pp.109.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix J-D, Goldschmidt-Clermout M, Merchant S. The Molecular Biology of Chloroplasts and Mitochoudria in Chlamydoueonas. Advances in Photosynthesis. Vol. 7. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. [Google Scholar]
- Sharkey TD, Berry JA. Carbon isotope fractionation of algae as influenced by an inducible CO2 concentrating mechanism. In: Lucas WJ, Berry JA, editors. Inorganic Carbon Uptake by Aquatic Photosynthetic Organisms. Rockville, MD: American Society of Plant Physiologists; 1985. pp. 389–401. [Google Scholar]
- Sheen J. Metabolic repression of transcription in higher plants. Plant Cell. 1990;2:1027–1038. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. Feedback control of gene expression. Photosynth Res. 1994;39:427–438. doi: 10.1007/BF00014596. [DOI] [PubMed] [Google Scholar]
- Spalding MH. CO2 acquisition. Acclimation to changing carbon availability. In: Rochaix J-D, Goldschmidt-Clermont M, Merchant S, editors. The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Advances in Photosynthesis. Vol. 7. Dordrecht, The Netherlands: Kluwer Academic Publications; 1998. pp. 528–547. [Google Scholar]
- Weger HG, Chadderton AR, Lin M, Guy RD, Turpin DH. Cytochrome and alternative pathway respiration during transient ammonium assimilation by N-limited Chlamydomonas reinhardtii. Plant Physiol. 1990a;94:1131–1136. doi: 10.1104/pp.94.3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weger HG, Guy RD, Turpin DH. Cytochrome and alternative pathway respiration in green algae. Plant Physiol. 1990b;93:356–360. doi: 10.1104/pp.93.1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman A, Gillham NW, Boynton JE. Nuclear mutations affecting mitochondrial structure and function in Chlamydomonas. J Cell Biol. 1977;73:56–77. doi: 10.1083/jcb.73.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S, Kalman-Rotem N, Yakir D, Ziv M. Autotrophic and heterotrophic carbon assimilation of in vitro grown potato (Solanum tuberosum L.) plants. J Plant Physiol. 1998;153:574–580. [Google Scholar]
- Yakir D, Giles L, Osmond CB. Autotrophy in maize husk leaves: evaluation using natural abundance of stable isotopes. Plant Physiol. 1991;97:1196–1198. doi: 10.1104/pp.97.3.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]