Abstract
We applied a recently developed tool to examine the reduction in climate risk to biodiversity in moving from a 2°C to a 1.5°C target. We then reviewed the recent literature examining the impact of (a) land-based mitigation options and (b) land-based greenhouse gas removal options on biodiversity. We show that holding warming to 1.5°C versus 2°C can significantly reduce the number of species facing a potential loss of 50% of their climatic range. Further, there would be an increase of 5.5–14% of the globe that could potentially act as climatic refugia for plants and animals, an area equivalent to the current global protected area network. Efforts to meet the 1.5°C target through mitigation could largely be consistent with biodiversity protection/enhancement. For impacts of land-based greenhouse gas removal technologies on biodiversity, some (e.g. soil carbon sequestration) could be neutral or positive, others (e.g. bioenergy with carbon capture and storage) are likely to lead to conflicts, while still others (e.g. afforestation/reforestation) are context-specific, when applied at scales necessary for meaningful greenhouse gas removal. Additional effort to meet the 1.5°C target presents some risks, particularly if inappropriately managed, but it also presents opportunities.
This article is part of the theme issue ‘The Paris Agreement: understanding the physical and social challenges for a warming world of 1.5°C above pre-industrial levels'.
Keywords: biodiversity, climate change targets, land, greenhouse gas removal
1. Introduction
Perhaps the most remarkable outcome of the Paris Climate Agreement was a renewed focus on the aim of limiting global average temperature increase to ‘well below 2°C above pre-industrial levels’, and ‘pursuing efforts’ to limit it to 1.5°C. This has stimulated a new focus on understanding the challenges of achieving this target, but also differential costs and benefits of such ambitious climate targets. It is clear that immediate and aggressive mitigation action in all sectors is necessary to meet the 2°C target, with even this target probably requiring some atmospheric greenhouse gas removal during this century [1–3]. For the 1.5°C target, additional atmospheric greenhouse gas removal is even more likely to be required, and in greater quantity, to supplement immediate and aggressive mitigation [3].
Biodiversity is known to be sensitive to a range of global change drivers [4], including climate [5], land-use change [6] and land management [7]. Widespread biodiversity loss would be expected to significantly reduce ecosystem services globally, because even small declines in species populations can negatively impact service provision [8], and such loss of function in turn threatens human well-being [9]. Climate impacts may have differential direct impacts on biodiversity at 1.5°C and 2°C, but striving to achieve the 1.5°C target might also have indirect impacts on biodiversity via changes in land use and management implemented to help reach the 1.5°C target.
In this paper, we examine both direct impacts and indirect impacts of striving to reach the 1.5°C target. We examine (§2) the difference in risks to biodiversity of a 1.5°C world, relative to a 2°C world, and (§3) impacts on biodiversity of land-based efforts to reach the 1.5°C target. In the final section, we discuss the relative importance of direct and indirect impacts of the 1.5°C target on biodiversity, and we highlight some exciting current developments in this field and propose areas for future research. Owing to limitations in the datasets available, and our focus on climate envelopes, we focus our analysis on land regions, though we know that one of the most important reductions of risk to biodiversity under a 1.5°C target may be found in the marine biosphere, in particular through reduced levels of ocean acidification rather than through climate. The benefits to terrestrial biodiversity discussed here build on the previously established benefits for ocean biodiversity, which are not further discussed in this paper.
2. Difference in impacts on biodiversity of a 1.5°C world relative to a 2°C world
In this section, we examine the difference in risks to biodiversity of a 1.5°C world relative to a 2°C world incurred as a result of direct climate change impacts. Climate change poses risks to biodiversity, globally, regionally and locally [10,11]. Changes in phenology [12,13], species' ranges [14,15], ecological interactions [16] and primary productivity [17] have already been observed, and many studies have attributed such changes (as a whole or in part) to anthropogenic climate change (see [18] for a synthesis). Species face a challenge in being able to track their preferred climate space across what is often an increasingly fragmented landscape [17], in terms of both the speed of movement required to adapt and obstacles to movement. Furthermore, some of these barriers to movement are likely to increase in the future (see §3). Many studies have examined potential future impacts of climate change on biodiversity using a variety of modelling techniques. This includes models showing the potential for losses of range exceeding 50% across large fractions of species globally or regionally due to climate change (e.g. among 50 000 species studied, 57 ± 5% of plants and 34 ± 7% of animals were projected to lose over half their climatic range for a warming of approximately 3.6°C above pre-industrial levels [19]). Other studies have looked specifically at the potential for increasing risks of extinction at higher warming levels. A meta-analysis suggested 20–30% of plant and animal species would be at risk of extinction if global mean temperatures exceeded a warming of 2–3°C above pre-industrial levels [14], with another study predicting 32–34% of Western Hemisphere birds with increased risks of extinction for warming of approximately 3°C above pre-industrial levels [20]. Traits-based analyses have also produced similarly large estimates of the proportions of coral, bird and amphibian species with increased extinction risks for warming of approximately 3°C above pre-industrial levels [21]. Even for mid-range warming of 1.8–2°C, studies have shown predictions of 15–37% of endemic species being ‘committed to extinction’, demonstrating the potential severity of even moderate levels of climate change [22].
All such studies are couched in uncertainties. There remains uncertainty and debate about the plasticity of species' physiologies and ability to maintain or newly construct biotic interactions under climate change, and the relationship between ‘commitment to extinction’ and actual extinction rates. Nevertheless, such exercises are valuable in highlighting regions and taxa of high relative risk and which may be foci of conservation or climate adaptation actions.
While many studies have specifically looked at climate impacts on biodiversity at higher temperatures, or over different future time periods (see [23] for a meta-analysis), few have specifically looked at the benefits of climate mitigation with respect to potential impacts on biodiversity [19,24]. The Wallace Initiative is a global effort to model the potential impacts of climate change on biodiversity, to identify areas of climatic refugia for biodiversity and to analyse the potential impacts on protected areas worldwide. The first analyses with these data specifically looked at the proportion of species that are projected to lose over 50% of their bioclimatic range (at a spatial resolution of approx. 50 km × 50 km) due to climate change alone, calculated separately for plants and animals, for seven climate models (CMIP3) and a range of emission scenarios [19]. In this study, limiting warming to 2°C as opposed to 4°C was found to reduce the proportion of animals losing over half their climatic range from 42% (±7%) to 12% (±3%), and plants from 57% (±6%) to 23% (±4%) of the 50 000 species studied globally.
It should be noted that projection of range shifts of species in the lowland tropics (the location with most present biodiversity and projected biodiversity loss) carries particularly high uncertainty. This is because climate warming carries many tropical regions into novel climates [25], i.e. warmer climates not currently experienced on Earth. Hence the upper temperature range of species ranges is defined by currently observed temperatures, rather than by observed geographical ranges, without clear biogeographical insight into whether species will be able to adapt to temperatures a few degrees warmer. This necessarily causes massive decline in diversity in the lowland humid tropics (e.g. Amazonia or Central Africa [26]). There is substantial uncertainty as to whether most tropical organisms will be able to cope with a few degrees of warming. On the one hand, many tropical species may be able to cope with slightly higher temperatures because of rapid adaptation or generational turnover, or because there is no biotic pressure from in-migrating species adapted to higher temperatures [27]. On the other hand, other strands of evidence suggest that tropical organisms may have physiologies uniquely sensitive to small net increases in temperature, because of unusually low seasonal and inter-annual variability in temperature [28]. Some taxa such as plants may be more plastic in their physiology than others such as arthropods. This scientific uncertainty means that predictions of large-scale loss of biodiversity in the tropics under moderate warming should be treated with caution. Despite this uncertainty, and with these caveats in mind, it is still useful for such biogeographical analyses to highlight potentially sensitive and resilient regions, and in particular to examine how risk shifts between different climate scenarios.
In this paper, we apply a similar tool to plant [24] to examine the question of how much reduction there is in climate risk to Warren et al. biodiversity in moving from a 2°C to a 1.5°C target, and then examine regional differences in the relative impacts. In so doing, we follow the procedure of Warren et al. [19] by, respectively, including or excluding an ability for species to disperse in order to track shifting climates across the Earth's surface at rates informed by the literature (for further detail see [19]).
(a). Global benefits to reducing potential impacts on biodiversity at 1.5°C versus 2°C
We reanalysed the original Wallace Initiative data [19] to look at the potential benefits of constraining global mean temperature rise to 1.5°C versus 2°C. While pathways to 1.5°C were not specifically examined for this project, the potential impacts on biodiversity at 1.5°C can be estimated from the time-series scenarios examined. The main difference from looking at specific pathways, versus the approach presented here, comes in the overall potential for species (primarily birds and mammals) to move to keep up (or catch up) with climate change and colonize new territories. This is dependent on not only the pathway to reaching 1.5°C (whether it is slow and gradual, quick with a long plateau, or an overshoot with a decline to 1.5°C) but also whether movement pathways are blocked by barriers, especially in the form of incompatible land uses. For this reason we are only presenting a preliminary estimate of the results for the no-dispersal scenario for plants, which disperse in general only slowly, in this paper.
While a temperature difference of 0.5°C may seem small, the potential global biodiversity risks avoided by constraining warming to 1.5°C rather than 2°C above pre-industrial levels may be significant. At 2°C the models project that around 23% (±4%) of plants would lose more than half of their climatic range, while at 1.5°C risks to plants could be reduced to an estimated 7–13%. Thus, risks from climate change to plants (in terms of loss of climatic range) are estimated to be significantly reduced at 1.5°C versus 2°C (estimated from Warren et al. [19], no-dispersal scenario). Similar reductions might be expected for animals. Further, there would be an increase of 5.5–14% of the globe that could potentially act as climatic refugia for plants and animals, which is an area equivalent to the current global protected area network. These benefits would be reduced or even negated if 1.5°C was reached in an overshoot scenario [3], depending on the length of time and temperature reached during the overshoot.
(b). Regional differences
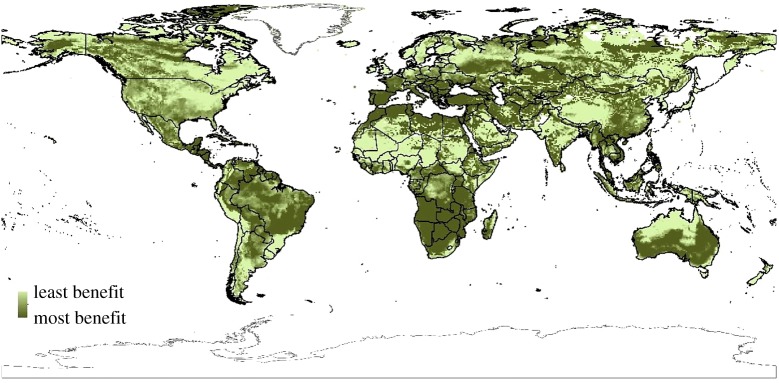
The benefits of mitigation to biodiversity conservation are not evenly distributed across the globe. An example of this, comparing 4°C versus 2.5°C, can be seen in Warren et al. [19], fig. 3. Furthermore, while constraining warming to 1.5° versus 2°C will have global benefits, some regions will continue to benefit more than others. Figure 1 shows regions with the greatest reduction in potential species richness loss for plants at 1.5°C versus 2°C.
Figure 1.
The areas showing the greatest benefit to maintaining plant species richness at 1.5°C versus 2°C. The darker the green, the greater the benefit in mean (out of seven models) species richness preserved. Areas that are lighter green show the same approximate numbers of species at 1.5°C and 2°C [19]. Full details of the methods used can be found in Warren et al. [19].
The approach described in this section provides an assessment of possible changes of moving from a 2°C to a 1.5°C target, but it should be noted that there are many metrics of local and regional change, and selecting different metrics can yield different outcomes. Garcia et al. [29], for example, compared six metrics of change at local (climate anomalies, climate extremes, and changes in seasonality) and regional (change in area of analogous climate, novel climates, and change in distance to analogous climates) level, and found that they provide different and complementary information. Despite the variety of different approaches available, and the presentation of only one such method in this section, the findings outlined here are indicative of potential changes, suggesting that further detailed study is required to assess fully the implications for biodiversity of moving from a 2°C to a 1.5°C target.
Having reviewed the risks to biodiversity of a 1.5°C world relative to a 2°C world incurred as a result of direct climate change impacts in this section, in §3 we examine the effects on biodiversity of land-based efforts to meet the 1.5°C target.
3. Impacts on biodiversity of land-based efforts to reach the 1.5°C target
In addition to the direct differential impacts upon biodiversity of 1.5°C versus 2°C warming described in §2, there are likely to also be indirect impacts, driven by efforts to achieve the 1.5°C target. These biodiversity impacts could be driven by land-use change or land-management change through, for example, changes in agricultural management aiming to deliver greenhouse gas mitigation [30], or by land-use change arising from implementation of land-based greenhouse gas removal (sometimes referred to as negative emissions) technologies [1,31,32]. In this section, we examine the evidence for potential impacts on biodiversity, of land-use and land-management change, driven by efforts to achieve the 1.5°C target through land-based options. While it is difficult to quantify in absolute terms the difference in impact between a 1.5°C versus a 2°C target, the higher level of mitigation or greenhouse gas removal ambition required to achieve 1.5°C would necessitate that such actions be applied more aggressively and more widely. We present the likely impacts of these actions on biodiversity, and assess the implications of their more aggressive/widespread application to meet a 1.5°C target.
(a). Impacts on biodiversity of additional climate mitigation in the agriculture, forestry and other land-use sector to reach the 1.5°C target
Climate mitigation in the agriculture, forestry and other land-use (AFOLU) sector is implemented via change in management of land, or via land-use change [33]. Land-use change options that provide mitigation benefits include the restoration of farmed organic peatlands, restoration of degraded land [34], afforestation (though we discuss this in §3b) [33], and the protection of large carbon stocks through, for example, wetland/peatland protection [35] or reduced deforestation and degradation [36]. Most of these land-use change activities are likely to provide improvements for biodiversity status [37], because they all involve de-intensification of land use and/or reversion to a state closer to the undisturbed ecosystem, which is a positive outcome for most biodiversity indicators. Proper planning, tied with the findings from §2, would actually provide adaptation benefits concomitant with mitigation. Most mitigation in the AFOLU sector, however, is realized through land-management change, i.e. the land use remains the same (e.g. cropland, grazing land, forest), but the management interventions are modified to reduce greenhouse gas emissions.
On croplands, mitigation practices include improved rotations with the greater use of cover crops and nitrogen-fixing crops, reducing the intensity of tillage, improved residue management, optimized fertilization (correct amount, placement and timing), the better use of organic amendments such as manure and straw, and optimized water management (particularly for rice). These are described and reviewed by Smith et al. [33,34]. Of these practices, reducing the use of mineral fertilizer could have beneficial impacts on some microbial components of biodiversity, with, for example, methane oxidizers known to be suppressed by large quantities of nitrogen fertilizer [38]. Over-fertilization (mineral or organic) is known to have significant off-site effects, particularly through the eutrophication of watercourses and water bodies [39], so optimization of fertilizer use as a climate mitigation measure is likely to have positive impacts on biodiversity locally, but also off-site regionally (within the same watershed/catchment [39]), and over larger scales, because atmospheric N deposition originating from agriculture adversely affects biodiversity across terrestrial biomes [40]. There may, however, be trade-offs between yield and biodiversity if fertilizer applications are reduced below plant requirements.
Improved, diversified rotations, including shorter periods where the ground is left bare, are also likely to improve soil biodiversity, because plant cover is provided all year round and carbon inputs will be higher [41]. The exact impact of the improved rotations will depend upon the crops included in the rotation and how they are grown (grown in sequence/under-sown, etc.), but relative to a rotation where the ground is bare for significant parts of the year, the biodiversity impact is expected to be positive. Given that plant cover is known to reduce soil loss through water and wind erosion [42], and that large deposits of eroded soil are known to increase turbidity [43] and disadvantage biodiverse watercourses [44] and coastal waters (e.g. [45]), improved rotations might also be expected to benefit biodiversity at the watershed/catchment scale or at coastal margins. Management systems emphasizing crop diversity can also reduce insect pests and provide refuge for natural enemies and pollinators, especially if mass-flowering crops are used in rotations [46].
As with improved rotations, reduced tillage intensity, particularly when combined with improved residue management (leaving crop residues on the field), is likely to reduce erosion [47], so the local and watershed/catchment-scale benefits will be similar. Reduced tillage can also increase soil organic matter, which increases soil quality through improved structure and increases soil microbial activity, which is negatively influenced by soil tillage [48]. Additional local soil biodiversity benefits might also be realized through greater number and diversity of earthworms and mesofauna, which are known to be more prevalent under reduced tillage systems compared to conventionally tilled soils [49]. One indirect impact might arise from reduced tillage if additional herbicide is required to reduce weeds as a result of reduced mechanical weeding by ploughing [50], which would be likely to have a negative impact on plant and insect biodiversity.
On grazing lands, mitigation practices include manipulation of grazing intensity, optimized fertilization (correct amount, placement and timing), the better use of organic amendments such as manure, fire management, use of deeper-rooting species and increased legume shares in the sward (reviewed in [33,34]). Reduction of over-fertilization on grazing lands (and replacing external nitrogen inputs by increasing legume shares) will have similar beneficial impacts as for croplands described above, providing both local and watershed/catchment-scale benefits for biodiversity. Rotational grazing can also more effectively imitate natural feeding patterns of migratory herbivores, resulting in increased plant biodiversity and improved soil quality [51]. The relationship between grazing intensity and biodiversity is complex, but overgrazed systems lose biodiversity relative to optimally grazed grasslands [52], and for some grasslands, highly valued biodiversity is maintained by moderate grazing [52]. If grazing intensity is optimized, it is therefore likely to improve biodiversity relative to un-optimized practice. Overgrazing can also lead to bare soil, which will increase erosion risk [53]—so impacts on reduced erosion through optimal grazing will be similar to those described for croplands above. Deeper-rooting species might also reduce erosion risk and the concomitant impacts on local and watershed/catchment-scale biodiversity. The impact of fire management on biodiversity is also complex. In some systems, frequent fire prevents woody encroachment and maintains biodiverse grassland ecosystems [54], though there is also evidence that fire suppression can increase fuel load, so that when fire does occur, the fire burns more intensely (though see [55]). This can have a negative impact on biodiversity by changing the community structure, increasing the risk of extinction. Furthermore, increasing levels of CO2 are leading to woody plants in grasslands becoming more fire-resistant. Burning can also accelerate soil erosion [53], with the negative biodiversity consequences noted above.
Forestry measures for climate mitigation include reduced deforestation and degradation, and improved forest management. Reduced deforestation and degradation are likely to benefit biodiversity in that they preserve habitat in a condition closer to natural [36,56]. The potential benefits of reduced deforestation and degradation to biodiversity have been mapped, although these findings do not take into account any changes due to climate change [57]. Improved forest management includes practices such as reduced impact logging [58]. Such practices are also likely to have less impact on biodiversity than clear-fell [59], and also leave more necromass in the forest, which is important for forestry food webs and biogeochemical cycling.
In addition to the practice-specific impacts described above, there are also systemic changes to food/fibre production that have been proposed as climate mitigation measures. These include sustainable intensification [60,61], whereby environmental impact is reduced relative to the unit of product. In greenhouse gas terms, this is expressed through decreased emission intensity (GHG emitted per unit of product) [33]. By targeting areas of low efficiency, environmental impacts of intensive agriculture could be limited and the need for agricultural expansion would be reduced [62]. For this intensification to be sustainable, by definition, it must have no adverse effects on biodiversity. Sustainable intensification, then, would at worst be neutral in terms of biodiversity on the land on which it is practised, and could potentially spare land for other uses if food production can be concentrated on a smaller land area [63–66]. Closing yield gaps through sustainable intensification would decrease global cropland and grassland areas required for food production by 37% and 10%, respectively [66], relative to current yield trends without yield gap closure, while adding an estimated 2.3 billion tonnes of new production—a 58% increase [62]. However, closing the yield gap without environmental degradation will require new approaches, including the reform of conventional agriculture, adopting lessons from organic systems and precision agriculture as well as overcoming challenges such as distribution of inputs and seed varieties [62]. Other systemic changes in the food system with considerable mitigation potential are dietary changes (largely via reduction in livestock product consumption [67]) and waste reduction [66]. Both of these demand-side measures also reduce the pressure on land [30,68,69]. Combining dietary change (to healthy global diets) and waste reduction by 50% could reduce global cropland and grassland areas by 17% and 36%, respectively, compared to yield gap closure alone [68]. This creates the opportunity for land sparing [66], potentially providing considerable benefits for wildlife conservation and biodiversity [66], though there could be rebound effects, as the land spared does not necessarily match with priority areas for biodiversity conservation.
For all of the measures discussed in this section, it is possible that all might be used in efforts to meet the 2°C target, which could mean little difference to biodiversity. However, given the known challenges of meeting a 1.5°C target, we might expect efforts to reach 1.5°C to lead to them being applied to their maximum extent and over large geographical areas, with the impacts on biodiversity discussed in this section thereby accentuated. Table 1 summarizes the likely biodiversity impact associated with more aggressive/widespread implementation of the mitigation measures discussed in this section.
Table 1.
Summary of the likely biodiversity impacts associated with more aggressive/widespread implementation of land-based climate change mitigation measures.
| mitigation type | mitigation measure | impact on processes affecting biodiversity | local biodiversity impact | catchment-scale biodiversity impact | regional/global biodiversity impact |
|---|---|---|---|---|---|
| land-use change | restoration of farmed organic peatlands | peatland restoration/rewetting | + | + | + |
| land-use change | restoration of degraded land | soil/vegetation cover restored | + | + | + |
| land-use change | protection of large carbon stocks (peatlands/reduced deforestation and degradation) | peatland/forest protection | + | + | + |
| cropland management | improved rotations | reduced soil erosion risk—reduced turbidity | + | + | |
| cropland management | reduced tillage intensity | reduced impact on earthworms and mesofauna; requirement for additional herbicides; reduced soil erosion risk—reduced turbidity | +/− | +/− | +/− |
| cropland management | improved residue management | reduced soil erosion risk—reduced turbidity | + | + | |
| cropland management | optimized fertilization | reduced adverse impact on soil microbiota; reduced nutrient loss reducing eutrophication; reduced N deposition | + | + | + |
| cropland management | better use of organic amendments | reduced nutrient loss reducing eutrophication | + | + | |
| grazing land management | manipulation of grazing intensity | reducing overgrazing; greater plant production and less bare soil; reduced soil erosion risk—reduced turbidity | + | + | |
| grazing land management | optimized fertilization | reduced adverse impact on soil microbiota; reduced nutrient loss reducing eutrophication | + | + | + |
| grazing land management | better use of organic amendments | reduced nutrient loss reducing eutrophication | + | + | |
| grazing land management | fire management | prevention of woody encroachment; fewer but more intense fires | +/− | +/− | +/− |
| grazing land management | deeper-rooting species | reduced soil erosion risk—reduced turbidity | + | + | |
| forest management | REDD+ | reduced deforestation and degradation | + | + | + |
| forest management | selective/low-impact logging | less impact than clear-fell, more necromass; reduced erosion losses—reduced turbidity | + | + | + |
| food system change | sustainable intensification | concentrated production on smaller land area | +/− | +/− | + |
| food system change | dietary change | fewer livestock products in the diet—more efficient production – less pressure on land—land sparing | + | + | + |
| food system change | food waste reduction | less pressure on land—land sparing | + | + | + |
With a few exceptions, the majority of mitigation measures summarized in table 1 confer biodiversity benefits, either locally, at catchment scale or globally. Global impacts arise because local impacts are spread over large areas when there is widespread application of practices (e.g. by improved cropland or grassland management over the 40–50% of the global land surface used for agriculture), or because the impacts of the mitigation measure can occur in distant locations (e.g. eutrophication of distant water bodies or N deposition from changed fertilizer management [40]). The implementation of these measures in an attempt to meet the 1.5°C target would, therefore, largely be consistent with biodiversity protection, though governance will be critical to achieving mitigation and biodiversity conservation goals [36,70].
(b). Impacts on biodiversity of implementation of land-based greenhouse gas removal technologies to reach the 1.5°C target
Terrestrial greenhouse gas removal technologies [1,2,32] include: (1) bioenergy with carbon capture and storage (BECCS [71]); (2) direct air capture of CO2 from ambient air by engineered chemical reactions [72,73]; (3) enhanced weathering of minerals [74–76], where natural weathering to remove CO2 from the atmosphere is accelerated, and the products are stored in soils, or buried in land/deep ocean; (4) afforestation and reforestation [70,77,78] to fix atmospheric carbon in biomass and soils; (5) soil carbon sequestration through changed agricultural practices (which include activities such as less invasive tillage with residue management, organic amendment, improved rotations/deeper-rooting cultivars, optimized stocking density, fire management, optimized nutrient management and restoration of degraded lands) [32,34]; and (6) converting biomass to recalcitrant biochar, for use as a soil amendment [32,79]. There will be an interaction between the use of greenhouse gas removal technologies and the direct impacts described in §2, because greenhouse gas emissions are used to manage an overshoot of emissions/temperature increase. Therefore, scenarios that employ greenhouse gas removal to manage overshoot are likely to result in additional direct impacts on biodiversity compared to those that do not result in overshoot, because temperature will be higher than 1.5°C for a period of time [3]. These issues will be examined in the forthcoming Intergovernmental Panel on Climate Change Special Report on meeting the 1.5°C target.
Of these options, some have an obvious land footprint and, when implemented, prevent the land being used for other purposes (e.g. afforestation and reforestation, BECCS, the mineral mining components of enhanced weathering), while others use land but do not prevent it from being used for its current purpose (e.g. soil carbon sequestration, biochar, the land spreading component of enhanced weathering), and still others may have an indirect footprint (a direct capture plant has a small land footprint, but if powered by renewable energy such as wind or solar, the indirect land footprint could be very large [80]). Table 2 presents the land footprint of greenhouse gas removal technologies, expressed per tonne of CO2 carbon removed from the atmosphere, with notes on whether or not the land can be used for other purposes when the technology/practice is implemented on it [1,32,81].
Table 2.
Land footprint of greenhouse gas removal technologies, expressed per tonne of CO2 carbon removed from the atmosphere (data from [1,32,79] and references therein).
| GHG removal rate per unit land |
land area per unit of GHG removal |
||||
|---|---|---|---|---|---|
| low | high | low | high | ||
| technology | t-Ceq./ha | t-Ceq./ha | ha/t-Ceq. | ha/t-Ceq. | current land use still possible? |
| BECCS | 3 | 12 | 0.1 | 0.4 | no |
| afforestation/reforestation | 3.4 | 3.4 | 0.1 | 0.6 | no |
| soil carbon sequestration | 0.03 | 1 | 1 | 33 | yes |
| biochar | 1.15 | 7.5 | 0.13 | 0.87 | yes |
| direct air capture | 1818 | 1818 | 0.001 | 0.001 | no |
| enhanced mineral weathering | 0.82 | 10.91 | 1.22 | 0.09 | no/yesa |
aNo for mineral mining sites; yes for land upon which the ground rock is spread.
The land footprint of direct air capture technologies is small, so the direct impact on biodiversity is also likely to be small. The land area required for mining minerals for enhanced weathering is small globally, but mining has devastating local biodiversity impacts where it occurs due to habitat destruction unless ameliorated, though site restoration is possible after mining ceases [82]. For land upon which the crushed/ground mineral is spread, current activity on the land could continue if rates are relatively low. When applied at low rates of 10 t rock ha−1 yr−1 [76], agricultural/forestry use of the land could continue, though these rates are still higher than those regularly used in agriculture for liming [83] and could have negative impacts on biodiversity. However, high application rates of 50 t rock ha−1 yr−1 [76] are incompatible with agriculture, so only low rates would not lead to pressure on land, and thereby minimize biodiversity impacts. Soil carbon sequestration is a headline indicator for soil quality [84] and soil health [85], and hence is likely to support local biodiversity. If soil carbon sequestration is used to help restore degraded lands, global biodiversity benefits could follow. Biochar could have negative impacts on soil biota if contaminated, but when created from clean feedstock, it is generally regarded as beneficial to soil microbiota by providing microsites for colonization [86]. Soil carbon sequestration and biochar addition, when practised sustainably, would, therefore, have neutral to positive impacts on biodiversity.
BECCS and afforestation/reforestation are the greenhouse gas removal technologies that occupy land so that it cannot be used for other purposes, though some forms of afforestation, e.g. agroforestry, still allow agricultural production [87]. Vast land areas could be used for greenhouse gas removal if greenhouse gas removal on the scale of approximately 10 Gt CO2 is necessary [1]. Such levels of implementation would require land areas of 380–700 Mha for BECCS and 970 Mha for afforestation/reforestation [1]. To put these figures in context, the total agricultural land area in 2000 was approximately 4960 Mha, with an area of arable and permanent crops of approximately 1520 Mha [88], so the area for BECCS represents 7–25% of agricultural land, and 25–46% of arable plus permanent crop area. Afforestation at a level to deliver equivalent greenhouse gas removal (970 Mha) represents 20% of the total agricultural land, and 64% of arable plus permanent crop area [1]. For both BECCS and afforestation/reforestation, then, the land footprint, and therefore the biodiversity impact, could be very large—unless afforestation is implemented in a way that supports biodiversity [89].
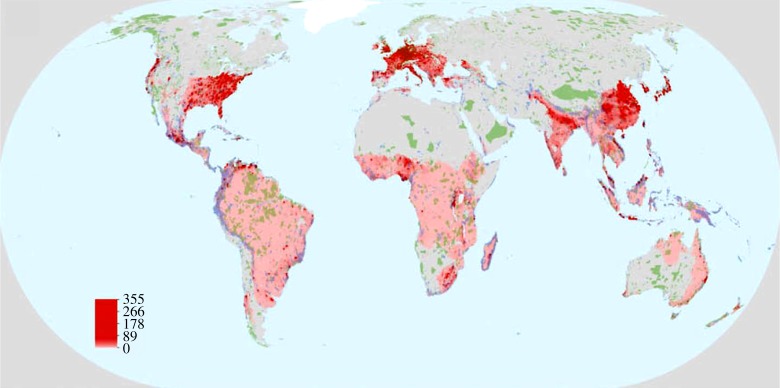
For BECCS, though some bioenergy crops have been shown to provide biodiversity benefits when grown on former cropland [90], very large-scale deployment is often suggested to be associated with potential biodiversity loss [91], either directly, or indirectly by displacing food production to other areas where it replaces natural ecosystems [92]. Santangeli et al. [93] recently examined the potential conflicts between renewable energy production and biodiversity by examining overlap between energy production potential and current and future protected areas, with bioenergy showing the largest potential conflicts with biodiversity (e.g. figure 2). If biomass is extracted from existing forests for BECCS, fears have been expressed that this could lead to forest degradation [94], with concomitant loss of biodiversity. The areas identified as having the highest potential BECCS, in terms of biomass production potential from dedicated energy crops, in figure 2 (showing Miscanthus as the example bioenergy crop) can be compared with the areas reserved for biodiversity benefits in figure 1. The overlap and potential impacts on biodiversity are high for plants in the United States, Central America, West and South Africa, United Kingdom, India and Indonesia. For animals, the degree of overlap between refugia at 2°C and power generation potential is high, with most areas that have been identified as having high power generation potential also being climatic refugia for most animals. Thus, land-use changes on the order of that required by BECCS to meet a 1.5°C target (380–700 Mha [1]) would have potentially significant impacts on biodiversity.
Figure 2.
Overlap between power generation potential (GJ ha−1 yr−1; red colour gradient; see legend) for bioenergy (here represented by Miscanthus × giganteus as simulated by the MiscanFor model), constrained by energy demand, costs and carbon, overlaid with current protected areas (green shading) and global top 17% areas for protected area expansion (blue shading). Areas with no power generation potential are in grey. For bioenergy, no data were available for Greenland. (Reproduced with permission from Pedroli et al. [91].) Full details of the methods used can be found in Pedroli et al. [91].
For afforestation/reforestation, biodiversity impacts will depend upon how afforestation occurs (species planted, method of establishment) and the previous use of the afforested land. Although, in most cases, afforestation would benefit biodiversity, in some cases it could have negative impacts on native flora and fauna; for example, if based on tree plantations, naturally open habitats would be replaced by low-biodiversity ecosystems with a high water uptake [95]. Biodiversity is known to be lower in production forestry relative to natural forest, but can be managed [96]. If monoculture production forestry is used to maximize carbon dioxide removal, biodiversity could suffer, but if biodiverse mixed forestry is used, biodiversity could be enhanced by afforestation/reforestation. There is likely to be a trade-off between maximizing greenhouse gas removals and providing a wider range of ecosystem services, including biodiversity [96]. Previous land use also plays a role; the widespread planting of production forestry on UK peatlands during the 1940s–1980s is widely regarded as a conservation disaster [97], and planting on deep peats has since been discontinued [98]. Planting of biodiverse mixed woodland on degraded former cropland, however, is likely to enhance biodiversity, can even be inserted in agricultural landscapes [99] and might act as an adaptation in providing connectivity between habitats to potentially allow some taxa to shift with climatic changes. The impacts of afforestation/reforestation on biodiversity is therefore quite context-specific, but could usually be implemented in a way that enhances biodiversity [89].
At the planetary level, greenhouse gas removal technologies could have other indirect effects. If the concentration of atmospheric carbon dioxide is reduced, CO2 could be released from the oceans [100]. As this CO2 in the ocean is driving ocean acidification, with damaging impacts on biodiversity [101], ocean outgassing of CO2 could relieve some ocean acidification, providing an indirect benefit for ocean biodiversity. Table 3 summarizes the likely biodiversity impacts of widespread implementation of greenhouse gas removal technologies.
Table 3.
Summary of the likely biodiversity impacts of widespread implementation of land-based greenhouse gas removal technologies.
| technology type | greenhouse gas removal technology | impact on processes affecting biodiversity | local biodiversity impact | catchment- scale biodiversity impact | regional/ global biodiversity impact |
|---|---|---|---|---|---|
| greenhouse gas removal | direct air capture | land footprint | 0 | 0 | 0 |
| greenhouse gas removal | enhanced mineral weathering | local impact of mining mineral/impact of spreading on land | −/0 | −/0 | 0 |
| greenhouse gas removal | soil carbon sequestration | enhancing soil organic matter stocks | +/0 | +/0 | +/0 |
| greenhouse gas removal | biochar | providing microsites for soil microbiota | + | + | + |
| greenhouse gas removal | BECCS | large land footprint; direct and indirect effects | − | − | − |
| greenhouse gas removal | afforestation/ reforestation | large land footprint; direct and indirect effects | +/− | +/− | +/− |
| Earth system feedback | outgassing of CO2 from the ocean | de-acidification of the oceans | +/0 | n.a. | + |
While most mitigation measures listed in §3a tend to have positive local biodiversity impacts, greenhouse gas removal technologies are more mixed. BECCS will almost certainly lead to biodiversity conflicts if implemented at scales necessary to achieve greenhouse gas removals of approximately 10 Gt CO2, which would require 380–700 Mha of land [1]. In terms of impacts on biodiversity, soil carbon sequestration is neutral to positive, direct air capture neutral, and indirect effects of all greenhouse gas removal on ocean biodiversity might be positive; but for the others, evidence is mixed or, more often, the outcome is context-specific. For enhanced weathering, effects of spreading minerals are neutral, but mining will have an adverse local biodiversity impact. Afforestation/reforestation will depend on what tree species/mix are planted and what land use the new woodland replaces, but there are usually options to benefit biodiversity by woodland establishment [89]. In terms of mitigation–adaptation interactions, improving soil carbon sequestration and afforestation/reforestation are likely to show significant co-benefits, while BECCS, if implemented at scale, would probably yield some trade-offs [1,33].
4. Conclusion
Holding warming to 1.5°C versus 2°C can reduce significantly, the number of species facing a potential loss of 50% of their climatic range. Furthermore, there would be an increase of between 5.5% and 14% of the amount of the globe that could potentially act as climatic refugia for plants and animals. This is the equivalent, in terms of km2, to the current global protected area network [19]. From the additional analysis here, we further conclude that efforts to meet the 1.5°C target through mitigation efforts in the land sector would largely be consistent with biodiversity protection/enhancement depending on the mitigation approach used. Additional effort to meet the 1.5°C target using some greenhouse gas removal technologies (e.g. soil carbon sequestration) would be neutral or positive, whereas others are likely to lead to biodiversity conflicts (e.g. BECCS) when applied at scales necessary for meaningful greenhouse gas removal. However, if greenhouse gas removal technologies are used to manage an overshoot of emissions/temperature increase, there could be additional direct impacts on biodiversity compared to those that do not result in overshoot [3], because temperature will be higher than 1.5°C for a period of time, so scenarios that avoid overshoot would lead to fewer adverse impacts than those that result in overshoot. Many of the areas previously identified as showing high potential for some bioenergy crops directly overlap many of the areas of climatic refugia for many biodiversity taxa. Other land-based greenhouse gas removal options, such as afforestation/reforestation, are context-specific, but there is enough knowledge to implement these options in a manner that protects or enhances biodiversity, potentially offering adaptation benefits. Additional effort to meet the 1.5°C target, therefore, does present some risks, particularly if inappropriately managed, but it also presents some opportunities. More spatially explicit research is needed to look at the overlap between bioenergy, food security and biodiversity conservation.
Article 2 of the United Nations Framework Convention on Climate Change (UNFCCC) states that greenhouse gas concentrations be stabilized at … ‘a level that would prevent dangerous anthropogenic (human induced) interference with the climate system’ and ‘Such a level should be achieved within a time frame sufficient to allow ecosystems to adapt naturally to climate change, to ensure that food production is not threatened and to enable economic development to proceed in a sustainable manner’. This will require careful planning and cooperation that simultaneously deals with mitigation, energy, food, biodiversity and sustainable development—a point recognized in the spread of the Sustainable Development Goals. Thus, efforts must be taken to ensure that mitigation of greenhouse gas emissions is Article 2-compliant.
Acknowledgements
We thank Jason Lowe and Dan Bernie at the UK MetOffice, who produced the climate change data used in the biodiversity analysis in §2.
Data accessibility
Wallace Initiative data can be accessed at http://Wallaceinitiative.org.
Authors' contributions
P.S. coordinated contributions and led analysis and writing of §3, with contributions from A.M. J.P. led analysis and writing of §2, with contributions from R.W. and Y.M. All the authors (P.S., J.P., A.M., R.W., Y.M.) contributed to the design, drafting and revision of the article, and have given their final approval of the version to be published.
Competing interests
We declare we have no competing interests.
Funding
The input of P.S. contributes to the following projects: DEVIL [NE/M021327/1], MAGLUE [EP/M013200/1], U-GRASS [NE/M016900/1], Assess-BECCS [funded by UKERC] and Soils-R-GRREAT [NE/P019455/1]. A.M. is supported by a BBSRC EastBio studentship. J.P. was supported by the EU FP7 HELIX project (grant agreement number 603864). Climate data used in the biodiversity analysis were produced during the Avoiding Dangerous Climate Change project funded by the former UK Department of Energy and Climate Change.
References
- 1.Smith P, et al. 2016. Biophysical and economic limits to negative CO2 emissions. Nat. Clim. Change 6, 42–50. ( 10.1038/nclimate2870) [DOI] [Google Scholar]
- 2.Fuss S, et al. 2016. Research priorities for negative emissions. Environ. Res. Lett. 11, 115007 ( 10.1088/1748-9326/11/11/115007) [DOI] [Google Scholar]
- 3.Obersteiner M, et al. 2018. How to spend a dwindling greenhouse gas budget. Nat. Clim. Change 8, 7–10. ( 10.1038/s41558-017-0045-1) [DOI] [Google Scholar]
- 4.Sala OE, et al. 2000. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774. ( 10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 5.Field CB, et al. (eds) 2014. Summary for policymakers. In Climate change 2014: impacts, adaptation, and vulnerability. Part A: Global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, pp. 1–32. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Souza DM, Teixeira RFM, Ostermann OP. 2015. Assessing biodiversity loss due to land use with life cycle assessment: are we there yet? Glob. Change Biol. 21, 32–47. ( 10.1111/gcb.12709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polasky S, et al. 2008. Where to put things? Spatial land management to sustain biodiversity and economic returns. Biol. Conserv. 141, 1505–1524. ( 10.1016/j.biocon.2008.03.022) [DOI] [Google Scholar]
- 8.Gaston KJ, Fuller RA. 2008. Commonness, population depletion and conservation biology. Trends Ecol. Evol. 23, 14–19. ( 10.1016/j.tree.2007.11.001) [DOI] [PubMed] [Google Scholar]
- 9.Díaz S, Fargione J, Chapin FS III, Tilman D. 2006. Biodiversity loss threatens human well-being. PLoS Biol. 4, e277 ( 10.1371/journal.pbio.0040277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oppenheimer M, et al. 2014. Emergent risks and key vulnerabilities. In Climate change 2014: impacts, adaptation, and vulnerability. Part A: Global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Field CB. et al), pp. 1039–1099. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 11.O'Neill BC, et al. 2017. Key risks of climate change: the IPCC reasons for concern. Nat. Clim. Change 7, 28–37. ( 10.1038/nclimate3179) [DOI] [Google Scholar]
- 12.Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. 2003. Fingerprints of global warming on wild animals and plants. Nature 42, 57–60. ( 10.1038/nature01333) [DOI] [PubMed] [Google Scholar]
- 13.Parmesan C. 2007. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob. Change Biol. 13, 1860–1872. ( 10.1111/j.1365-2486.2007.01404.x) [DOI] [Google Scholar]
- 14.Fischlin A, et al. 2007. Ecosystems, their properties, goods and services. In Climate change 2007: impacts, adaptation and vulnerability (eds Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE), pp. 211–272. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 15.Chen I-C, Hill JK, Ohlemuller R, Roy DB, Thomas CD. 2001. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026. ( 10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 16.Post E. 2013. Ecology of climate change: the importance of biotic interactions. Princeton, NJ: Princeton University Press. [Google Scholar]
- 17.Settele J, et al. 2014. Terrestrial and inland water systems. In Climate change 2014: impacts, adaptation, and vulnerability. Part A: Global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Field CB. et al), pp. 271–359. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 18.Cramer W, et al. 2014. Detection and attribution of observed impacts. In Climate change 2014: impacts, adaptation, and vulnerability. Part A: Global and sectoral aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Field CB, et al.), pp. 979–1037. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 19.Warren R, et al. 2013. Quantifying the benefit of early mitigation in avoiding biodiversity loss. Nat. Clim. Change 3, 678–682. ( 10.1038/nclimate1887) [DOI] [Google Scholar]
- 20.Sekercioglu CH, Schneider SH, Fay JP, Loarie SR. 2008. Climate change, elevational range shifts, and bird extinctions. Conserv. Biol. 22, 140–150. ( 10.1111/j.1523-1739.2007.00852.x) [DOI] [PubMed] [Google Scholar]
- 21.Foden WB, et al. 2013. Identifying the world's most climate change vulnerable species: a systematic trait-based assessment of all birds, amphibians and corals. PLoS ONE 8, e65427 ( 10.1371/journal.pone.0065427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas CD, et al. 2004. Extinction risk from climate change. Nature 427, 145–148. ( 10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 23.Urban M. 2015. Accelerating extinction risk from climate change. Science 348, 571–573. ( 10.1126/science.aaa4984) [DOI] [PubMed] [Google Scholar]
- 24.Warren R, Price J, VanDerWal J, Cornelius S, Sohl H. In press. The implications of the United Nations Paris Agreement on Climate Change for key biodiversity areas. Clim. Change.
- 25.Williams JW, Jackson ST. 2007. Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Environ. 5, 475–482. ( 10.1890/070037) [DOI] [Google Scholar]
- 26.Feeley KJ, Silman MR. 2010. Biotic attrition from tropical forests correcting for truncated temperature niches. Glob. Change Biol. 16, 1830–1836. ( 10.1111/j.1365-2486.2009.02085.x). [DOI] [Google Scholar]
- 27.Malhi Y. 2012. The productivity, metabolism and carbon cycle of tropical forest vegetation. J. Ecol. 100, 65–75. ( 10.1111/j.1365-2745.2011.01916.x). [DOI] [Google Scholar]
- 28.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia RA, Cabeza M, Rahbek C, Araújo MB. 2014. Multiple dimensions of climate change and their implications for biodiversity. Science 344, 1247579 ( 10.1126/science.1247579). [DOI] [PubMed] [Google Scholar]
- 30.Smith P, et al. 2013. How much land based greenhouse gas mitigation can be achieved without compromising food security and environmental goals? Glob. Change Biol. 19, 2285–2302. ( 10.1111/gcb.12160) [DOI] [PubMed] [Google Scholar]
- 31.Popp A, et al. 2014. Land-use transition for bioenergy and climate stabilization: model comparison of drivers, impacts and interactions with other land use based mitigation options. Clim. Change 123, 495–509. ( 10.1007/s10584-013-0926-x) [DOI] [Google Scholar]
- 32.Smith P. 2016. Soil carbon sequestration and biochar as negative emission technologies. Glob. Change Biol. 22, 1315–1324. ( 10.1111/gcb.13178) [DOI] [PubMed] [Google Scholar]
- 33.Smith P, et al. 2014. Agriculture, forestry and other land use (AFOLU). In Climate change 2014: mitigation of climate change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Edenhofer O, et al.), pp. 811–922 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 34.Smith P, et al. 2008. Greenhouse gas mitigation in agriculture. Phil. Trans. R. Soc. B 363, 789–813. ( 10.1098/rstb.2007.2184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bain CG, et al. 2011. IUCN UK Commission of Inquiry on peatlands. Edinburgh, Scotland: IUCN UK Peatland Programme. [Google Scholar]
- 36.Bustamante M, et al. 2014. Co-benefits, trade-offs, barriers and policies for greenhouse gas mitigation in the agriculture, forestry and other land use (AFOLU) sector. Glob. Change Biol. 20, 3270–3290. ( 10.1111/gcb.12591) [DOI] [PubMed] [Google Scholar]
- 37.Smith P, et al. 2007. Policy and technological constraints to implementation of greenhouse gas mitigation options in agriculture. Agric. Ecosyst. Environ. 118, 6–28. ( 10.1016/j.agee.2006.06.006) [DOI] [Google Scholar]
- 38.Hütsch BW, Webster CP, Powlson DS. 1993. Long-term effects of nitrogen fertilization on methane oxidation in soil of the broadbalk wheat experiment. Soil Biol. Biochem. 25, 1307–1315. ( 10.1016/0038-0717(93)90045-D) [DOI] [Google Scholar]
- 39.Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, Lancelot C, Likens GE. 2009. Controlling eutrophication: nitrogen and phosphorus. Science 323, 1014–1015. ( 10.1126/science.1167755) [DOI] [PubMed] [Google Scholar]
- 40.Bobbink R, et al. 2010. Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol. Appl. 20, 30–59. ( 10.1890/08-1140.1) [DOI] [PubMed] [Google Scholar]
- 41.Maeder P, Fliessbach A, Dubois D, Gunst L, Fried P, Niggli U. 2002. Soil fertility and biodiversity in organic farming. Science 296, 1694–1697. ( 10.1126/science.1071148) [DOI] [PubMed] [Google Scholar]
- 42.Durán Zuazo VH, Rodríguez Pleguezuelo CR. 2008. Soil-erosion and runoff prevention by plant covers. A review . Agron. Sustain. Dev. 28, 65–86. ( 10.1051/agro:2007062) [DOI] [Google Scholar]
- 43.Clark EH. 1985. The offsite costs of soil erosion. J. Soil Water Conserv. 40, 19–22. [Google Scholar]
- 44.Newcombe CP, Macdonald DD. 1991. Effects of suspended sediments on aquatic ecosystems. North Am. J. Fish Manage. 11, 72–82. ( 10.1577/1548-8675(1991)011%3C0072:EOSSOA%3E2.3.CO;2) [DOI] [Google Scholar]
- 45.Fabricius KE, De'ath G. 2001. Biodiversity on the Great Barrier Reef: large-scale patterns and turbidity-related total loss of soft coral taxa. In Oceanographic processes of coral reefs: physical and biological links in the Great Barrier Reef (ed. Wolanski E.), pp. 127--144 Boca Raton, FL: CRC Press. [Google Scholar]
- 46.Power AG. 2010. Ecosystem services and agriculture: tradeoffs and synergies. Phil. Trans. R. Soc. B 365, 2959–2971. ( 10.1098/rstb.2010.0143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langdale GW, West LT, Bruce RR, Miller WP, Thomas AW. 1992. Restoration of eroded soil with conservation tillage. Soil Technol. 5, 81–90. ( 10.1016/0933-3630(92)90009-P) [DOI] [Google Scholar]
- 48.Ferreira MC, Andrade DDS, Chueire LMDO, Takemura SM, Hungria M. 2000. Tillage method and crop rotation effects on the population sizes and diversity of bradyrhizobia nodulating soybean. Soil Biol. Biochem. 32, 627–637. ( 10.1016/S0038-0717(99)00189-3) [DOI] [Google Scholar]
- 49.Kladivko EJ. 2001. Tillage systems and soil ecology. Soil Tillage Res. 61, 61–76. ( 10.1016/S0167-1987(01)00179-9) [DOI] [Google Scholar]
- 50.Witt WW. 1984. Response of weeds and herbicides under no-tillage conditions. In No-tillage agriculture: principles and practices (eds Phillips RE, Phillips SH), pp. 152--170 New York, NY: Van Nostrand Reinhold. [Google Scholar]
- 51.Machovina B, Feeley KJ, Ripple WJ. 2015. Biodiversity conservation: the key is reducing meat consumption. Sci. Total Environ. 536, 419–431. ( 10.1016/j.scitotenv.2015.07.022) [DOI] [PubMed] [Google Scholar]
- 52.Matera E, Sakowski T, Słoniewski K, Romanowicz B. 2010. Grazing as a tool to maintain biodiversity of grassland—a review. Anim. Sci. Papers Rep. 28, 315–334. [Google Scholar]
- 53.Evans R. 1990. Soils at risk of accelerated erosion in England and Wales. Soil Use Manage. 6, 125–131. ( 10.1111/j.1475-2743.1990.tb00821.x) [DOI] [Google Scholar]
- 54.Heisler JL, Briggs JM, Knapp AK. 2002. Long-term patterns of shrub expansion in a C4-dominated grassland: fire frequency and the dynamics of shrub cover and abundance. Am. J. Bot. 90, 423–428. ( 10.3732/ajb.90.3.423) [DOI] [PubMed] [Google Scholar]
- 55.Keeley JE, Fotheringham CJ, Morais M. 1999. Reexamining fire suppression impacts on brushland fire regimes. Science 284, 1829–1832. ( 10.1126/science.284.5421.1829) [DOI] [PubMed] [Google Scholar]
- 56.Harvey CA, Dickson B, Kormos C. 2010. Opportunities for achieving biodiversity conservation through REDD. Conserv. Lett. 3, 53–61. ( 10.1111/j.1755-263X.2009.00086.x) [DOI] [Google Scholar]
- 57.Strassburg BBN, et al. 2010. Global congruence of carbon storage and biodiversity in terrestrial ecosystems. Conserv. Lett. 3, 98–105. ( 10.1111/j.1755-263X.2009.00092.x) [DOI] [Google Scholar]
- 58.Holmes TP, Blate GM, Zweede JC, Pereira R J, Barreto P, Boltz F, Bauch R. 2002. Financial and ecological indicators of reduced impact logging performance in the eastern Amazon. Forest Ecol. Manage. 163, 93–110. ( 10.1016/S0378-1127(01)00530-8) [DOI] [Google Scholar]
- 59.Putz FE, Blate GM, Redford KH, Fimbel R, Robinson J. 2001. Tropical forest management and conservation of biodiversity: an overview. Conserv. Biol. 15, 7–20. ( 10.1046/j.1523-1739.2001.00018.x) [DOI] [Google Scholar]
- 60.Garnett T, et al. 2013. Sustainable intensification in agriculture. Science 341, 33–34. ( 10.1126/science.1234485) [DOI] [PubMed] [Google Scholar]
- 61.Smith P. 2013. Delivering food security without increasing pressure on land. Glob. Food Secur. 2, 18–23. ( 10.1016/j.gfs.2012.11.008) [DOI] [Google Scholar]
- 62.Foley JA, et al. 2011. Solutions for a cultivated planet. Nature 478, 337–342. ( 10.1038/nature10452) [DOI] [PubMed] [Google Scholar]
- 63.Tilman D, Balzer C, Hill J, Befort BL. 2011. Global food demand and the sustainable intensification of agriculture. Proc. Natl Acad. Sci. USA 108, 20 260–20 264. ( 10.1073/pnas.1116437108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phalan B, Onial M, Balmford A, Green RE. 2011. Reconciling food production and biodiversity conservation: land sharing and land sparing compared. Science 333, 1289–1291. ( 10.1126/science.1208742) [DOI] [PubMed] [Google Scholar]
- 65.Mueller ND, Gerber JS, Johnston M, Ray DK, Ramankutty N, Foley JA. 2012. Closing yield gaps through nutrient and water management. Nature 490, 254–257. ( 10.1038/nature11420) [DOI] [PubMed] [Google Scholar]
- 66.Lamb A, et al. 2016. Land sparing could help eliminate net greenhouse gas emissions from farming and land-use change. Nat. Clim. Change 6, 488–492. ( 10.1038/nclimate2910) [DOI] [Google Scholar]
- 67.Aleksandrowicz L, Green R, Joy EJ, Smith P, Haines A. 2016. The impacts of adopting environmentally sustainable and healthy diets on greenhouse gas emissions, land use, and water use: a systematic review. PLoS ONE 11, e0165797 ( 10.1371/journal.pone.0165797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bajželj B, Richards KS, Allwood JM, Smith P, Dennis JS, Curmi E, Gilligan CA. 2014. The importance of food demand management for climate mitigation. Nat. Clim. Change 4, 924–929. ( 10.1038/nclimate2353) [DOI] [Google Scholar]
- 69.Stehfest E, Bouwmann L, van Vuuren D, den Elzen MGJ, Eikhout B, Kabat P. 2009. Climate benefits of changing diet. Clim. Change 95, 83–102. ( 10.1007/s10584-008-9534-6) [DOI] [Google Scholar]
- 70.Canadell JG, Raupach MR. 2008. Managing forests for climate change mitigation. Science 320, 1456–1457. ( 10.1126/science.1155458) [DOI] [PubMed] [Google Scholar]
- 71.Obersteiner M, et al. 2001. Managing climate risk. Science 294, 786–787. ( 10.1126/science.294.5543.786b) [DOI] [PubMed] [Google Scholar]
- 72.Keith D. 2009. Why capture CO2 from the atmosphere. Science 325, 1654–1655. ( 10.1126/science.1175680) [DOI] [PubMed] [Google Scholar]
- 73.Socolow R, et al. 2011. Direct air capture of CO2 with chemicals: a technology assessment for the APS Panel on Public Affairs. College Park, MD: American Physical Society. [Google Scholar]
- 74.Schuiling RD, Krijgsman P. 2006. Enhanced weathering: an effective and cheap tool to sequester CO2. Clim. Change 74, 349–354. ( 10.1007/s10584-005-3485-y) [DOI] [Google Scholar]
- 75.Kelemen PB, Matter JM. 2008. In situ carbonation of peridotite for CO2 storage. Proc. Natl Acad. Sci. USA 105, 17 295–17 300. ( 10.1073/pnas.0805794105) [DOI] [Google Scholar]
- 76.Taylor LL, et al. 2016. Enhanced weathering strategies for stabilizing climate and averting ocean acidification. Nat. Clim. Change 6, 402–406. ( 10.1038/nclimate2882) [DOI] [Google Scholar]
- 77.Arora VK, Montenegro A. 2011. Small temperature benefits provided by realistic afforestation efforts. Nat. Geosci. 4, 514–518. ( 10.1038/ngeo1182) [DOI] [Google Scholar]
- 78.Jackson RB, et al. 2008. Protecting climate with forests. Environ. Res. Lett. 3, 044006 ( 10.1088/1748-9326/3/4/044006) [DOI] [Google Scholar]
- 79.Woolf D, Amonette JE, Street-Perrott A, Lehmann J, Joseph S. 2010. Sustainable biochar to mitigate global climate change. Nat. Commun. 1, 56 ( 10.1038/ncomms1053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.National Academy of Sciences. 2015. Climate intervention: carbon dioxide removal and reliable sequestration. Washington, DC: National Academies Press. [Google Scholar]
- 81.Smith P, Haszeldine RS, Smith SM. 2016. Preliminary assessment of the potential for, and limitations to, terrestrial negative emission technologies in the UK. Environ. Sci. Processes Impacts 18, 1400–1405. ( 10.1039/C6EM00386A) [DOI] [PubMed] [Google Scholar]
- 82.Rainey HJ, et al. 2014. A review of corporate goals of no net loss and net positive impact on biodiversity. Oryx 49, 232–238. ( 10.1017/S0030605313001476) [DOI] [Google Scholar]
- 83.Renforth P. 2012. The potential of enhanced weathering in the UK. Int. J. Greenh. Gas Control 10, 229–243. ( 10.1016/j.ijggc.2012.06.011) [DOI] [Google Scholar]
- 84.Franzluebbers AJ. 2002. Soil organic matter stratification ratio as an indicator of soil quality. Soil Tillage Res. 66, 95–106. ( 10.1016/S0167-1987(02)00018-1) [DOI] [Google Scholar]
- 85.Acton DF, Gregorich LJ (eds). 1995. The health of our soils—toward sustainable agriculture in Canada, pp. 5–10. Ottawa, ON: Centre for Land and Biological Resources Research, Research Branch, Agriculture and Agri-Food Canada. [Google Scholar]
- 86.Lehmann J, Joseph S (eds). 2015. Biochar for environmental management: science, technology and implementation. London, UK: Earthscan. [Google Scholar]
- 87.Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C. 2005. Landscape perspectives on agricultural intensification and biodiversity–ecosystem service management. Ecol. Lett. 8, 857–874. ( 10.1111/j.1461-0248.2005.00782.x) [DOI] [Google Scholar]
- 88.FAOSTAT. 2016. Food and agriculture data. See http://faostat3.fao.org/home/E (accessed 1 December 2016).
- 89.Griscom BW, et al. 2017. Natural pathways to climate mitigation. Proc. Natl Acad. Sci. USA 114, 11 645–11 650. ( 10.1073/pnas.1710465114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rowe RL, Street NR, Taylor G. 2009. Identifying potential environmental impacts of large-scale deployment of dedicated bioenergy crops in the UK. Renew. Sustain. Energy Rev. 13, 271–290. ( 10.1016/j.rser.2007.07.008) [DOI] [Google Scholar]
- 91.Pedroli B, et al. 2013. Energy cropping in Europe compatible with biodiversity?—Opportunities and threats to biodiversity from land-based production of biomass for bioenergy purposes. Biomass Bioenergy 55, 73–86. ( 10.1016/j.biombioe.2012.09.054) [DOI] [Google Scholar]
- 92.Fargione J, Hill J, Tilman D, Polasky S, Hawthorne P. 2008. Land clearing and the biofuel carbon debt. Science 319, 1235–1238. ( 10.1126/science.1152747) [DOI] [PubMed] [Google Scholar]
- 93.Santangeli A, Toivonen T, Montesino Pouzols F, Pogson M, Hastings A, Smith P, Moilanen A. 2016. Global change synergies and trade-offs between renewable energy and biodiversity. Glob. Change Biol. Bioenergy 8, 941–951. ( 10.1111/gcbb.12299) [DOI] [Google Scholar]
- 94.Jonsell M. 2007. Effects on biodiversity of forest fuel extraction, governed by processes working on a large scale. Biomass Bioenergy 31, 726–732. ( 10.1016/j.biombioe.2007.06.018) [DOI] [Google Scholar]
- 95.Jackson RB, et al. 2005. Trading water for carbon with biological carbon sequestration. Science 310, 1944–1947. ( 10.1126/science.1119282) [DOI] [PubMed] [Google Scholar]
- 96.Brockerhoff EG, Jactel H, Parrotta JA, Quine CP, Sayer J. 2008. Plantation forests and biodiversity: oxymoron or opportunity? Biodivers. Conserv. 17, 925–951. ( 10.1007/s10531-008-9380-x) [DOI] [Google Scholar]
- 97.Patterson G, Anderson R. 2000. Forests and peatland habitats, 16pp Edinburgh, Scotland: Forestry Commission; See http://www.forestry.gov.uk/pdf/fcgn1.pdf/$FILE/fcgn1.pdf (accessed 1 December 2016). [Google Scholar]
- 98.Forestry Commission. 2016. UK Woodland Carbon Code. See: http://www.forestry.gov.uk/carboncode (accessed 1 December 2016).
- 99.Rey Benayas JM, Bullock JM, Newton AC. 2008. Creating woodland islets to reconcile ecological restoration, conservation, and agricultural land use. Front. Ecol. Environ. 6, 329–336. ( 10.1890/070057) [DOI] [Google Scholar]
- 100.Jones CD. et al 2016. Simulating the Earth system response to negative emissions. Environ. Res. Lett. 11, 095012 ( 10.1088/1748-9326/11/9/095012) [DOI] [Google Scholar]
- 101.Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. ( 10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Wallace Initiative data can be accessed at http://Wallaceinitiative.org.