Abstract
Among 149 men who survived Ebola virus disease (EVD) and donated semen 260–1016 days after EVD onset, Ebola virus (EBOV) ribonucleic acid (RNA) was detected in 13 (9%). Of 137 men who donated semen 2 years after EVD onset, 11 (8%) had an EBOV RNA-positive specimen. The mechanism underlying the persistence of EBOV RNA in semen is unclear, and it is unclear whether the detection of viral RNA represents the presence of infectious virus.
Keywords: Ebola, PCR, semen, viral persistence
The well documented sexual transmission of Ebola virus (EBOV) by 2 men [1–3] who survived the 2013–2016 West Africa epidemic of Ebola virus disease (EVD) coupled with the detection of EBOV ribonucleic acid (RNA) in semen months after recovery from EVD has raised concern for the continued risk of sexual transmission of EBOVs by asymptomatic survivors [1–9].
Semen collected in cohort studies of EVD survivors in Guinea, Sierra Leone, and Liberia have been analyzed for the presence of EBOV, and, as observed during earlier outbreaks, viral RNA has been detected during the early phase after recovery [4, 6, 8, 9]. Among the small number of men sampled within approximately 3 months of acute EVD, as many as 28% to 100% have been reported to harbor EBOV RNA in their semen [4, 8, 9].
The reported proportions of men with detectable viral RNA in their genital fluid later in the convalescent course have varied widely. Deen et al [4] detected EBOV RNA in the semen of 11 (26%) of 43 men sampled at 7 to 9 months after EVD onset, whereas Sow et al [9] in Guinea found evidence of EBOV RNA in semen collected from only 2 of 31 (7%) men within a similar time period. There are even fewer data regarding the presence of EBOV RNA in the semen of EVD survivors further out from their disease onset, although cases of detectable viral RNA in the semen of men 407 to 565 days after Ebola Treatment Unit (ETU) release have been reported [2, 7, 10, 11].
Few studies have attempted to correlate the presence of EBOV RNA with the presence of replication-competent virus. Infectious virus has been detected in the semen of survivors up to 82 days after onset of EVD symptoms using Vero E6 cell culture models [6]. More recently, EBOV RNA was detected in the blood and organs of severe combined immunodeficiency (SCID) mice inoculated with EBOV RNA-positive semen collected as long as 233 days after symptom onset and in a second group of SCID mice inoculated with lung homogenate from these animals [6, 7].
The prolonged presence of potentially infectious EBOVs in the male genital tract has implications for both personal and public health. We aimed to determine the prevalence of late persistence of EBOV RNA in the semen of EVD survivors beyond 2 years from the onset of acute illness using an assay validated in West Africa to detect EBOV RNA in semen.
METHODS
Men age 18 years and older participating in a longitudinal cohort study of EVD survivors conducted at the Eternal Love Winning Africa Hospital in Monrovia, Liberia were recruited. Cohort entry required written documentation of prior EVD as evidenced by an ETU discharge certificate verified by photo identification. All participants provided written informed consent for the semen testing described. Research protocols were approved by the Institutional Review Boards at the University of North Carolina at Chapel Hill and the University of Liberia.
Clinical history, physical examination, and collection of semen were performed every 3 months. The dates of EVD onset and ETU discharge were self-reported. For 7 participants who could recall the month but not the day of EVD onset, the 15th of that month was used. In an additional 6 cases, both the month and day of EVD onset were not recalled. To approximate the EVD onset date for these participants, the mean time from EVD onset to discharge from the ETU among the 142 participants with both dates available was calculated (26 days) and then was subtracted from the ETU release date. None of the participants with EBOV RNA detected in their semen required imputation of EVD onset date.
Demographics, time from ETU discharge to enrollment, and reports of the new onset of symptoms including vision problems, hearing loss, arthralgias, myalgias, fatigue, and headache at the time of enrollment and new since discharge from ETU were compared between those with and without evidence of EBOV RNA in semen.
We assessed the distributions of all factors overall and stratified by patient characteristics, including presence of Ebola RNA shedding in semen. For bivariable analyses, we contrasted factors using parametric and non-parametric tests, including the Student’s t, Wilcoxon-Mann-Whitney, Pearson’s χ2, and Fisher’s exact tests, as appropriate. Differences in means and medians were estimated using linear and quantile regression, respectively. Relative risks and associated 95% confidence intervals were calculated. All P values were 2-sided, and <.05 was considered statistically significant. Analyses were performed in SAS software, version 9.4 (SAS Institute, Cary, NC).
Semen was collected in sterile containers, stored at 2–8°C, and transported within 24 hours to the Phebe Hospital Laboratory, Bong County, Liberia. Semen was tested for EBOV RNA within 2 days of collection using procedures previously described [12]. In brief, the Cepheid Xpert Ebola Assay (Sunnyvale, CA) was used to detect Zaire EBOV total nucleic acid, targeting the glycoprotein (GP) and nucleoprotein (NP) genes. Test results were interpreted by the GeneXpert Dx System from measured fluorescence signals based on algorithms embedded within the assay software. Samples were considered positive if either target gene (GP or NP) was detected. During the validation study, there were no false positives among the 58 negative control semen specimens or 52 commercial controls tested.
RESULTS
From June 2016 to January 2017, 158 men with documented evidence of prior EVD entered the survivor cohort, and 149 (94%) donated semen at least once. Among those who provided semen for EBOV RNA polymerase chain reaction (PCR) testing, the median age at cohort entry was 32 years (range, 17–59), and the median time from self-reported onset of EVD symptoms to first and last semen donation was 681 days (range, 260–835) and 786 days (range, 367–1016), respectively. To date, participants donated an average of 3 (range, 1–6) semen specimens. Of the 149 men who have donated semen, 137 (92%) have provided a semen specimen more than 2 years (730 days) since EVD onset.
Ebola virus RNA was detected in the semen of 13 (9%) participants who donated semen (Figure 1). In 11 (85%) of the 13 men, EBOV RNA was detected in semen more than 2 years after EVD onset; the longest time from EVD onset to detectable EBOV in semen was 965 days. Detection of EBOV RNA was observed to be intermittent (a detectable PCR result preceded by an undetectable PCR result) in 8 of 13 (62%) participants who ever had a positive result, including 1 participant who initially had 2 samples test negative followed by a third that tested positive (participant 12; Figure 1).
Figure 1.
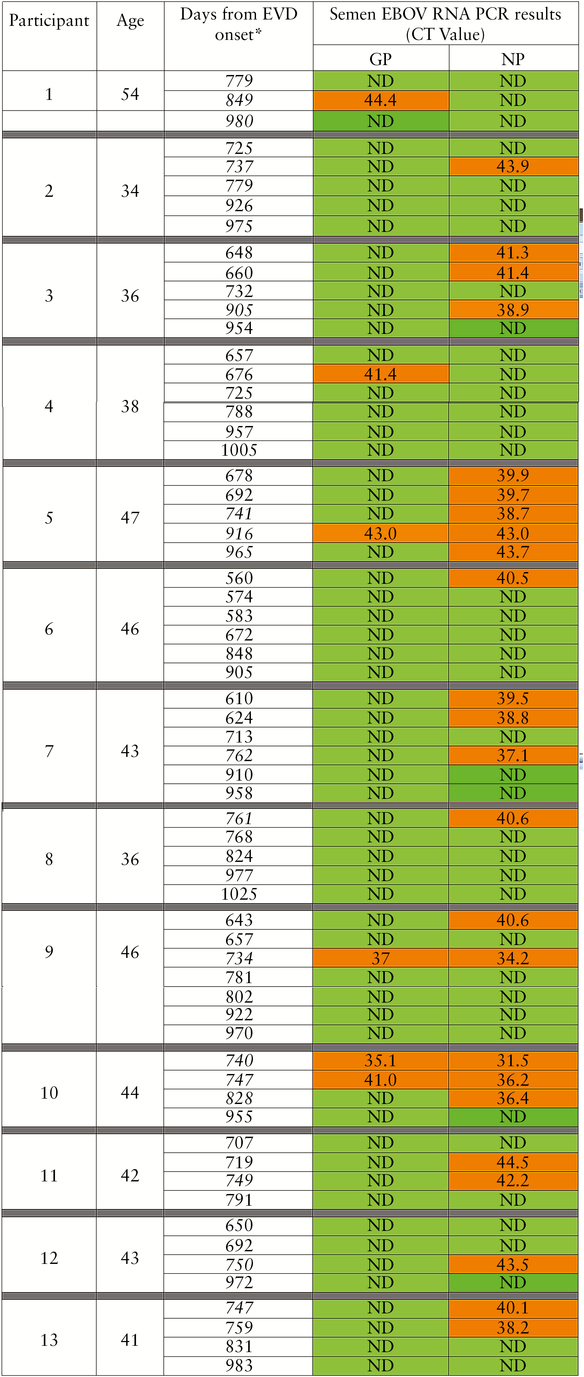
Ebola virus (EBOV) ribonucleic acid (RNA) detected in semen of long-term Ebola virus disease (EVD) survivors. *Bold and italicized numbers indicate detectable EBOV RNA 2 years or greater from onset of acute EVD symptoms. Abbreviations: CT, computed tomography; GP, glycoprotein; ND, not detected; NP, nucleoprotein; PCR, polymerase chain reaction.
There was no significant difference in the time from ETU discharge to first semen donation between men with EBOV RNA detected in semen and those whose semen never tested positive for EBOV RNA (median days 352 vs 368, respectively; P = .46). Male survivors with detectable EBOV RNA in semen were significantly older (median age 41.8 vs 31.2 years; P = .0004) and more likely to report vision problems but less fatigue compared with those whose semen tested negative (Table 1). There was no significant difference found in the proportion of men who reported headaches, hearing loss, numbness of hands or feet, muscle pain, or joint pain between those with or without EBOV RNA in semen.
Table 1.
Association Between Seminal EBOV RNA Detection and Age, Time From ETU Discharge, and Post-Ebola Clinical Complications
| Patient Characteristics/Symptoms | Men With Seminal EBOV RNA | Men Without Seminal EBOV RNA | P Value | RR (95% CI) |
|---|---|---|---|---|
| Agea | 41.8 (37.8–44.8) | 31.2 (25.2–38.5) | .0004 | |
| Days from ETU Discharge to Enrollmentb | 352 (203–538) | 368 (68–819) | .46 | |
| Symptomsc | N (%) | N (%) | ||
| Fatigue | 3 (23) | 76 (56) | .039 | 0.41 (0.15–1.13) |
| Headaches | 5 (38) | 65 (48) | .573 | 0.80 (0.40–1.64) |
| Vision | 7 (54) | 33 (24) | .043 | 2.22 (1.24–3.98) |
| Hearing | 1 (8) | 9 (7) | .999 | 1.16 (0.16–8.47) |
| Numbness hands | 4 (31) | 29 (21) | .486 | 1.44 (0.60–3.47) |
| Numbness feet | 3 (23) | 36 (26) | .999 | 0.87 (0.31–2.45) |
| Muscle pain | 4 (31) | 27 (20) | .472 | 1.55 (0.64–3.75) |
| Joint pain | 10 (77) | 71 (52) | .143 | 1.47 (1.05–2.07) |
Bold indicates statistical significance.
Abbreviations: CI, confidence interval; EBOV, Ebola virus; ETU, Ebola Treatment Unit; RNA, ribonucleic acid; RR, relative risk.
aMedian age with interquartile range; Mann-Whitney U test.
bDays with full range; Mann-Whitney U test.
cFishers exact test.
DISCUSSION
According to the World Health Organization (WHO), men who survive EVD should undertake measures such as abstinence and the use of condoms to prevent potential sexual transmission of EBOV for at least 12 months after the onset of EVD symptoms, or until their semen has tested negative for EBOV RNA twice [13]. Our study of long-term Ebola survivors confirms earlier findings that EBOV RNA can be detected beyond 12 months after EVD and also extends the duration of seminal EBOV RNA detection beyond 2 years. We have also found that EBOV RNA is often intermittently detected when using a validated assay with a lower limit of detection approximating 1000 copies/mL.
Overall, these findings have a number of important implications. Foremost, although the persistence of EBOV RNA in semen is concerning, it is not known whether the detection of EBOV RNA in genital fluids is a surrogate for the presence of infectious virus. In a recent study, Sissoko et al [7] reported the retrieval of EBOV RNA in the blood and organs after the passage of PCR-positive survivor semen in a SCID mouse model. In that study, EBOV RNA was not detected in samples collected beyond 233 days after onset of EVD, but a report of sexual transmission of EBOVs from a male survivor more than 500 days after the onset of his EVD illness suggests that infectious virus can persist beyond 1 year after acute Ebola infection. Further work to establish whether EBOV RNA detection correlates with the presence of infectious virus is needed to determine whether a diagnostic strategy based on PCR is sufficient to declare a survivor incapable of transmitting EBOV sexually. Meanwhile, the WHO recommendations regarding the prevention of sexual transmission of EBOV should be updated to acknowledge that the duration of the presence of EBOV RNA in semen is not known and that the detection of Ebola viral RNA can be intermittent even when tested using a validated diagnostic platform in accordance with Clinical and Laboratory Standards Institute and Good Clinical Laboratory Practice guidelines.
Second, the demonstration of the persistence of EBOV RNA in the male genital tract for more than 2 years after the clearance of viremia also requires a new approach to the study of this virus and its immunology. A comparison of the 13 men with EBOV RNA detected in their semen and the 136 without revealed important differences in age and post-EVD clinical sequelae. The finding that men with evidence of seminal EBOV RNA were significantly older than men without seminal EBOV RNA detected supports earlier findings from the US Centers for Disease Control and Prevention and Liberian Ministry of Health Men’s Health Study, and it may suggest that aging and the associated immune senescence may enable penetration of EBOVs into immune-privileged sites such as the testes [8]. Likewise, men with EBOV RNA detected semen were more likely to report vision problems, supporting a hypothesis that both the eye and the testes are sites of immune privilege where viable virus may evade systemic immune responses. Two cases of recrudescent EVD involving the cerebrospinal fluid and 1 case of anterior uveitis with ocular hypertension further support the potential for viral persistence in these and other immune privileged sites [14, 15]. Further research into viral penetrance of immune privileged sites and the role of aging and other host factors are needed to identify who is at risk of viral persistence, sexual transmission, and potentially recrudescent disease. In addition, the factors associated with EBOV persistence in semen must also be explored, including whether there is a differential in the neutralizing capability of antibodies in the circulation compared with the genital tract, the role of other immune deficiencies such as human immunodeficiency virus (HIV), as well as the potential role of sexually transmitted infections that are associated with increased HIV shedding in semen [16–19].
Finally, the impact of the potential for prolonged, intermittent seminal shedding of EBOV on the lives of EVD survivors must be considered. For many survivors, the physical manifestations of the disease have been compounded by the stigma encountered with their return to their communities. Survivor messaging regarding viral persistence, if demonstrated, must provide information that can be used to protect loved ones but at the same time not risk further ostracizing by society.
In addition to the lack of clarity as to the infectiousness of semen with detected EBOV RNA, which will be addressed in subsequent studies, there are other limitations that should be considered when interpreting these results. Although a validated assay was used to assess for EBOV in semen, the lower limit of detection was approximately 1000 copies/mL, and concentrations of viral RNA below this threshold are less likely to be detected. Furthermore, at this point, we remain unable to determine the complete duration of seminal EBOV RNA persistence because we have yet to observe a prolonged absence of detection of viral RNA in the semen of men participating in this ongoing, longitudinal cohort study.
CONCLUSIONS
In conclusion, EBOV RNA can be detected in the semen of some survivors of EVD for over 2 years after the onset of their infection. This unexpected and prolonged presence of EBOV RNA in the male genital tract should prompt discussion of revised approaches to the prevention of sexual transmission of Ebola and encourage novel investigations to determine the mechanisms by which this virus remains in this and other compartments, its infectiousness, and strategies for its elimination. Such data are necessary to inform public health recommendations that are used to counsel thousands of EVD survivors.
Acknowledgments
The research team is indebted to the staff at Eternal Love Winning Africa and Phebe hospitals including Drs. John Fankhauser, Rick Sacra, Jefferson Sibley, and Uriah Glaybo for support and dedicated care during and after the Ebola outbreak. Dr. Myron Cohen provided valuable insights. Alexandria Buller, Amanda Crooks, Darrius McMillian, and Darrell Hawks assisted with data quality and analyses. We are also grateful to the many EVD survivors who are generously participating in this research and those who thoughtfully represent them.
Financial support. This work was funded by the Bill and Melinda Gates Foundation as well as National Institutes of Health grants K23AI121516 (to W. A. F.), R01 AI123535 (to W. A. F. and D. A. W.), and K24DA037101 (to D. A. W.) and grants from Clinical Research Management.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Christie A, Davies-Wayne GJ, Cordier-Lassalle T et al. Possible sexual transmission of Ebola virus - Liberia, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:479–81. [PMC free article] [PubMed] [Google Scholar]
- 2. Diallo B, Sissoko D, Loman NJ et al. Resurgence of Ebola virus disease in Guinea linked to a survivor with virus persistence in seminal fluid for more than 500 days. Clin Infect Dis 2016; 63:1353–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mate SE, Kugelman JR, Nyenswah TG et al. Molecular evidence of sexual transmission of Ebola virus. N Engl J Med 2015; 373:2448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deen GF, Knust B, Broutet N et al. Ebola RNA persistence in semen of Ebola virus disease survivors—preliminary report. N Engl J Med 2015; doi: 10.1056/NEJMoa1511410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fischer WA II, Wohl DA. Confronting Ebola as a sexually transmitted infection. Clin Infect Dis 2016; 62:1272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriguez LL, De Roo A, Guimard Y et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis 1999; 179Suppl 1:S170–6. [DOI] [PubMed] [Google Scholar]
- 7. Sissoko D, Duraffour S, Kerber R et al. Persistence and clearance of Ebola virus RNA from seminal fluid of Ebola virus disease survivors: a longitudinal analysis and modelling study. Lancet Glob Health 2017; 5:e80–8. [DOI] [PubMed] [Google Scholar]
- 8. Soka MJ, Choi MJ, Baller A et al. Prevention of sexual transmission of Ebola in Liberia through a national semen testing and counselling programme for survivors: an analysis of Ebola virus RNA results and behavioural data. Lancet Glob Health 2016; 4:e736–43. [DOI] [PubMed] [Google Scholar]
- 9. Sow MS, Etard JF, Baize S et al. New evidence of long-lasting persistence of Ebola virus genetic material in semen of survivors. J Infect Dis 2016; 214:1475–6. [DOI] [PubMed] [Google Scholar]
- 10. Keita AK, Toure A, Sow MS et al. Extraordinary long-term and fluctuating persistence of Ebola virus RNA in semen of survivors in Guinea: implications for public health. Clin Microbiol Infect 2017; 23:412–3. [DOI] [PubMed] [Google Scholar]
- 11. Purpura LJ, Rogers E, Baller A et al. Ebola virus RNA in semen from an HIV-positive survivor of Ebola. Emerg Infect Dis 2017; 23:714–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loftis AJ, Quellie S, Chason K et al. Validation of the Cepheid GeneXpert for Detecting Ebola Virus in Semen. J Infect Dis 2017; 215:344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization. Interim advice on the sexual transmission of the Ebola virus disease 2016. Available from: http://www.who.int/reproductivehealth/topics/rtis/ebola-virus-semen/en/. Accessed July 30, 2017 [Google Scholar]
- 14. Jacobs M, Rodger A, Bell DJ et al. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet 2016; 388:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Varkey JB, Shantha JG, Crozier I et al. Persistence of Ebola Virus in ocular fluid during convalescence. N Engl J Med 2015; 372:2423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen MS, Hoffman IF, Royce RA et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet 1997; 349:1868–73. [DOI] [PubMed] [Google Scholar]
- 17. Dyer JR, Eron JJ, Hoffman IF et al. Association of CD4 cell depletion and elevated blood and seminal plasma human immunodeficiency virus type 1 (HIV-1) RNA concentrations with genital ulcer disease in HIV-1-infected men in Malawi. J Infect Dis 1998; 177:224–7. [DOI] [PubMed] [Google Scholar]
- 18. Pilcher CD, Joaki G, Hoffman IF et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS 2007; 21:1723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ping LH, Cohen MS, Hoffman I et al. Effects of genital tract inflammation on human immunodeficiency virus type 1 V3 populations in blood and semen. J Virol 2000; 74:8946–52. [DOI] [PMC free article] [PubMed] [Google Scholar]


