Abstract
Background:
Age-related losses of lean mass and shifts to central adiposity are related to functional decline and may predict mortality and/or explain part of the risk of weight loss. To determine how mortality risk is related to shifts in body composition, changes should be considered in the context of overall weight change.
Methods:
Five-year changes in body composition were assessed with computed tomography (cm2) and dual x-ray absorptiometry (kg) in 869 men and 934 women initially aged 70–79 years. All-cause mortality was monitored for up to 12 years (2002–2003 to September 30, 2014), and risk was assessed using sex-specific Cox models.
Results:
Both men and women lost weight, visceral fat area, thigh muscle area, lean mass, and fat mass (all p < .01) but gained intermuscular thigh fat area (p < .01). There were 995 deaths. After adjustment for total weight change, demographics, and chronic disease, losing thigh muscle area was associated with higher mortality in both men (1.21, 1.08–1.35) and women (1.18, 1.01–1.37, per 9.0cm2) and was especially strong in normal weight (body mass index < 25kg/m2) individuals and those losing weight. Losing intermuscular thigh fat was protective against mortality only in normal weight (0.66, 0.51–0.86) and weight stable men (0.79, 0.66–0.95, per 3.2cm2). Changes in visceral fat area were not associated with mortality.
Conclusions:
Older adults with greater loss of thigh muscle than expected for overall weight change had a higher mortality risk compared to those with relative thigh muscle preservation, suggesting that conservation of muscle mass is important for survival in old age.
Keywords: Body composition, Mortality, Muscle, Prospective cohort
Body composition changes are common and quite variable with aging, with a gradual loss of lean mass and a shift to central fat accumulation (1–3). Metrics of sarcopenia have not consistently predicted mortality when measured at a single point in time, possibly because one measure does not capture complex body composition changes or because of the insensitivity of dual-energy x-ray absorptiometry (DXA) compared with computed tomography (CT) (4–7). Weight loss is common in older adults (8) and can accelerate muscle loss (1,9). Observational studies clearly show that weight loss, regardless of starting weight, is associated with an increased mortality risk (8,10,11). To distinguish between risks related to muscle mass versus weight loss, it is important to consider whether the changes in muscle are proportional to or excess for a given weight change (1).
Visceral fat increases with age (12) and intermuscular fat (IMAT) increases with age even in those who lose weight (9). Both fat depots are associated with adverse health outcomes regardless of body size (2,12–16). Potentially, longitudinal shifts in fat distribution are early indicators of declining health in old age and could add information to the loss of lean mass in predicting mortality.
We examined 5-year changes in regional body composition from CT to determine the associations of loss of lean and rate of central and IMAT accumulation with mortality in the context of overall weight change. We hypothesized that excessive loss of lean mass would be associated with mortality. Because body fat seems to be protective in old age (5) and weight gain is indicative of an anabolic state, we hypothesized that that these associations may be stronger in thinner participants and mitigated by weight gain. We also hypothesized that relatively greater increases in IMAT and visceral fat would be independently associated with mortality.
Methods
Participants
The Health, Aging and Body Composition (Health ABC) Study is a prospective cohort study of 3,075 nondisabled black (41.7%) and white men and women (51.5%) from the Pittsburgh, PA and Memphis, TN areas, aged 70–79 years at baseline. Eligibility criteria included no self-reported difficulty walking a quarter mile, climbing 10 steps, or performing mobility-related activities of daily living, no reported use of a walking aid, and no active cancer treatment. The institutional review boards of the University of Pittsburgh, the University of Tennessee, the University of California–San Francisco Coordinating Center, and the National Institute on Aging approved the study, and all participants gave informed consent.
Of the 3,075 participants enrolled, 1,803 participants had a both a baseline (1997–1998) and a valid 2002–2003 (5-year follow-up) CT scan. Between baseline and follow-up, 378 participants died and 589 participants were unable to attend the 2002–2003 exam. Of the 2,108 participants with a 5-year follow-up CT scan, we excluded 25 participants with missing baseline thigh or abdominal CT data, 179 participants with invalid CT images, and 101 participants with inadequate follow-up thigh or abdomen positioning compared with baseline.
Assessment of Mortality
All-cause mortality surveillance started at the date of the 5-year follow-up visit and was conducted using annual in-person examinations, telephone interviews, and obituaries. Hospital records, death certificates, obituaries, and next-of-kin interviews were obtained and reviewed by a committee of geriatricians for all deaths occurring on or before September 30, 2014. Days of follow-up were defined as days from the follow-up clinic visit to date of death, date of last contact, or September 30, 2014, whichever occurred first.
Body Composition
Using CT as described previously, cross-sectional areas (cm2) of midline thigh muscle, IMAT, and subcutaneous fat of both thighs were measured and summed at baseline and follow-up. CT was also used to quantify visceral and subcutaneous abdominal fat using established methods (16).
For the abdomen, 1,107 (61.4%) and 1,050 (58.2%) participants had complete data for visceral and subcutaneous fat, respectively. We excluded 682 scans because of inadequate anatomical alignment between baseline and follow-up scans. We compared those with complete thigh and abdominal data (men n = 491, women n = 505, n = 996) to the analytic sample with either complete thigh or abdomen data (n = 1,803). We found no differences in regard to demographic, disease, physical activity or body composition or changes in thigh CT measures.
Bone-free lean and fat mass were measured by DXA (Hologic QDR 4500; Waltham, MA), as described previously (7).
Weight Change, Obesity Status, and DXA
Body height (cm) and weight (kg) were measured in light clothing without shoes on a calibrated balance beam scale and used to calculate body mass index (BMI; kg/m2). Participants were classified into three weight change groups: stable (<3% change), gainers (≥3% increase), and losers (≥3% decrease) (1). Participants were also classified into BMI groups: normal (BMI < 25.0kg/m2), overweight (BMI = 25.0–29.9kg/m2), and obese (BMI ≥ 30kg/m2). A small number (n = 20, 3.5%) of “underweight” individuals (BMI < 18.5) were included as normal weight.
Potential Confounders
Potential confounders included age, self-reported race, sex, baseline smoking (current/former), alcohol consumption (>1 drink per day), and physical activity (kcal/wk; climbing stairs and doing chores based on questionnaire data).
The prevalence of chronic conditions were updated from baseline to follow-up to account for body composition changes that may have been precipitated by disease and are described in online Supplementary Material.
Statistical Analyses
Means and SD or frequencies and percentages were calculated in men and women and compared using t tests and chi-square tests. In all analyses, men and women were examined separately, as there is little overlap in body composition between them and because of previously documented sex differences in factors associated with body composition change (1). Cox proportional hazards models were used to test the associations of changes in each aspect of body composition with all-cause mortality. Proportionality of hazards over time was tested and confirmed using the Kolmogorov-type supremum test. Potential confounders were included if they were bivariately associated with mortality at the p ≤ .15 level. Hazard ratios for body composition change were presented per SD of change with an increase as referent. Models were adjusted for age, race, site, smoking, chronic health conditions, and baseline body composition measure. We determined whether body composition changes were more strongly related to death in the presence of obesity versus normal weight and in the presence of weight loss versus gain or stability by testing interactions. To illustrate differences in subgroups, we stratified by baseline BMI and weight change direction.
Finally, we evaluated the degree to which changes in body composition explained the risk of mortality associated with weight change by examining the extent to which the hazard ratios were attenuated after adjusting for body composition depots that were significantly related to mortality. All analyses were conducted using SAS v9.3 (Cary, NC).
Results
Participant Characteristics and Body Composition Changes
At the start of follow-up, there were 869 men and 934 women and baseline characteristics can be found in Table 1. Men and women lost weight on average but with substantial variation (Table 1). There were also large variations in changes in lean and fat mass as well as regional body composition from CT. On average, men and women lost lean mass and thigh muscle area. Men gained a small amount of fat mass, whereas women lost fat. Both visceral and subcutaneous abdominal fat decreased in men and women, with women losing more visceral fat area than men. In both sexes, intermuscular thigh fat was the only body composition depot to increase.
Table 1.
Characteristics by Sex at Start of Follow-up, Body Composition at Baseline (1997–1998), and Change From Baseline to Start of Follow-up (2002–2003)
| Men (n = 869) | Women (n = 934) | |
|---|---|---|
| Characteristic | Value | Value |
| N (%) | N (%) | |
| Mean ± SD | Mean ± SD | |
| Demographics and health | ||
| Age | 78.5±2.8* | 78.1±2.8* |
| Race, black | 264 (30.4)* | 370 (39.6)* |
| Site, Memphis | 400 (46.0) | 450 (48.2) |
| Education, less than high school† | 193 (22.2) | 181 (19.4) |
| Smoking status, past | 510 (58.7) | 160 (31.7)* |
| Current | 47 (5.4) | 25 (5.0) |
| Alcohol, >1 drink per day† | 103 (11.9)* | 31 (3.3)* |
| Physical activity, kcal/wk | 1,108.0±1,776.7* | 589.3±1,108.9* |
| Diabetes, yes | 231 (26.6)* | 180 (19.3)* |
| Cardiovascular disease, yes | 296 (34.1)* | 207 (22.2)* |
| Hypertension, yes | 515 (59.3)* | 625 (66.9)* |
| COPD, yes | 68 (7.8) | 88 (9.4) |
| Osteoarthritis in knee or hip, yes | 183 (21.1) | 316 (33.8) |
| Depression, CES-D Score ever ≥10 | 194 (22.3)* | 279 (29.9)* |
| 3MSE Score | 91.0±7.7 | 91.5±7.9 |
| Body composition | ||
| Total body weight, kg† | 81.6±12.9* | 70.1±14.2* |
| BMI, kg/m2† | 27.1±3.8 | 27.4±5.3 |
| Baseline body composition, CT | ||
| Thigh muscle, cm2 | 265.1±42.8* | 184.7±34.1* |
| Intermuscular thigh fat, cm2 | 19.3±12.2 | 20.3±12.2 |
| Visceral abdominal fat, cm2 | 157.8±71.1* | 129.5±58.6 |
| Subcutaneous abdominal fat, cm2 | 228.8±86.6* | 334.1±117.4* |
| Subcutaneous thigh fat, cm2 | 95.2±40.4* | 212.5±88.4* |
| Baseline body composition, DXA | ||
| Total lean mass, kg | 54.5±7.0* | 39.2±5.8* |
| Total appendicular lean mass, kg | 23.9±3.5* | 16.5±3.4* |
| Total leg lean mass, kg | 16.5±3.1* | 12.5±2.4* |
| Total fat mass, kg | 24.4±7.1* | 29.0±9.0* |
| Change in body composition | ||
| Δ Total body weight, kg | −1.5±4.7 | −1.4±5.1 |
| % | −1.7±5.7 | −1.8±7.0 |
| Change from CT | ||
| Δ Thigh muscle area, cm2 | −13.5±19.2 | −6.3±14.5 |
| % | −5.0±7.1 | −3.1±7.6 |
| Δ Intermuscular thigh fat, cm2 | 6.2±5.7 | 3.5±5.7 |
| % | 48.7±56.8 | 29.3±40.5 |
| Δ Visceral abdominal fat, cm2 | −5.3±43.7 | −12.1±32.5 |
| % | −2.7±29.5 | −9.2±25.8 |
| Δ Subcutaneous abdominal fat, cm2 | −15.9±37.4 | −24.1±49.2 |
| % | −7.0±18.0 | −7.3±20.2 |
| Δ Subcutaneous thigh fat, cm2 | −1.6±18.4 | −6.9±32.9 |
| % | −1.7±19.9 | −2.4±16.7 |
| Change from DXA | ||
| Δ Total lean mass, kg | −1.4±2.4 | −0.5±1.9 |
| % | −2.6±4.2 | −1.3±4.7 |
| Δ Appendicular lean mass, kg | −1.0±1.3 | −0.6±1.0 |
| % | −3.9±5.1 | −3.2±5.9 |
| Δ Total leg lean mass, kg | −0.7±1.0 | −0.5±0.8 |
| % | −4.1±5.6 | −3.5±6.5 |
| Δ Total fat mass, kg | 0.4±3.3 | −0.5±3.8 |
| % | 2.0±14.1 | −1.1±13.5 |
Notes: CES-D = Center for Epidemiologic Studies-Depression scale; COPD = chronic obstructive pulmonary disease; 3MSE = Modified Mini-Mental State Examination; CT = computed tomography; DXA = dual x-ray absorptiometry.
*Significant difference between sexes at p < .05 level.
†Measured at baseline.
Relationship Between Body Composition Change and Mortality
Follow-up time in survivors was 11.5±0.9 years (range: 2.5–12.3 years); there were 995 deaths and average time to death was 6.7±3.2 years (range: 4 days–12.2 years). Mortality rates were higher in men than women (Table 2).
Table 2.
Deaths, Mortality Rates, and Hazards Associated With Decreases in Individual Body Composition Depots by Sex
| Separate Cox Models for Each Body Composition Measure | HR* (95% CI) for Men (n = 869) | HR* (95% CI) for Women (n = 934) | ||||||
|---|---|---|---|---|---|---|---|---|
| Deaths and Mortality Rate: 528 (71.4/1,000 Person Years) | Deaths and Mortality Rate: 467 (54.6/1,000 Person Years) | |||||||
| A | B | C | D | A | B | C | D | |
| Δ Total body weight (per 4.9kg) | 1.15 (1.04–1.26) | 1.12 (1.02–1.24) | — | 1.29 (1.18–1.42) | 1.27 (1.16–1.40) | — | ||
| Δ Thigh muscle area (per 9.0cm2) | 1.23 (1.13–1.35) | 1.21 (1.11–1.33) | 1.21 (1.08–1.35) | 1.19 (1.06–1.34) | 1.41 (1.25–1.59) | 1.32 (1.16–1.50) | 1.18 (1.01–1.37) | 1.16 (0.99–1.36) |
| Δ Intermuscular thigh fat (per 3.2cm2) | 0.97 (0.88–1.06) | 0.98 (0.89–1.08) | 0.93 (0.84–1.03) | 0.94 (0.84–1.05) | 1.10 (0.99–1.23) | 1.10 (0.98–1.22) | 0.96 (0.85–1.09) | 0.94 (0.82–1.07) |
| Δ Visceral abdominal fat (per 38.5cm2) | 1.06 (0.95–1.18) | 1.06 (0.96–1.18) | 1.03 (0.91–1.17) | — | 1.27 (1.09–1.48) | 1.26 (1.08–1.46) | 1.07 (0.88–1.29) | — |
| Δ Subcutaneous abdominal fat (per 43.9cm2) | 1.19 (1.02–1.38) | 1.21 (1.03–1.41) | 1.18 (0.96–1.47) | — | 1.30 (1.16–1.46) | 1.28 (1.15–1.44) | 1.15 (0.96–1.37) | — |
| Δ Subcutaneous thigh fat (per 13.5cm2) | 1.15 (0.99–1.34) | 1.16 (1.00–1.35) | 1.05 (0.85–1.29) | — | 1.16 (1.07–1.26) | 1.18 (1.09–1.28) | 1.04 (0.91–1.19) | — |
| Δ Total lean mass (per 2.2kg) | 1.10 (1.01–1.21) | 1.08 (0.99–1.18) | 1.01 (0.89–1.15) | — | 1.31 (1.17–1.46) | 1.25 (1.12–1.40) | 1.08 (0.92–1.26) | — |
| Δ Total appendicular lean mass (per 1.2kg) | 1.16 (1.06–1.27) | 1.14 (1.04–1.24) | 1.10 (0.98–1.23) | — | 1.29 (1.14–1.45) | 1.24 (1.10–1.39) | 1.06 (0.91–1.24) | — |
| Δ Total leg lean mass (per 0.9kg) | 1.13 (1.03–1.23) | 1.11 (1.02–1.21) | 1.07 (0.96–1.19) | — | 1.24 (1.11–1.38) | 1.20 (1.08–1.33) | 1.04 (0.90–1.19) | |
| Δ Total fat mass (per 3.6kg) | 1.16 (1.04–1.28) | 1.14 (1.03–1.26) | 1.12 (0.92–1.37) | — | 1.25 (1.14–1.38) | 1.24 (1.13–1.36) | 0.99 (0.78–1.25) | — |
Notes: 3MSE = Modified Mini-Mental State Examination; CI = confidence interval; CT = computed tomography; CVD = cardiovascular disease; HR = hazard ratio.
A: adjusted for age, race, study site, baseline CT measure, physical activity, smoking, and education (men only).
B: A, plus diabetes, CVD, hypertension, pulmonary disease (women only), depression (men only), 3MSE score.
C: B, plus change in total body weight.
D: C, plus both change in thigh intermuscular fat or muscle area.
*HRs are per SD of change over 5 years with an increase as the referent.
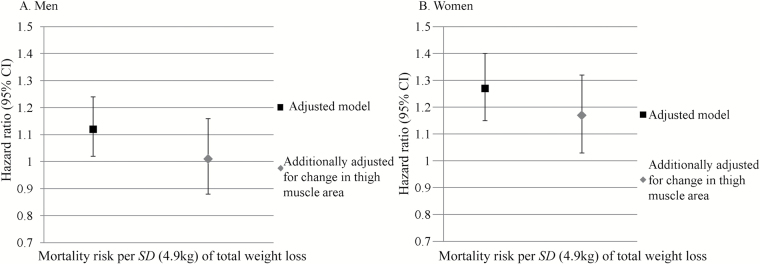
Weight loss was associated with higher mortality in both men and women (Table 2), though more strongly in women. Adjusting for thigh muscle area change attenuated mortality risk associated with weight loss by ~10% in both men and women (Figure 1).
Figure 1.
Hazard ratios for mortality associated with total weight loss before and after adjustment for thigh muscle area loss in men (A) and women (B). Hazard ratios are from Cox models and are per SD change over 5 years (4.9kg) with an increase in muscle area as the referent, adjusting for race, study site, physical activity, education (men only), smoking, baseline body composition measure, diabetes, cardiovascular disease, hypertension, chronic obstructive pulmonary disease (women only), depression (men only), Modified Mini-Mental State Examination score, and change in total body weight.
Loss of total thigh muscle area was associated with higher all-cause mortality risk in both men and women (Table 2, column A). Adjustment for chronic diseases had little effect on the relationships between loss of CT thigh muscle area changes and death (Table 2, column B), and the association was independent of total weight loss (Table 2, column C).
Change in abdominal visceral and subcutaneous fat and thigh subcutaneous fat was not associated with mortality. Though not statistically significant, the direction of the association between IMAT and mortality was opposite that of other fat depots, with decreasing IMAT being protective against death. Based on DXA, decreases in both lean and fat mass were associated with higher risk of death; however, after adjustment these relationships were attenuated to nonsignificance.
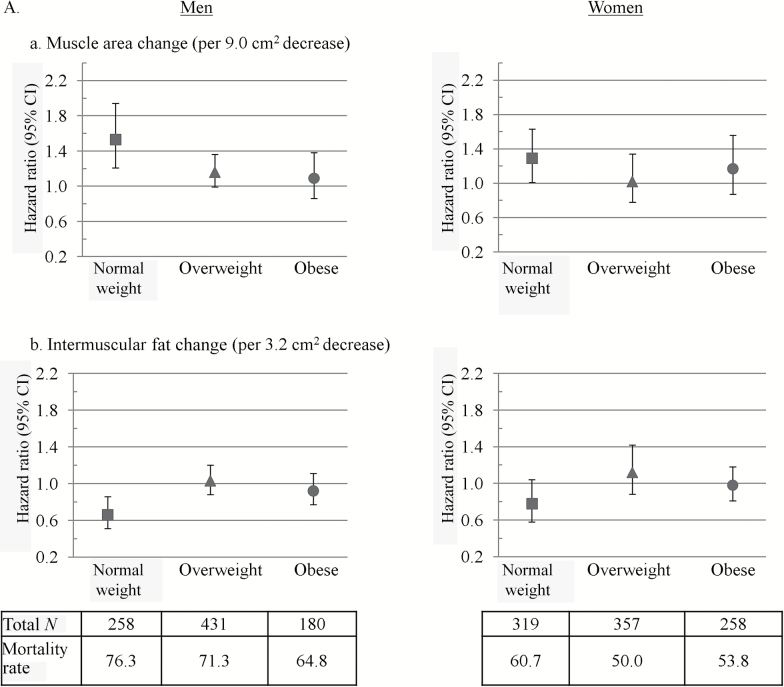
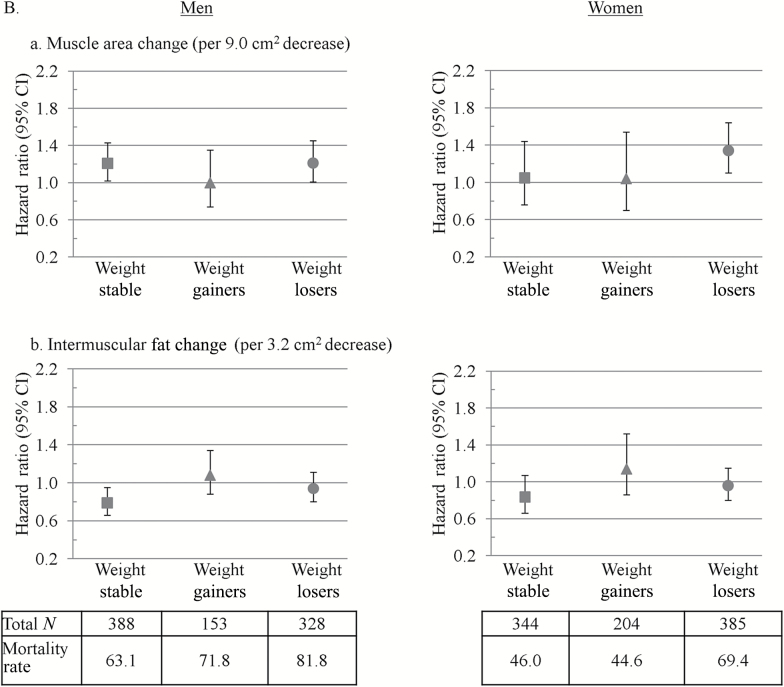
Finally, we found that associations between thigh muscle area decrease and mortality tended to be stronger in the normal weight (Figure 2a) and weight loser groups (Figure 2b). Associations between IMAT increases with mortality were significant in normal weight (Figure 2a) and weight stable men (Figure 2b).
Figure 2.
Hazard ratios for mortality associated with changes in (a) intermuscular fat and (b) thigh muscle in men and women by baseline (A) body mass index (BMI) and (B) weight change status. Hazard ratios are from Cox models and are per SD change over 5 years with an increase as the referent. Models were adjusted for age, race, study site, physical activity, education (men only), smoking, baseline body composition measure, diabetes, cardiovascular disease, hypertension, chronic obstructive pulmonary disease (women only), depression (men only), Modified Mini-Mental State Examination score, and change in total body weight. Mortality rates are deaths per 1,000 person years. Thigh muscle area and intermuscular fat were measured by computed tomography. BMI groups were defined as follows: normal weight (BMI < 25.0kg/m2), overweight (BMI = 25.0–29.9kg/m2), and obese (BMI ≥30kg/m2). p Values for BMI category by muscle and intermuscular fat change interactions on mortality were p = .04 and p = .12 in men and p = .57 and p = .36 in women, respectively. Weight change groups were defined as follows: weight stable (<3% weight change), weight gainers (≥3% weight increase), and weight losers (≥3% weight decrease). p Values for weight change direction by muscle and intermuscular fat change interactions on mortality were both p = .06 in men and p = .11 and p = .39 in women, respectively.
Discussion
We demonstrated that failure to appropriately conserve thigh muscle is specifically related to risk of death and this excess loss of muscle partly explains why weight loss is a risk factor for death. Of note, these associations were not detected with DXA, which is currently considered the standard for defining sarcopenia (17–19). Together these findings suggest that sarcopenia may be better defined longitudinally than cross-sectionally and using CT rather than DXA. Furthermore, the risk of mortality associated with loss of thigh muscle was higher in those who lost weight, suggesting that a more catabolic context of lean change is important in understanding its impact on health. The risk of decreasing thigh muscle area was also stronger in normal weight individuals; thus, preserving muscle may be particularly important for survival in those with lower BMI.
Cross-sectional measures of body composition have not proven to be strong predictors of mortality (5–7). However, several studies have shown an association between lean mass loss and mortality (11,20–22), but included only men or did not evaluate whether it is the excessive loss of muscle mass after accounting for weight loss that is important. This study contributes novel findings to the field as we show that loss of thigh muscle area was associated with higher mortality in both men and women, independent of chronic diseases and overall weight change. Thus, excessive loss of thigh muscle area in older age is an important, independent risk factor for death.
Another important finding was that weight loss appeared to be more strongly associated with mortality in women than men. Similarly, after adjustment for total weight change, the relationships between body composition changes (especially from DXA) and mortality were attenuated to a greater degree in women compared with men. This may be due to the fact that women are less lean at baseline, thus have a lower threshold for suboptimal body composition remodeling. Previous work from Health ABC showed that poorer physical function on performance measures in women compared with men is attributable to differences in body composition (23). Future work should specially investigate mechanisms underlying the stronger association between weight loss and mortality in women compared with men.
We also show that, except for IMAT, changes in body fat were not related to mortality. Loss (or lack of accumulation) of IMAT was protective against mortality in normal weight and weight stable men. Weight stability and loss mask the accumulation of IMAT (9); thus, IMAT increase may not be easily recognized as a risk factor. Exercise and weight loss decrease IMAT (24), and these decreases are directly associated with improved physical function (25). More work is needed to determine whether there are risk factors for accumulation of IMAT other than age and obesity that could be modified.
We found no relationship between visceral fat change and mortality. Several studies have shown a relationship between waist circumference and mortality (26–28). One CT study showed that mortality risk increased with higher visceral fat area, but did not assess longitudinal change (29). The Framingham Heart Study failed to show a relationship between baseline visceral fat and all-cause mortality (30). Although our study differs in that we measured change, it is possible that the older age of the cohort could explain the lack of association, as we may have missed a substantial period of body composition remodeling. Studies have shown visceral fat increases as early as the fourth decade of life (31). Second, small 5-year changes in visceral fat were observed, and our data do not suggest a propensity toward central adiposity after the age of 70 years. Studies that capture body composition changes earlier in life are needed to fully understand the impact of central fat accumulation on death and disease.
Strengths of this study include the large, community dwelling sample with repeat measures of body composition rigorously checked to ensure accuracy of changes. Participants were closely followed for over 17 years, including for over 12 years after follow-up CT, with a well-documented history of chronic diseases. Importantly, we carefully evaluated body composition changes in the context of total weight change dynamics. Finally, we were able to adjust for incident diseases during the change period that could have influenced both mortality risk and body composition change. Limitations included the fact that participants were relatively healthy at baseline, which precludes generalization to more frail or institutionalized populations. A considerable number were excluded from the analysis of abdominal CT data due to inadequate alignment of baseline and follow-up scans. Fortunately, the sample with aligned scans was representative and did not appear to be biased. Finally, newer methods such as Creatine-d3, ultrasound, and peripheral quantitative CT may be as accurate and more translatable to the clinical setting.
In conclusion, excess loss of thigh muscle area was the only aspect of age-related body composition change that was most strongly associated with higher mortality in older age and partly explained the association of weight loss with mortality. Thus, monitoring and preserving muscle mass could potentially reduce risk of death in older adults.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
Health ABC was supported by NIA Contracts (N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, NIA grant R01-AG-028050, and NINR grant R01-NR-012459). A.J.S. was supported by a NRSA (T32-AG-000181). A.B.N. was also supported by AG023629 and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center P30-AG-024827.
Conflict of Interest
S.B.K. is Editor in Chief of the Journals of Gerontology Series A: Medical Sciences (JG:MS). A.B.N. is an associate editor of JG:MS. B.H.G. and T.B.H. are on the editorial board of JG:MS. All other authors have no conflicts of interest to disclose.
Supplementary Material
References
- 1. Newman AB, Lee JS, Visser M, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82:872–878; quiz 915. [DOI] [PubMed] [Google Scholar]
- 2. Miljkovic I, Zmuda JM. Epidemiology of myosteatosis. Curr Opin Clin Nutr Metab Care. 2010;13:260–264. doi:10.1097/MCO.0b013e328337d826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vlassopoulos A, Combet E, Lean ME. Changing distributions of body size and adiposity with age. Int J Obes (Lond). 2014;38:857–864. doi:10.1038/ijo.2013.216 [DOI] [PubMed] [Google Scholar]
- 4. Newman AB, Kupelian V, Visser M, et al. ; Health ABC Study Investigators Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi:10.1046/j.1532-5415.2003.51534.x [DOI] [PubMed] [Google Scholar]
- 5. Rolland Y, Gallini A, Cristini C, et al. Body-composition predictors of mortality in women aged ≥75 y: data from a large population-based cohort study with a 17-y follow-up. Am J Clin Nutr. 2014;100:1352–1360. doi:10.3945/ajcn.114.086728 [DOI] [PubMed] [Google Scholar]
- 6. Cesari M, Pahor M, Lauretani F, et al. Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2009;64:377–384. doi:10.1093/gerona/gln031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. [DOI] [PubMed] [Google Scholar]
- 8. Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP; Cardiovascular Study Research Group Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49:1309–1318. doi:10.1046/j.1532-5415.2001.49258.x [DOI] [PubMed] [Google Scholar]
- 9. Delmonico MJ, Harris TB, Visser M, et al. ; Health, Aging, and Body Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi:10.3945/ajcn.2009.28047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008;12:487–491. doi:10.1007/bf02982710 [DOI] [PubMed] [Google Scholar]
- 11. Murphy RA, Patel KV, Kritchevsky SB, et al. ; Health, Aging, and Body Composition Study Weight change, body composition, and risk of mobility disability and mortality in older adults: a population-based cohort study. J Am Geriatr Soc. 2014;62:1476–1483. doi:10.1111/jgs.12954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi:10.1152/physrev.00033.2011 [DOI] [PubMed] [Google Scholar]
- 13. Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. doi:10.2337/diacare.26.2.372 [DOI] [PubMed] [Google Scholar]
- 14. Beavers KM, Beavers DP, Houston DK, et al. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. Am J Clin Nutr. 2013;97:552–560. doi:10.3945/ajcn.112.047860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buford TW, Lott DJ, Marzetti E, et al. Age-related differences in lower extremity tissue compartments and associations with physical function in older adults. Exp Gerontol. 2012;47:38–44. doi:10.1016/j.exger.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783. doi:10.1001/archinte.165.7.777 [DOI] [PubMed] [Google Scholar]
- 17. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi:10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. ; European Working Group on Sarcopenia in Older People Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi:10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi:10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee CG, Boyko EJ, Nielson CM, et al. ; Osteoporotic Fractures in Men Study Group Mortality risk in older men associated with changes in weight, lean mass, and fat mass. J Am Geriatr Soc. 2011;59:233–240. doi:10.1111/j.1532-5415.2010.03245.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Szulc P, Munoz F, Marchand F, Chapurlat R, Delmas PD. Rapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: the prospective MINOS study. Am J Clin Nutr. 2010;91:1227–1236. doi:10.3945/ajcn.2009.28256 [DOI] [PubMed] [Google Scholar]
- 22. Allison DB, Zannolli R, Faith MS, et al. Weight loss increases and fat loss decreases all-cause mortality rate: results from two independent cohort studies. Int J Obes Relat Metab Disord. 1999;23:603–611. [DOI] [PubMed] [Google Scholar]
- 23. Tseng LA, Delmonico MJ, Visser M, et al. Body composition explains sex differential in physical performance among older adults. J Gerontol A Biol Sci Med Sci. 2014;69:93–100. doi:10.1093/gerona/glt027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murphy JC, McDaniel JL, Mora K, Villareal DT, Fontana L, Weiss EP. Preferential reductions in intermuscular and visceral adipose tissue with exercise-induced weight loss compared with calorie restriction. J Appl Physiol. 2012;112:79–85. doi:10.1152/japplphysiol.00355.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Santanasto AJ, Newman AB, Strotmeyer ES, Boudreau RM, Goodpaster BH, Glynn N. Effects of changes in regional body composition on physical function in older adults: a pilot randomized controlled trial. J Nutr Health Aging. 2015;19:913–921. doi:10.1007/s12603-015-0523-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bigaard J, Frederiksen K, Tjønneland A, et al. Waist circumference and body composition in relation to all-cause mortality in middle-aged men and women. Int J Obes (Lond). 2005;29:778–784. doi:10.1038/sj.ijo.0802976 [DOI] [PubMed] [Google Scholar]
- 27. Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69:373–380.10075319 [Google Scholar]
- 28. Staiano AE, Reeder BA, Elliott S, et al. Body mass index versus waist circumference as predictors of mortality in Canadian adults. Int J Obes (Lond). 2012;36:1450–1454. doi:10.1038/ijo.2011.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring). 2006;14:336–341. doi:10.1038/oby.2006.43 [DOI] [PubMed] [Google Scholar]
- 30. Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62:921–925. doi:10.1016/j.jacc.2013.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lemieux S, Prud’homme D, Nadeau A, Tremblay A, Bouchard C, Després JP. Seven-year changes in body fat and visceral adipose tissue in women. Association with indexes of plasma glucose-insulin homeostasis. Diabetes Care. 1996;19:983–991. doi:10.2337/diacare.19.9.983 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.