Abstract
AIM
To verify the validity of the endoscopy guidelines for patients taking warfarin or direct oral anticoagulants (DOAC).
METHODS
We collected data from 218 patients receiving oral anticoagulants (73 DOAC users, 145 warfarin users) and 218 patients not receiving any antithrombotics (age- and sex-matched controls) who underwent polypectomy. (1) We evaluated post-polypectomy bleeding (PPB) risk in patients receiving warfarin or DOAC compared with controls; (2) we assessed the risks of PPB and thromboembolism between three AC management methods: Discontinuing AC with heparin bridge (HPB) (endoscopy guideline recommendation), continuing AC, and discontinuing AC without HPB.
RESULTS
PPB rate was significantly higher in warfarin users and DOAC users compared with controls (13.7% and 13.7% vs 0.9%, P < 0.001), but was not significantly different between rivaroxaban (13.2%), dabigatran (11.1%), and apixaban (13.3%) users. Two thromboembolic events occurred in warfarin users, but none in DOAC users. Compared with the continuing anticoagulant group, the discontinuing anticoagulant with HPB group (guideline recommendation) had a higher PPB rate (10.8% vs 19.6%, P = 0.087). These findings were significantly evident in warfarin but not DOAC users. One thrombotic event occurred in the discontinuing anticoagulant with HPB group and the discontinuing anticoagulant without HPB group; none occurred in the continuing anticoagulant group.
CONCLUSION
PPB risk was similar between patients taking warfarin and DOAC. Thromboembolism was observed in warfarin users only. The guideline recommendations for HPB should be re-considered.
Keywords: High-risk endoscopic procedures, Novel oral anticoagulants, Endoscopic guideline validation, Post-procedure gastrointestinal bleeding
Core tip: First, we found that anticoagulant (AC) users were at higher risk of post-polypectomy bleeding (PPB) than controls. Second, PPB risk was similar between warfarin users and direct oral anticoagulant users, whereas thromboembolism risk was observed only in warfarin users. Third, PPB risk was not significantly different between rivaroxaban, dabigatran, and apixaban users. Fourth, the strategy of discontinuing AC with heparin bridge as recommended in the endoscopy guidelines showed a higher bleeding rate than continuing AC alone and had one thrombotic event, thus indicating that heparin bridge increased bleeding and may not prevent thromboembolism.
INTRODUCTION
The number of oral anticoagulants (AC) used for prophylaxis or treatment of thromboembolic events is expected to increase as the population ages[1,2]. Along with this, the number of colonoscopic polypectomies, the most common high-risk endoscopic procedure, is also expected to increase in patients receiving AC[3-5]. Physicians are thus confronted with the issue of striking a balance between performing procedures with bleeding risk, such as polypectomy, and temporarily discontinuing AC agents to mitigate thromboembolic risk[4,6-8]. Among the AC agents commonly prescribed, warfarin requires careful and complex management because of its intricate pharmacodynamics and narrow therapeutic range[2,9], whereas direct oral anticoagulants (DOAC) offer easier management because of the rapid onset of anticoagulation and short half-lives[10]. However, whether post-polypectomy bleeding (PPB) or thromboembolic risk differs between warfarin and DOAC users remains unknown.
Several endoscopy guidelines recommend that warfarin be discontinued and replaced by heparin bridge (HPB) in patients at high thromboembolic risk during polypectomy[6-8]. In one study, DOAC were also stopped in one-third of patients who underwent HPB for a high-risk endoscopic procedure[11]. As yet however, the guideline recommendation on AC management for polypectomy has not been confirmed by a validation study. In addition, the situation is further complicated in the real-world clinical setting as some physicians may choose to continue the AC agent or to discontinue it without HPB in the peri-endoscopic period[11]. Previous data suggest that patients undergoing HPB are at higher risk of procedural-related bleeding than those not undergoing HPB or continuing their warfarin[12,13]. Therefore, continuing the AC strategy without HPB may be acceptable for polypectomy. However, there are currently no data available on the comparative risks of bleeding and thromboembolism between patients discontinuing AC with HPB, continuing AC, or discontinuing AC without HPB.
To address these gaps in our knowledge, in this study we first evaluated PPB risk in patients receiving warfarin or DOAC compared with patients not receiving any antithrombotics (controls). Second, we assessed the risks of PPB and thromboembolism between the three AC management methods mentioned above, discontinuing AC with HPB (guideline recommendation), continuing AC, and discontinuing AC without HPB.
MATERIALS AND METHODS
Study design, setting, and participants
We conducted a retrospective cohort study at the Department of Gastroenterology, National Center for Global Health and Medicine (NCGM), Japan. NCGM, with 900 beds, is the largest emergency hospital in the Tokyo metropolitan area. We collected clinical and endoscopic data using an electronic medical database (MegaOak online imaging system, NEC, Japan) and an electronic endoscopic database (SolemioEndo, Olympus, Japan). Physicians or nurses input all findings immediately after clinical evaluation or endoscopy into the electronic medical and endoscopic reports. Staff also completed a detailed questionnaire that included patient background factors and medication information during a face-to-face interview with each patient at the endoscopy unit on the same day as pre-colonoscopy[14,15]. Patient selection and the study flow are shown in Figure 1. From the databases, we identified 5950 patients who underwent colonoscopic polypectomy at our institution between August 2010 and December 2016. Of these, 3120 provided responses to the questionnaire during the interview. We identified 227 patients receiving oral AC (cases) and 1981 patients not receiving any antithrombotics (controls). Then, we reviewed the clinical and endoscopic data for each patient and excluded the following patients: among cases, 6 patients whose clinical information could not be accurately collected and 3 patients who underwent polypectomy plus endoscopic submucosal dissection (ESD) simultaneously; among controls, 959 patients whose clinical information could not be accurately collected, 93 patients who underwent polypectomy plus another endoscopic procedure simultaneously, and 31 patients who were lost to follow-up. Then, controls (non-users of antithrombotics) were randomly selected from the cases (AC users) matched for decennial age and sex at a ratio of 1:1. Ultimately, data from a total of 436 patients (218 AC users and 218 controls) were analyzed.
Figure 1.
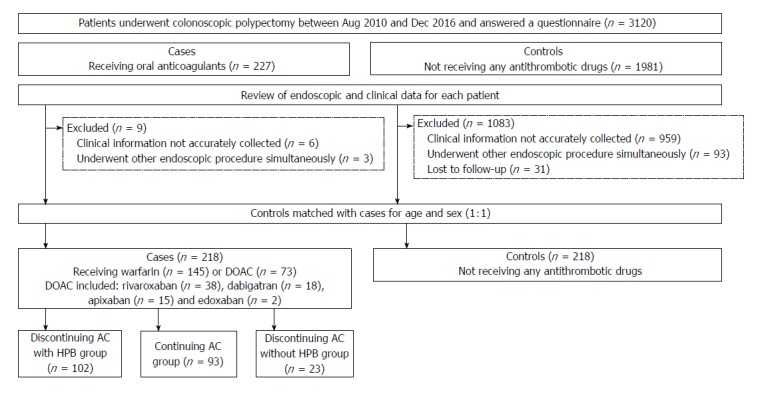
Patient selection and flow. AC: Anticoagulants; DOAC: Direct oral anticoagulants; HPB: Heparin bridge.
This study was approved by the institutional review board of NCGM and patient consent was waived as this was a retrospective study (approval number 2176).
Patient characteristics
Using the electronic database and prospectively collected questionnaire results, we assessed the following factors: height, weight, body mass index (BMI), alcohol, smoking, 14 comorbidities or past history (diabetes mellitus, hypertension, dyslipidemia, chronic kidney disease, abnormal liver function, stroke, bleeding past history, chronic heart disease, vascular disease, acute coronary syndrome, pulmonary embolism, peripheral arterial disease, deep vein disease and advanced cancer), and medication [warfarin, rivaroxaban, dabigatran, apixaban, edoxaban, antiplatelet, and non-steroidal anti-inflammatory drugs (NSAIDs)]. We also evaluated laboratory data before colonoscopy [platelet count, prothrombin time-international normalized ratio (PT-INR), and creatinine clearance (Ccr)] and calculated the HAS-BLED[16] and CHA2DS2-VASc2[17] scores. During hospitalization, data were collected on the following AC management factors: HPB use, HPB duration, drug continuation/discontinuation, and use of reversal agent (vitamin K).
Endoscopic factors
After full bowel preparation, polypectomies were done with or without local injection of saline using a high-resolution colonoscope (CF260AI or CF260AZI, Olympus Co., Tokyo, Japan), snare (SnareMaster, Olympus Co.), and electrosurgical device (ERBE ICC-350, Somo Technology Inc., Tokyo, Japan or ESG-100, Olympus Co.). After polypectomy, patients routinely underwent prophylactic clipping. Number of polyps and polyp size were evaluated from data in the endoscopic database. Advanced adenoma was defined as adenoma ≥ 1 cm with villous components (tubulovillous or villous) or high-grade or severe dysplasia[18].
AC management and heparin bridge
American, European, and Asian guidelines[6-8] recommend that patients discontinue AC and be bridged with heparin before polypectomy, and to confirm the validity of this strategy, we classified patients during the peri-endoscopic period into three main AC management groups: (1) Discontinuing AC with HPB (as recommended by the guidelines); (2) continuing AC alone (i.e., without HPB) before endoscopy; and (3) discontinuing AC for > 24 h without HPB before endoscopy. Which of these strategies was adopted was at the discretion of the treating physician.
For HPB, patients received prophylactic unfractionated heparin infusion intravenously (because low-molecular-weight heparin is not covered by Japan’s health care insurance system[8,19]), with the exception of 1 patient who received low-molecular-weight heparin because of heparin-induced thrombocytopenia.
In our institution, we carry out anticoagulant management during high-risk endoscopy in accordance with the Japanese Endoscopy Guidelines[8]; warfarin was stopped 3-5 d before endoscopy and DOAC was stopped 24-48 h after endoscopy. Heparin was administered after cessation of anticoagulants[8]. INR value before polypectomy was set at < 1.5 in warfarin users[8]. In these users, heparin was continued until INR was optimal after polypectomy. Because the guidelines do not recommend HPB for DOAC users[8], some DOAC users continued heparin for one day and others did not use heparin after polypectomy. The HPB period included the entire period before and after polypectomy.
Clinical outcomes
The main outcomes of interest were PPB within 30 d of polypectomy. PPB was defined as massive, continuous, or frequent hematochezia after polypectomy[20]. Not all patients underwent additional colonoscopy when PPB occurred, but those with unstable vital signs or in need of transfusion tended to undergo colonoscopy. Major bleeding was defined according to the International Society on Thrombosis and Haemostasis (ISTH) bleeding scale as (1) fatal bleeding; and/or (2) symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome; and/or (3) bleeding causing a fall in hemoglobin level of 20 g/L (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or red cells[21]. In addition, we defined late PPB as bleeding occurring more than 24 h after polypectomy and all other cases as early PPB[22]. We defined a thromboembolic event as the occurrence of acute coronary syndrome, stroke, transient ischemic attack, pulmonary embolism, deep vein thrombosis, or arterial thromboembolism. We also evaluated mortality at 30 d after polypectomy. Date and cause of death were ascertained from the electronic medical records and death certificates.
Statistical analysis
Pearson’s chi-squared test or Fisher’s exact test was used for categorical data to assess the difference in risk factors between subjects. Continuous data were compared with Mann-Whitney U test. Risk factors were examined by univariate and multivariate analysis. Odds ratios (OR) and 95% confidence intervals (CI) were estimated.
First, we compared baseline characteristics and clinical outcomes between AC users and controls. Second, we compared baseline characteristics and clinical outcomes between the following groups: discontinuing AC with HPB and continuing AC group alone and between discontinuing AC with HPB group and discontinuing AC without HPB group. These comparisons were also evaluated for the subgroups of warfarin and DOAC users.
Third, to determine the risk factors for PPB, we conducted univariate and multivariate analysis. In multivariate analysis, we developed multivariate models adjusting for propensity score for each strategy. Although there are four different propensity score methods-matching, stratification, inverse probability treatment weighting, and covariates adjustment[23,24]-we used propensity score as a covariate rather than perform a regression adjustment with all of the covariates (traditional covariate adjustment[25]), because many covariates were associated with a small number of bleeding outcomes in this study and we did not want to lose the observations of patients as typically occurs in matching. Propensity score as a covariate method allows for a large number of baseline variables to be included in the regression model, which are not adequately adjusted for when there are insufficient numbers of outcomes[23,24]. To estimate the propensity score, we employed a logistic regression model including potentially clinically important variables. Some of these were shown to differ (P < 0.10) between groups. We evaluated the area under the receiver operating characteristic (ROC) curve for each propensity score in each group.
A P value of < 0.05 was considered statistically significant. All statistical analyses were conducted using STATA version 14 software (StataCorp, College Station, TX, United States).
RESULTS
Baseline characteristics and outcomes of AC users and controls
There were some significant differences in baseline characteristics between AC users and controls (Table 1). In terms of outcomes, there were 32 patients with PPB and only 2 patients with major bleeding, both of whom were warfarin users and received HPB. Four patients had early PPB (bleeding within 24 h) and 28 with late PPB: 9 cases at day 2, 9 cases at day 3, 6 cases at day 4, 1 case at day 5, 2 cases at day 6, and 1 case at day 8. The 4 patients with early PPB were all warfarin users. Compared with controls, there were a significantly higher rate among AC users of PPB (13.7% vs 0.9%, P < 0.001; Figure 2). Adjusting for propensity score between groups, AC users had a significantly increased PPB risk (adjusted OR = 18.9, P < 0.001; Table 2). Two thromboembolic events occurred in AC users, but none in controls. Thromboembolism occurred in 2 warfarin users and no DOAC users. No mortality events were noted in either group.
Table 1.
Baseline characteristics of oral anticoagulant users, warfarin users, direct oral anticoagulants users, and controls not taking any antithrombotic drugs (n = 436) n (%)
| Factors | Controls (n = 218) | AC users (n = 218) | P value Control vs AC users | Warfarin users (n = 145) | P value Control vs warfarin users | DOAC users (n = 73) | P value Control vs DOAC users |
| Age ≥ 75 yr | 104 (47.1) | 113 (51.8) | 0.389 | 79 (54.5) | 0.206 | 34 (46.6) | 0.867 |
| Male | 157 (72.0) | 157 (72.0) | 1.000 | 103 (71.0) | 0.839 | 54 (74.0) | 0.746 |
| BMI ≥ 25 | 54 (24.8) | 69 (31.7) | 0.110 | 44 (30.3) | 0.241 | 25 (34.2) | 0.115 |
| Drinker | 119 (54.6) | 131 (62.1) | 0.115 | 77 (55.4) | 0.881 | 54 (75.0) | 0.002 |
| Smoker | 36 (16.5) | 32 (14.8) | 0.626 | 21 (14.6) | 0.622 | 11 (15.3) | 0.805 |
| Laboratory data | |||||||
| Platelet < 10 × 104 μL | 6 (2.8) | 5 (2.3) | 1.000 | 3 (2.1) | 1.000 | 2 (2.7) | 1.000 |
| Ccr < 30 mL/min | 9 (4.1) | 24 (11.0) | 0.007 | 20 (13.8) | 0.001 | 4 (5.48) | 0.743 |
| Comorbidities | |||||||
| Diabetes mellitus | 45 (20.6) | 52 (23.9) | 0.420 | 39 (26.9) | 0.166 | 13 (17.8) | 0.600 |
| Hypertension | 121 (55.5) | 148 (67.9) | 0.008 | 94 (64.8) | 0.077 | 54 (74.0) | 0.005 |
| Dyslipidemia | 74 (33.9) | 102 (46.8) | 0.006 | 67 (46.2) | 0.019 | 35 (48.0) | 0.037 |
| Chronic kidney disease | 49 (22.5) | 37 (17.0) | 0.149 | 32 (22.1) | 0.927 | 5 (6.9) | 0.003 |
| Abnormal liver function | 15 (6.9) | 8 (3.7) | 0.134 | 3 (2.1) | 0.047 | 5 (6.9) | 0.993 |
| Stroke | 10 (4.6) | 47 (21.6) | < 0.001 | 29 (20.0) | < 0.001 | 18 (24.7) | < 0.001 |
| Bleeding past history | 21 (9.6) | 13 (6.0) | 0.153 | 10 (6.9) | 0.361 | 3 (4.1) | 0.217 |
| Chronic heart failure | 1 (0.5) | 56 (25.7) | < 0.001 | 46 (31.7) | < 0.001 | 10 (13.7) | < 0.001 |
| Vascular disease | 6 (2.8) | 56 (25.7) | < 0.001 | 49 (33.8) | < 0.001 | 7 (9.6) | 0.014 |
| Acute coronary syndrome | 6 (2.8) | 34 (15.6) | < 0.001 | 28 (19.3) | < 0.001 | 6 (8.2) | 0.042 |
| Pulmonary embolism | 0 (0.0) | 7 (3.2) | 0.008 | 6 (4.1) | 0.004 | 1 (1.4) | 0.251 |
| Peripheral arterial disease | 0 (0.0) | 7 (3.2) | 0.008 | 6 (4.1) | 0.004 | 1 (1.4) | 0.251 |
| Deep vein thrombosis | 0 (0.0) | 14 (6.4) | < 0.001 | 14 (9.7) | < 0.001 | 0 | NA |
| Advanced carcinoma | 7 (3.2) | 33 (15.1) | < 0.001 | 21 (14.5) | < 0.001 | 12 (16.4) | < 0.001 |
| Medications | |||||||
| Antiplatelet | 0 (0.0) | 53 (24.3) | < 0.001 | 43 (30.0) | < 0.001 | 10 (13.7) | < 0.001 |
| Low-dose aspirin | 0 (0.0) | 40 (18.4) | < 0.001 | 33 (22.8) | < 0.001 | 7 (9.6) | < 0.001 |
| Thienopyridine1 | 0 (0.0) | 5 (2.3) | 0.025 | 5 (3.5) | 0.006 | 0 (0.0) | NA |
| Other antiplatelets2 | 0 (0.0) | 11 (5.1) | 0.001 | 8 (5.5) | < 0.001 | 3 (4.1) | 0.003 |
| NSAIDs | 21 (9.6) | 7 (3.2) | 0.006 | 3 (2.1) | 0.004 | 4 (5.5) | 0.341 |
| Endoscopic factors | |||||||
| Number of polyps | 2.0 ± 1.4 | 8.3 ± 5.3 | 0.019 | 2.4 ± 1.8 | 0.063 | 2.5 ± 1.8 | 0.041 |
| Number of polyps ≥ 5 | 13 (6.0) | 28 (12.8) | 0.014 | 17 (11.7) | 0.078 | 11 (15.1) | 0.014 |
| Polyp size | 6.0 ± 3.3 | 6.3 ± 3.4 | < 0.001 | 8.7 ± 5.9 | < 0.001 | 7.4 ± 3.7 | 0.001 |
| Polyp size ≥ 10 mm | 28 (12.8) | 69 (31.7) | < 0.001 | 47 (32.4) | < 0.001 | 22 (30.1) | 0.001 |
| Advanced adenoma3 | 27 (12.4) | 64 (29.4) | < 0.001 | 43 (29.7) | < 0.001 | 21 (28.8) | 0.001 |
Thienopyridine includes ticlopidine, clopidogrel, and prasugrel;
Other antiplatelets are antiplatelets other than low-dose aspirin and thienopyridine;
Advanced adenoma is adenoma ≥ 1 cm with villous components (tubulovillous or villous) or high-grade or severe dysplasia. Values in parentheses are percentages. Values presented with a plus/minus sign are means ± SD. Bold type indicates statistical significance (P < 0.05). AC: Anticoagulant; DOAC: Direct oral anticoagulants; BMI: Body mass index; Ccr: Creatinine clearance; NSAIDs: Non-steroidal anti-inflammatory drugs.
Figure 2.
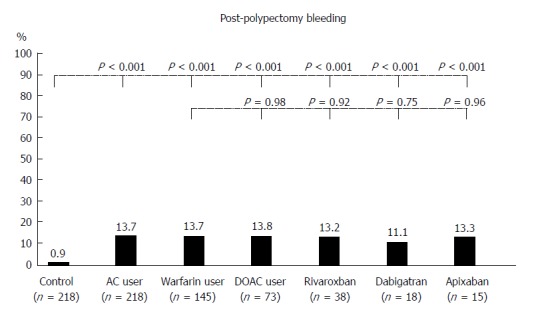
Thirty-day post-polypectomy bleeding in controls (n = 218), anticoagulants users (n = 218) and subgroups of warfarin (n = 145) and direct oral anticoagulants users [n = 73: rivaroxaban (n = 38), dabigatran (n = 18), and apixaban (n = 15)]. P-values for comparison of each group with controls and for comparison of direct oral anticoagulants users with warfarin users. AC: Anticoagulants; DOAC: Direct oral anticoagulants.
Table 2.
Crude and adjusted odds ratios for post-polypectomy bleeding in controls (n = 218), anticoagulant users (n = 218), warfarin users (n = 145), and direct oral anticoagulants users (n = 73)
| Subjects | Crude OR (95%CI) | P value | Propensity score-adjusted OR1 (95%CI) | P value |
| Controls | 1 (referent) | 1 (referent) | ||
| AC users | 17.2 (4.1-73.1) | < 0.001 | 18.9 (4.2-85.5) | < 0.001 |
| Warfarin users | 17.3 (4.0-75.2) | < 0.001 | 18.6 (3.8-89.9) | < 0.001 |
| DOAC users | 17.1 (3.7-80.3) | < 0.001 | 17.8 (3.2-98.8) | 0.001 |
Propensity score estimations. Values in parentheses are percentages. Values presented with a plus/minus sign are means ± SD; bold type indicates statistical significance (P < 0.05). AC users vs controls: Logistic regression model included 17 factors that are potentially clinically important variables; area under the receiver operating characteristic (ROC) curve for propensity scores for AC users was 0.81 (95%CI: 0.77-0.85); Warfarin users vs controls: Logistic regression model included 18 factors that are potentially clinically important variables; area under the ROC curve for propensity scores for warfarin users was 0.83 (95%CI: 0.78-0.88); DOAC users vs controls: Logistic regression model included 14 factors that are potentially clinically important variables; area under the ROC curve for DOAC user propensity scores was 0.85 (95%CI: 0.80-0.90). NA: Not applicable; AC: Anticoagulants; DOAC: Direct oral anticoagulants; HPB: Heparin bridge; OR: Odds ratio.
Warfarin users vs DOAC users
In the subgroup analysis of warfarin users, there were some significant differences in baseline characteristics with controls (Table 1). In terms of outcomes, warfarin users had a significantly higher rate of PPB (13.7% vs 0.9%, P < 0.001; Figure 2); a significantly increased PPB risk when adjusting for propensity score (adjusted OR = 18.6, P < 0.001; Table 2). In the subgroup analysis of DOAC users, there were also some significant differences in baseline characteristics with controls (Table 1). As for outcomes, DOAC users had a significantly higher rate of PPB (13.8% vs 0.9%, P < 0.001; Figure 2); significantly increased PPB risk when adjusting for propensity score (adjusted OR = 17.8, P = 0.001; Table 2). PPB rates did not differ significantly between rivaroxaban, dabigatran, and apixaban users (Figure 2).
Differences in baseline characteristics and clinical outcomes between the three AC management strategies
Discontinuing AC with HPB (guideline recommendation) vs continuing AC: There were some significant differences in baseline characteristics between strategies (Supplementary Table 1). The discontinuing AC with HPB group showed a higher rate of PPB (19.6% vs 10.8%, P = 0.087; Figure 3A); a higher PPB risk when adjusting for propensity score (adjusted OR = 2.2, P = 0.069; Table 3).
Figure 3.
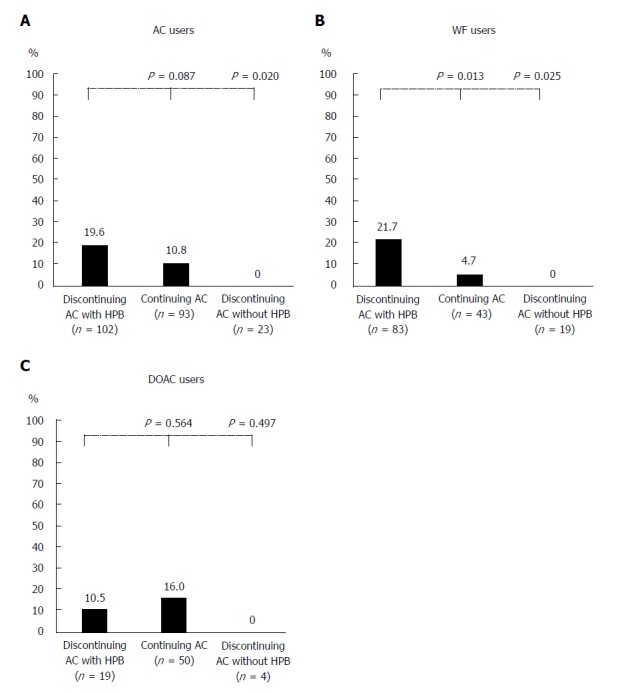
Post-polypectomy bleeding according to the three main anticoagulants management strategies in anticoagulants (A), warfarin (B), and direct oral anticoagulants (C) users. For the 218 patients, 102 patients (46.8%) in the discontinuing anticoagulants with heparin bridge group, 93 (42.7%) in the continuing anticoagulants group, and 23 (10.6%) in the discontinuing anticoagulants without heparin bridge group. AC: Anticoagulants; DOAC: Direct oral anticoagulants; HPB: Heparin bridge.
Table 3.
Crude and adjusted odds ratios for post-polypectomy bleeding in anticoagulant users (n = 218), warfarin users (n = 145), and direct oral anticoagulants users (n = 73)
| AC management during peri-endoscopic period | Crude OR (95%CI) | P value | Propensity score-adjusted OR1 (95%CI) | P value |
| AC users | ||||
| Discontinuing AC with HPB vs continuing AC | 2.0 (0.9-4.6) | 0.091 | 2.2 (0.9-5.2) | 0.069 |
| Discontinuing AC with HPB vs discontinuing AC without HPB | 7.7 (1.3-Inf) | 0.023 | NA | NA |
| Warfarin users | ||||
| Discontinuing warfarin with HPB vs continuing warfarin | 5.7 (1.3-25.8) | 0.024 | 4.7 (1.0-22.1) | 0.049 |
| Discontinuing warfarin with HPB vs discontinuing warfarin without HPB | 7.2 (1.1-Inf) | 0.033 | NA | NA |
| DOAC users | ||||
| Discontinuing DOAC with HPB vs continuing DOAC | 0.6 (0.1-3.2) | 0.567 | 0.7 (0.1-4.5) | 0.664 |
| Discontinuing DOAC with HPB vs discontinuing DOAC without HPB | 0.5 (0.4-Inf) | 1.000 | NA | NA |
Propensity score estimations. Values in parentheses are percentages. Values presented with a plus/minus sign are means ± SD; bold type indicates statistical significance (P < 0.05). Continuing AC group vs standard group: Logistic regression model included 8 factors that are potentially clinically important variables; area under the ROC curve for propensity scores for the continuing AC group was 0.71 (95%CI: 0.63-0.79); standard group vs continuing warfarin group: Logistic regression model included 6 factors that are potentially clinically important variables; area under the ROC curve for propensity scores for the continuing warfarin group was 0.63 (95%CI: 0.53-0.73); standard group vs continuing DOAC group: Logistic regression model included 6 factors that are potentially clinically important variables; area under the ROC curve for propensity scores for the continuing DOAC group was 0.90 (95%CI: 0.82-0.98). NA: Not applicable; AC: Anticoagulants; CI: Confidential interval; DOAC: Direct oral anticoagulants; HPB: Heparin bridge; Inf: Infinity; OR: Odds ratio.
In the warfarin subgroups, the discontinuing warfarin with HPB group showed a significantly higher rate of PPB (21.7% vs 4.7%, P = 0.013; Figure 3B); increased PPB risk on multivariate analysis (Table 3). In the subgroup of DOAC users, there were no significant differences between the two groups in PPB risk (Figure 3C), and multivariate models adjusted for propensity score also revealed no significant difference (Table 3).
Discontinuing AC with HPB (guideline recommendation) vs discontinuing AC without HPB: The discontinuing AC with HPB group showed a significantly higher rate of PPB (19.6% vs 0.0%, P = 0.020; Figure 3A); increased PPB risk on univariate analysis (OR = 7.7, P = 0.023; Table 3).
In the warfarin subgroups, the discontinuing AC with HPB group had a significantly higher rate of PPB (21.7% vs 0%, P = 0.025; Figure 3B); increased PPB risk on univariate analysis (OR = 7.2, P =0.033; Table 3). In the DOAC subgroups, there were no significant differences in PPB risk between the two subgroups (Table 3).
Association of rate of PPB with HPB duration and INR value at endoscopy
The rate of PPB increased significantly with longer duration of HPB (P = 0.015 for trend; Figure 4). This trend was also found in warfarin and DOAC users (Figure 4). Rate of PPB was 18.7% for INR < 1.5, 0% for INR 1.5-1.9, 25% for INR 2.0-2.4, and 0% for INR > 2.5. INR value at pre-endoscopy did not predict PPB (P = 0.431 for trend; Supplementary Figure 1).
Figure 4.
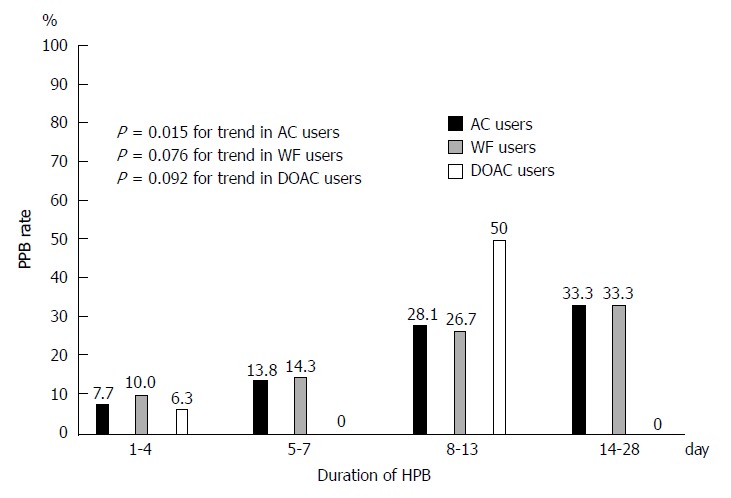
Association of post-polypectomy bleeding rate with duration of heparin bridge in anticoagulants, warfarin, and direct oral anticoagulants users. AC: Anticoagulants; WF: Warfarin; DOAC: Direct oral anticoagulants; HPB: Heparin bridge; PPB: Post-polypectomy bleeding.
DISCUSSION
The four main findings of the study are as follows: (1) AC users were at higher risk of PPB than controls; (2) PPB risk was similar between warfarin users and DOAC users, whereas thromboembolism risk was observed only in warfarin users; (3) PPB risk was not significantly different between rivaroxaban, dabigatran, and apixaban users; and (4) the recommended strategy of discontinuing AC with HPB showed a higher bleeding rate than continuing AC alone and had one thrombotic event, indicating that HPB increased bleeding and may not prevent thromboembolism. These findings were significantly evident in warfarin users compared with DOAC users.
In agreement with past studies, our AC users had a significantly higher OR for PPB than did controls (adjusted OR = 18.9). Witt et al[26] reported that PPB occurred more often in AC users than non-AC users (adjusted OR = 11.6). Hui et al[27] demonstrated that warfarin use was an independent risk factor for PPB (adjusted OR = 13.4). The ORs in these studies were lower than ours because their control subjects included antiplatelet users.
We revealed for the first time in this study that PPB risk was similar between warfarin and DOAC users compared with controls. A meta-analysis study indicated a higher risk of non-procedural-related bleeding in DOAC users than in warfarin users[28]. Thus, bleeding risk might be different between procedure-related and non-procedure-related bleeding. Only limited data are available on differences in post-endoscopic bleeding between DOAC and warfarin users. In this study, we found that 14% of DOAC and warfarin users had PPB. In agreement with this, Nagata et al[29] showed that 14% of DOAC users had PPB and 16.9% of warfarin users had PPB (P = 0.324). However, post-polypectomy-related bleeding differ according to site of the bleed in the upper or lower GI tract, because upper GI polypectomy-related bleeding was higher in warfarin users than in DOAC users (P = 0.06)[29].
Several endoscopy guidelines recommend that AC be discontinued with HPB[6-8]. However, in our study, following this guideline strategy showed a higher bleeding risk and longer hospital stay compared with the continuing AC strategy, and one thrombotic event occurred with the guideline strategy and none in the continuing AC strategy. These findings suggest that continuing oral AC might be acceptable for polypectomy.
Consistent with our results, a meta-analysis[30] showed that HPB was associated with a higher rate of PPB and did not prevent thromboembolism. A randomized study[13] found that post-procedural bleeding risk was higher in patients with HPB than in those without it, and thromboembolic risk was similar in both groups. Taken together, the evidence suggests that the recommendation of several endoscopic guidelines[6-8] should be re-evaluated.
It is not clear why following the guideline strategy was associated with increased PPB risk in warfarin users but not DOAC users. One possible explanation is that in warfarin users, it takes several days for the anticoagulant effect to be sufficient, whereas onset is rapid with DOAC and therapeutic anticoagulation is achieved in a few hours[31]. The criterion for discontinuing heparin in warfarin users is that INR reaches the effective range, but the time to reach this range varies among patients. Therefore, heparin may need to be used for a long time after the procedure; the time is much shorter in DOAC users. Also, simultaneously administering warfarin and heparin (double anticoagulation effect) can increase bleeding risk. From these considerations, GI bleeding risk is high when HPB is performed in warfarin users compared with DOAC users. These prior findings, together with ours here, suggest that warfarin should be switched to DOAC before high-risk endoscopic procedures are performed.
One of the strengths of our study was the analysis of detailed clinical and endoscopic data that was collected and that we could adjust for propensity score by including these factors in the multivariate models. Another was that we identified a difference in clinical outcomes between the three main AC management strategies investigated. We also recognize limitations. First, this was a retrospective study conducted at a single site. Second, the AC users were heterogeneous and included those with atrial fibrillation, valvular disease, or with low or high thromboembolic risk. Third, we have no data on subcutaneous heparin because intravenous heparin is used in Japan. However, a previous study reported a similar incidence of major bleeding between patients treated with subcutaneous unfractionated heparin and those treated with intravenous unfractionated heparin (OR 0.91).
In conclusion, patients receiving oral AC had higher risks of bleeding after colonoscopic polypectomy compared with patients not receiving any antithrombotics. PPB risk was similar between warfarin and DOAC users, whereas thromboembolism risk was observed in warfarin users only. HPB increased bleeding risk, and may not prevent thromboembolism and therefore the current guideline recommendation should be re-considered. Continuing oral AC may be acceptable for polypectomy.
ARTICLE HIGHLIGHTS
Research background
The number of oral anticoagulants (AC) used increases as the population ages, and the number of colonoscopic polypectomies is expected to increase in patients receiving AC.
Research motivation
Whether post-polypectomy bleeding (PPB) or thromboembolic risk differs between warfarin and direct oral anticoagulant (DOAC) users remains unknown.
Research objectives
We evaluated PPB risk in patients receiving warfarin or DOAC compared with patients not receiving any antithrombotics (controls). We also assessed the risks of PPB and thromboembolism between the three AC management methods mentioned above, discontinuing AC with heparin bridge (guideline recommendation), continuing AC, and discontinuing AC without heparin bridge.
Research methods
We conducted a retrospective cohort study and collected data from 218 patients receiving oral anticoagulants (73 DOAC users, 145 warfarin users) and 218 patients not receiving any antithrombotics (age- and sex-matched controls) who underwent polypectomy.
Research results
PPB rate was significantly higher in both warfarin users and DOAC users compared with controls. Two thromboembolic events occurred in warfarin users, but none in DOAC users. Compared with the continuing anticoagulant group, the discontinuing anticoagulant with heparin bridge group (guideline recommendation) had a higher PPB rate. One thrombotic event occurred in the discontinuing anticoagulant with heparin bridge group and the discontinuing anticoagulant without heparin bridge group; none occurred in the continuing anticoagulant group.
Research conclusions
Patients receiving oral anticoagulant had higher risks of bleeding after colonoscopic polypectomy compared with patients not receiving any antithrombotics. PPB risk was similar between warfarin and DOAC users, whereas thromboembolism risk was observed in warfarin users only. Heparin bridge increased bleeding risk, and may not prevent thromboembolism.
Research perspectives
The current guideline recommendation for heparin bridge should be re-considered, and continuing oral anticoagulant may be acceptable for polypectomy.
ACKNOWLEDGMENTS
The authors thank clinical research coordinators Kuniko Miki, Kenko Yoshida, Eiko Izawa, and Hisae Kawashiro, for their help with data collection.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Supported by Grant-in-Aid for Research from the National Center for Global Health and Medicine (29-2001) partly. The funding agency played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: The study design was approved by the ethics committee of the National Center for Global Health and Medicine (Approval No. 2176).
Informed consent statement: This study was a retrospective observational study, and informed consent to participate was obtained by the opt-out method at our institution.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Peer-review started: February 21, 2018
First decision: March 9, 2018
Article in press: March 25, 2018
P- Reviewer: Chuah SK, Cui J, Qi XS, Zuniga VL S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
Contributor Information
Naohiro Yanagisawa, Department of Gastroenterology and Hepatology, National Center for Global Health and Medicine, Tokyo 162-8655, Japan.
Naoyoshi Nagata, Department of Gastroenterology and Hepatology, National Center for Global Health and Medicine, Tokyo 162-8655, Japan. nnagata_ncgm@yahoo.co.jp.
Kazuhiro Watanabe, Department of Gastroenterology and Hepatology, National Center for Global Health and Medicine, Tokyo 162-8655, Japan.
Tatsuhiro Iida, Department of Gastroenterology and Hepatology, National Center for Global Health and Medicine, Tokyo 162-8655, Japan.
Mariko Hamada, Department of Gastroenterology and Hepatology, National Center for Global Health and Medicine, Tokyo 162-8655, Japan.
Sakurako Kobayashi, Department of Gastroenterology and Hepatology, National Center for Global Health and Medicine, Tokyo 162-8655, Japan.
Takuro Shimbo, Ohta Nishinouchi Hospital, Fukushima 963-8022, Japan.
Junichi Akiyama, Department of Gastroenterology and Hepatology, National Center for Global Health and Medicine, Tokyo 162-8655, Japan.
Naomi Uemura, Department of Gastroenterology and Hepatology, National Center for Global Health and Medicine, Kohnodai Hospital, Chiba 272-8516, Japan.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Humbert X, Roule V, Chequel M, Fedrizzi S, Brionne M, Lelong-Boulouard V, Milliez P, Alexandre J. Non-vitamin K oral anticoagulant treatment in elderly patients with atrial fibrillation and coronary heart disease. Int J Cardiol. 2016;222:1079–1083. doi: 10.1016/j.ijcard.2016.07.212. [DOI] [PubMed] [Google Scholar]
- 3.Abraham NS, Castillo DL. Novel anticoagulants: bleeding risk and management strategies. Curr Opin Gastroenterol. 2013;29:676–683. doi: 10.1097/MOG.0b013e328365d415. [DOI] [PubMed] [Google Scholar]
- 4.Baron TH, Kamath PS, McBane RD. Management of antithrombotic therapy in patients undergoing invasive procedures. N Engl J Med. 2013;368:2113–2124. doi: 10.1056/NEJMra1206531. [DOI] [PubMed] [Google Scholar]
- 5.Kwok A, Faigel DO. Management of anticoagulation before and after gastrointestinal endoscopy. Am J Gastroenterol. 2009;104:3085–3097; quiz 3098. doi: 10.1038/ajg.2009.469. [DOI] [PubMed] [Google Scholar]
- 6.Veitch AM, Vanbiervliet G, Gershlick AH, Boustiere C, Baglin TP, Smith LA, Radaelli F, Knight E, Gralnek IM, Hassan C, et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Endoscopy. 2016;48:385–402. doi: 10.1055/s-0042-102652. [DOI] [PubMed] [Google Scholar]
- 7.ASGE Standards of Practice Committee. Acosta RD, Abraham NS, Chandrasekhara V, Chathadi KV, Early DS, Eloubeidi MA, Evans JA, Faulx AL, Fisher DA, Fonkalsrud L, Hwang JH, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Shergill AK, Wang A, Cash BD, DeWitt JM. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016;83:3–16. doi: 10.1016/j.gie.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto K, Fujishiro M, Kato M, Higuchi K, Iwakiri R, Sakamoto C, Uchiyama S, Kashiwagi A, Ogawa H, Murakami K, et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc. 2014;26:1–14. doi: 10.1111/den.12183. [DOI] [PubMed] [Google Scholar]
- 9.Phillips KW, Ansell J. Outpatient management of oral vitamin K antagonist therapy: defining and measuring high-quality management. Expert Rev Cardiovasc Ther. 2008;6:57–70. doi: 10.1586/14779072.6.1.57. [DOI] [PubMed] [Google Scholar]
- 10.Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206–232. doi: 10.1007/s11239-015-1310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heublein V, Pannach S, Daschkow K, Tittl L, Beyer-Westendorf J. Gastrointestinal endoscopy in patients receiving novel direct oral anticoagulants: results from the prospective Dresden NOAC registry. J Gastroenterol. 2018;53:236–246. doi: 10.1007/s00535-017-1346-x. [DOI] [PubMed] [Google Scholar]
- 12.Li HK, Chen FC, Rea RF, Asirvatham SJ, Powell BD, Friedman PA, Shen WK, Brady PA, Bradley DJ, Lee HC, et al. No increased bleeding events with continuation of oral anticoagulation therapy for patients undergoing cardiac device procedure. Pacing Clin Electrophysiol. 2011;34:868–874. doi: 10.1111/j.1540-8159.2011.03049.x. [DOI] [PubMed] [Google Scholar]
- 13.Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS, Garcia DA, Jacobson A, Jaffer AK, Kong DF, et al. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N Engl J Med. 2015;373:823–833. doi: 10.1056/NEJMoa1501035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagata N, Niikura R, Aoki T, Shimbo T, Kishida Y, Sekine K, Tanaka S, Okubo H, Watanabe K, Sakurai T, et al. Lower GI bleeding risk of nonsteroidal anti-inflammatory drugs and antiplatelet drug use alone and the effect of combined therapy. Gastrointest Endosc. 2014;80:1124–1131. doi: 10.1016/j.gie.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 15.Nagata N, Niikura R, Aoki T, Shimbo T, Sekine K, Okubo H, Watanabe K, Sakurai T, Yokoi C, Yanase M, et al. Association between colonic diverticulosis and bowel symptoms: A case-control study of 1629 Asian patients. J Gastroenterol Hepatol. 2015;30:1252–1259. doi: 10.1111/jgh.12941. [DOI] [PubMed] [Google Scholar]
- 16.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 17.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 18.Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Haug U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840,149 screening colonoscopies. Gut. 2007;56:1585–1589. doi: 10.1136/gut.2007.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ono K, Hidaka H, Koyama Y, Ishii K, Taguchi S, Kosaka M, Okazaki N, Tanimoto W, Katayama A. Effects of heparin bridging anticoagulation on perioperative bleeding and thromboembolic risks in patients undergoing abdominal malignancy surgery. J Anesth. 2016;30:723–726. doi: 10.1007/s00540-016-2187-0. [DOI] [PubMed] [Google Scholar]
- 20.Feagins LA, Iqbal R, Harford WV, Halai A, Cryer BL, Dunbar KB, Davila RE, Spechler SJ. Low rate of postpolypectomy bleeding among patients who continue thienopyridine therapy during colonoscopy. Clin Gastroenterol Hepatol. 2013;11:1325–1332. doi: 10.1016/j.cgh.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 22.Park SK, Seo JY, Lee MG, Yang HJ, Jung YS, Choi KY, Kim H, Kim HO, Jung KU, Chun HK, et al. Prospective analysis of delayed colorectal post-polypectomy bleeding. Surg Endosc. 2018 doi: 10.1007/s00464-018-6048-9. 17: Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, Robins JM. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163:262–270. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 25.Elze MC, Gregson J, Baber U, Williamson E, Sartori S, Mehran R, Nichols M, Stone GW, Pocock SJ. Comparison of Propensity Score Methods and Covariate Adjustment: Evaluation in 4 Cardiovascular Studies. J Am Coll Cardiol. 2017;69:345–357. doi: 10.1016/j.jacc.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 26.Witt DM, Delate T, McCool KH, Dowd MB, Clark NP, Crowther MA, Garcia DA, Ageno W, Dentali F, Hylek EM, et al. Incidence and predictors of bleeding or thrombosis after polypectomy in patients receiving and not receiving anticoagulation therapy. J Thromb Haemost. 2009;7:1982–1989. doi: 10.1111/j.1538-7836.2009.03598.x. [DOI] [PubMed] [Google Scholar]
- 27.Hui AJ, Wong RM, Ching JY, Hung LC, Chung SC, Sung JJ. Risk of colonoscopic polypectomy bleeding with anticoagulants and antiplatelet agents: analysis of 1657 cases. Gastrointest Endosc. 2004;59:44–48. doi: 10.1016/s0016-5107(03)02307-1. [DOI] [PubMed] [Google Scholar]
- 28.Holster IL, Valkhoff VE, Kuipers EJ, Tjwa ET. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology. 2013;145:105–112.e15. doi: 10.1053/j.gastro.2013.02.041. [DOI] [PubMed] [Google Scholar]
- 29.Nagata N, Yasunaga H, Matsui H, Fushimi K, Watanabe K, Akiyama J, Uemura N, Niikura R. Therapeutic endoscopy-related GI bleeding and thromboembolic events in patients using warfarin or direct oral anticoagulants: results from a large nationwide database analysis. Gut. 2017;pii:gutjnl–2017-313999. doi: 10.1136/gutjnl-2017-313999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaruvongvanich V, Assavapongpaiboon B, Wijarnpreecha K, Ungprasert P. Heparin-bridging therapy and risk of post-polypectomy bleeding: Meta-analysis of data reported by Japanese colonoscopists. Dig Endosc. 2017;29:743–748. doi: 10.1111/den.12882. [DOI] [PubMed] [Google Scholar]
- 31.Desai J, Granger CB, Weitz JI, Aisenberg J. Novel oral anticoagulants in gastroenterology practice. Gastrointest Endosc. 2013;78:227–239. doi: 10.1016/j.gie.2013.04.179. [DOI] [PubMed] [Google Scholar]


