TO THE EDITOR:
Band 3, the anion exchanger (AE1) encoded by SLC4A1, is expressed in the erythrocyte membrane and in the collecting ducts of the kidney where it mediates transmembrane electroneutral chloride-bicarbonate exchange and serves as a linker between the plasma membrane and the cytoskeleton. Despite determination of the crystal structure of the human Band 3 membrane domain, specific amino acids that mediate anion binding and exchange remain largely unknown.1 Studying human variants of AE1 may provide insights into the specific amino acids required for transport. In this study, we determined that a homozygous mutation, Ser725Arg, located in the anion-binding site of Band 3, causes anemia and distal renal tubular acidosis with loss of anion transport function likely due to an anomalous ionic interaction with the essential residue Glu681.
Heterozygous mutations in Band 3 have been linked to several phenotypes, including hereditary spherocytosis (HS), a subtype of hereditary cryohydrocytosis, Southeast Asian ovalocytosis (SAO), acanthocytosis, and distal renal tubular acidosis (dRTA). In a few cases, patients exhibit both hemolytic anemia and dRTA, typically with homozygous or compound heterozygous mutations associated with Band 3 deficiency due to abnormal trafficking from the endoplasmic reticulum (ER) to the plasma membrane.2-6
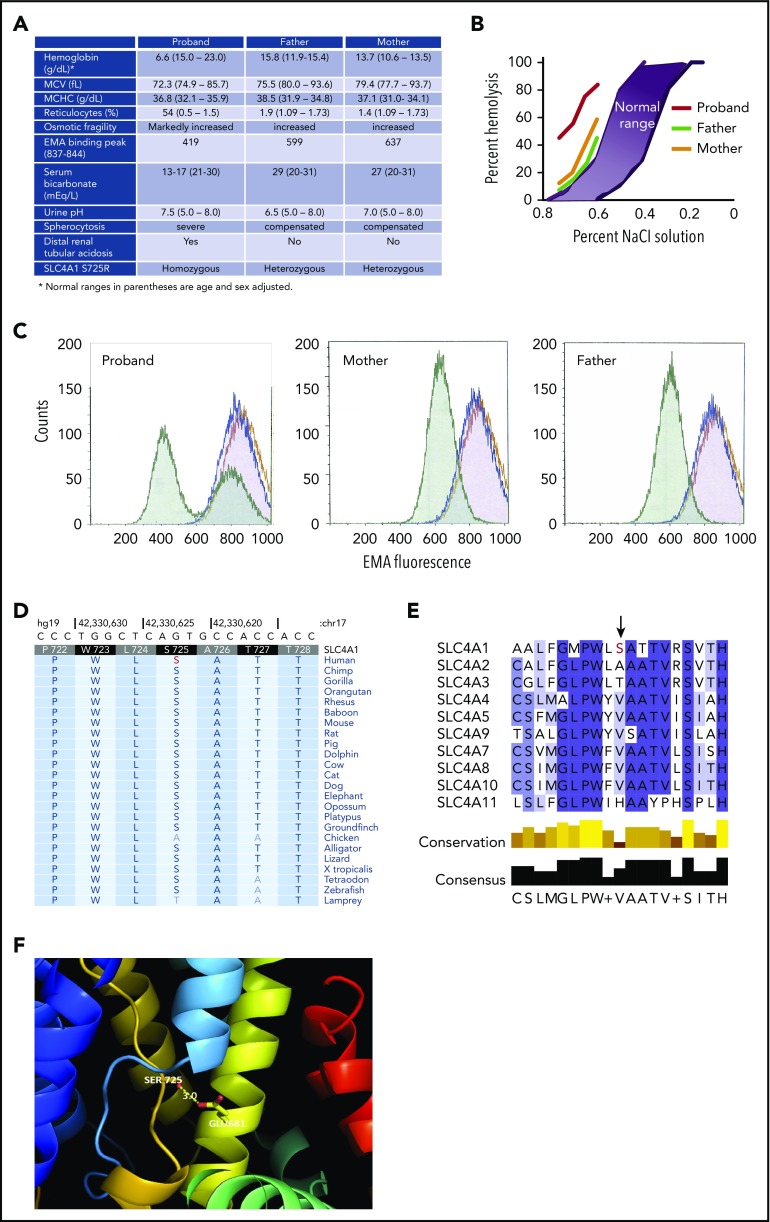
A 6-day-old male infant presented with tachypnea, intermittent hypoxia, scleral icterus, splenomegaly, and severe anemia, hemoglobin 6.6 g/dL, and marked spherocytosis. Serum bicarbonate was 17 mEq/L with anion gap 10 (Figure 1A). Erythrocytes demonstrated increased osmotic fragility and decreased eosin 5-maleimide (EMA) binding, a marker of membrane Band 3 content (Figure 1B-C). Monthly transfusions were required during the first year of life. Growth was poor, at the third percentile.
Figure 1.
Hereditary spherocytosis due to a mutation in Band 3. (A) Clinical and laboratory data. (B) Incubated osmotic fragility. (C) EMA binding. Normal control cells (purple); stain control (gold); proband and parents’ cells (green). The 2 green peaks in the proband panel represent transfused cells (higher EMA fluorescence) and patient’s endogenous cells (lower EMA fluorescence). (D) Sequence conservation of Ser725 in SLC4A1 across vertebrate species, including the clades of placental mammals and the extant Eutherians. (E) Lack of sequence conservation of Ser725 in SLC4 transporters. There is no serine at position 725 in any of the other 9 SLC4 transporters. (F) Structure of SLC4A1 showing the location of Ser725 in the substrate-binding site of Band 3 between the N-termini of TM3 (light blue) and TM10 (gold). The side chain of Ser725 is only 3 Å away from Glu681, the proton-binding site located on TM8 (yellow). Mutation of serine to arginine at 725 is predicted to form a salt-bridge with Glu681, blocking anion transport. MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume.
The parents are first cousins from Pakistan. There was no family history of hematologic disease. Hematologic indices, mildly increased osmotic fragility, and mildly decreased EMA binding indicate that both parents have compensated HS (Figure 1).
At 6 months of age, the proband’s serum bicarbonate was 16 mEq/L, urine pH was 8.0, and renal ultrasound revealed small kidneys with diffuse, bilateral nephrocalcinosis, findings suggestive of dRTA. Urine electrolytes confirmed positive anion gap. Sodium citrate was prescribed with good benefit. Serum bicarbonate increased, and weight gain improved. In the first, second, and third years of life, the patient received 13, 6, and 6 transfusions, respectively. In the fourth year of life, the patient maintained hemoglobin at ∼7.5 g/dL, with transfusions given twice for worsening anemia during intercurrent illness.
Sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) of erythrocyte membrane proteins from the proband (pretransfusion), his mother, and his father revealed Band 3 deficiency (32%, 61%, and 48% of control, respectively). Next-generation sequencing identified a homozygous A-to-C substitution in the SLC4A1 gene (nucleotide position chr17:42330624), changing Ser (AGT) to Arg (CGT) at residue 725 (NM_000342:c.A2173C:p.S725R; Figure 1A). This SLC4A1 missense mutation was predicted to be highly pathogenic (CADD Phred score 28.4) and damaging by SIFT, PolyPhen2, and other mutation prediction algorithms. It is novel and unique, not present in the National Heart, Lung, and Blood Institute Exome Sequencing Project database, the 1000 Genomes database, or the ExAC (>60 000 exomes) database.
Both parents were heterozygous for the S725R variant, which we name Band 3Punjab. These studies were approved by the Yale University School of Medicine institutional review board (12377).
Ser725 is a highly conserved residue in SLC4A1 proteins across vertebrates, clades of placental mammals, the extant Eutherians, birds, reptiles, and frogs, with a highly significant vertebrate phastCons score of 1.0 and a highly significant PhyloP conservation score of 1.06 (Figure 1D). However, it is not conserved among other members of the SLC4 transporters, being Ala in SLC4A2, Thr in SLC4A3, a hydrophobic residue in the sodium bicarbonate cotransporters, and His in SLC4A11 (Figure 1E).
The crystal structure of the membrane domain of Band 3 shows that Ser725 is located in an extended region of transmembrane (TM) 10 just NH2-terminal to a half-helix beginning at Thr728 (Figure 1F).1 The NH2-termini of TM10 and TM3 helices face each other in the middle of the protein, and their positive helix dipoles are likely involved in binding anions.7 TM10 also contains Arg730, an essential residue that points back toward the anion-binding site. The side chain of Ser725 is only 3 Å away from Glu681 in TM8, the proton-binding site required for sulfate uptake.8,9 Mutation of the homologous glutamate in murine AE1, E669Q, abrogated chloride-mediated anion exchange.10,11 This suggests that Ser725 is located within the anion-binding site of Band 3 and that there could be a hydrogen-bonding interaction between these 2 residues. Molecular modeling predicts that S725R mutation would result in the formation of an ionic bond between Arg725 and Glu681, thereby impairing anion transport.
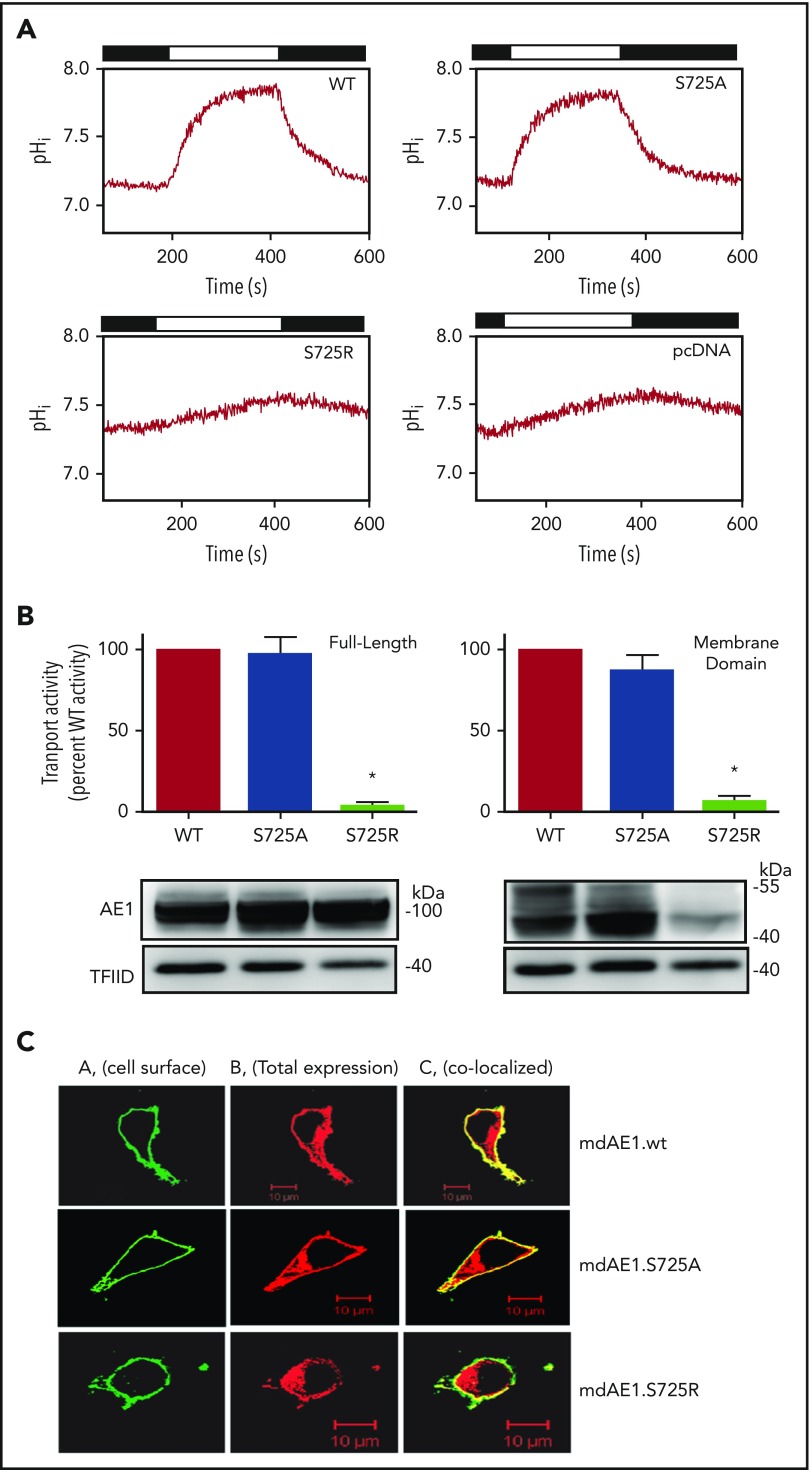
Transport function was assessed in a Cl−/HCO3− exchange assay in HEK293 cells transfected with Band 3 or an external HA-tagged membrane domain (md) of Band 3 with wild-type (WT), S725R, S725A, or pcDNA (Thermo-Fisher) empty vector control, as has been described.12,13 Fluorescence measurements were converted to intracellular pH (pHi) by the nigericin-high potassium method, and anion-exchange activity was calculated after protein expression level normalization.14 Normalized transport activity of Band 3 variants was calculated as H+-equivalent flux/Band 3 cellular abundance = H+-equivalent flux/(Band 3 intensity/transcription factor II D [TFIID] intensity). The S725A mutant in the Band 3 construct had normal expression levels, as did the S725R mutant, whereas the S725R mutant in the mdAE1 construct was expressed at ∼50% the level of WT. Anion transport was completely abolished in the S725R mutant in both Band 3 and the membrane domain construct, whereas the S725A mutants had normal transport activity (Figure 2A-B).
Figure 2.
Bicarbonate transport activity and cell surface expression of wild-type, S725R, or S725A mutants in Band 3 or membrane-domain constructs. HEK293 cells were grown on glass coverslips and transiently transfected with complementary DNA plasmids encoding the full length or membrane-domain constructs indicated. (A) Cells were loaded with the pH-sensitive dye, BCECF-AM, and fluorescence was monitored as cells were alternately perfused with chloride-containing Ringer's buffer (black bars) or chloride-free Ringer's buffer (white bars). HCO3− transport rates were monitored by the initial rate of change of intracellular pH (pHi) induced upon switching to chloride-free medium. Perfusion solutions were bubbled with 5% CO2. (B) (Top) HCO3− transport activity of WT and mutant Band 3 or membrane-domain constructs were corrected for background activity of vector-transfected cells, normalized to protein expression level, and expressed as percentage of wild-type rate. Error bars indicate standard errors of the mean (n = 3). *Significant difference in transport rate from WT-Band 3 or membrane-domain construct. (Bottom) Cell lysates from cells used for transport experiments were probed for Band 3 with IVF12 monoclonal antibody and for endogenous transcription factor IID (TFIID) on immunoblots. (C) Immunofluorescence localization of HA-tagged Band 3 membrane domain and S725R and S725A mutants in transfected HEK cells. We transfected 2 µg of mdAE1 WT and mutants by lipofectamine LTX & PLUS for 24 hours. Fixed HEK cells were incubated with subsaturating amounts of mouse anti-HA (1/500 dilution) antibody, followed by anti-mouse immunoglobulin G (IgG) Alexa 488 (green) to detect cell surface AE1. Then cells were washed, permeabilized by Triton X-100, and incubated with a second aliquot of mouse anti-HA antibody (1/500 dilution), followed by anti-mouse IgG-Cy3 (red) antibody to detect total AE1 expression. Cell images were examined using an LSM 510 confocal microscope. Colocalization (yellow) was calculated by Image J JACoP program using Pearson’s and Manders’s coefficients. Column A indicates cell surface mdAE1 (HA-Alexa 488 in green); column B indicates total mdAE1 (HA-cy3 in red); and column C indicates colocalization of cell surface mdAE1 and total mdAE1 (in yellow) on the cell surface. The average Pearson’s coefficients are WT 0.741, S725A 0.661, and S725R 0.419. TFIID, transcription factor IID.
Immunofluorescence microscopy using an anti–human influenza hemagglutinin antibody revealed HEK cells transfected with either the S725R or S725A mutant demonstrated staining at the cell surface in intact cells,15,16 confirming trafficking of the mutants to the plasma membrane (Figure 2C). Permeabilized cells revealed intracellular protein localized to the ER; the overlap in staining at the cell surface was used to estimate the cell surface expression levels. The S725A mutant and the S725R mutant were expressed at the cell surface at 90% and 60% of the percentage found for the wild-type protein, consistent with decreased endogenous expression observed in EMA binding and SDS-PAGE in mutant erythrocytes. Thus, although the S725R mutant can traffic to the cell surface, it lacks any transport activity.
Homozygous mutations in Band 3 have led to different effects on membrane levels of Band 3 and anion exchange in the erythrocyte and kidney but have not shed light on specific transport-related residues.2-6 Band 3Coimbra, V488M, and Band 3Vienna, S477X, lead to complete loss of membrane-associated Band 3, resulting in transfusion dependence and dRTA.2,6 Patients with homozygous A858D exhibit acanthocytic anemia and dRTA,4,17,18 whereas homozygous Band 3Courconnes leads to HS and incomplete dRTA,3 with both mutants exhibiting ER retention leading to decreased membrane Band 3. Homozygous Band 3Neapolis, a splicing mutation 5′ to the kidney Band 3 isoform, leads to erythrocyte membrane Band 3 deficiency and severe HS but no dRTA.19 Homozygous Band 3SAO, in-frame deletion of 9 amino acids at the cytosolic and membrane domain hinge region causing severe misfolding, leads to transfusion-dependent anemia and RTA.5
The S725R mutant leads to Band 3 protein deficiency and exhibits near-complete lack of ion transport function, likely due to the formation of a salt bridge between the positively charged arginine at 725 and the nearby Glu681. Decreased Band 3 expression and lack of transport activity lead to anemia and dRTA, respectively, in the homozygous state.
These results raise the question of whether anion exchange influences erythrocyte membrane integrity and function. In vitro studies of human erythrocytes demonstrate that under conditions of complete inhibition of anion transport by DIDS (4,4'-diisothiocyanostilbene-2,2'-disulfonic acid), ∼10% of spectrin-ankyrin membrane complexes are not extractable, suggesting a possible role for anion transport in Band 3–cytoskeletal interactions.20 Cows and mice with total lack of Band 3 are viable, suffering from spherocytosis and dRTA.21-23 Band 3–deficient cows exhibit mild acidosis, worsened after exercise or dietary acid load, with bicarbonate concentration and total CO2 content in the low-normal range. CO2 saturation of blood is infrequent; thus the additional CO2 load facilitated by Band 3 is likely not critical except under very high stress conditions. Demonstration of elevated total CO2 in Band 3–deficient erythrocytes lacking anion transport would begin to address these questions.
Acknowledgments
Supported in part by a grant from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (DK104046) (P.G.G.) and by grants from the Canadian Institutes of Health Research (FRN102493 [R.A.F.R.] and FRN153393 [J.R.C.]).
Authorship
Contribution: E.Y. and P.S.-M. phenotyped, diagnosed, and collected additional information on the patient; E.Y. cowrote the manuscript; K.E.B. performed experiments, analyzed data, and prepared figures; J.L. performed experiments, analyzed data, and prepared figures; J.R.C. designed experiments, analyzed data, prepared figures, and edited the manuscript; R.A.F.R. designed experiments, analyzed data, prepared figures, and edited the manuscript; K.L.-G. designed and performed experiments; and P.G.G. designed experiments, analyzed data, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Patrick G. Gallagher, Department of Pediatrics, Yale University School of Medicine, 333 Cedar St, LCI 401, PO Box 208064, New Haven, CT 06520-8064; e-mail: patrick.gallagher@yale.edu.
References
- 1.Arakawa T, Kobayashi-Yurugi T, Alguel Y, et al. . Crystal structure of the anion exchanger domain of human erythrocyte band 3. Science. 2015;350(6261):680-684. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro ML, Alloisio N, Almeida H, et al. . Severe hereditary spherocytosis and distal renal tubular acidosis associated with the total absence of band 3. Blood. 2000;96(4):1602-1604. [PubMed] [Google Scholar]
- 3.Toye AM, Williamson RC, Khanfar M, et al. . Band 3 Courcouronnes (Ser667Phe): a trafficking mutant differentially rescued by wild-type band 3 and glycophorin A. Blood. 2008;111(11):5380-5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fawaz NA, Beshlawi IO, Al Zadjali S, et al. . dRTA and hemolytic anemia: first detailed description of SLC4A1 A858D mutation in homozygous state. Eur J Haematol. 2012;88(4):350-355. [DOI] [PubMed] [Google Scholar]
- 5.Picard V, Proust A, Eveillard M, et al. . Homozygous Southeast Asian ovalocytosis is a severe dyserythropoietic anemia associated with distal renal tubular acidosis. Blood. 2014;123(12):1963-1965. [DOI] [PubMed] [Google Scholar]
- 6.Kager L, Bruce LJ, Zeitlhofer P, et al. . Band 3 nullVIENNA, a novel homozygous SLC4A1 p.Ser477X variant causing severe hemolytic anemia, dyserythropoiesis and complete distal renal tubular acidosis. Pediatr Blood Cancer. 2017;64(3):e26227. [DOI] [PubMed] [Google Scholar]
- 7.Reithmeier RA, Casey JR, Kalli AC, Sansom MS, Alguel Y, Iwata S. Band 3, the human red cell chloride/bicarbonate anion exchanger (AE1, SLC4A1), in a structural context. Biochim Biophys Acta. 2016;1858(7 Pt A):1507-1532. [DOI] [PubMed] [Google Scholar]
- 8.Jennings ML, Smith JS. Anion-proton cotransport through the human red blood cell band 3 protein. Role of glutamate 681. J Biol Chem. 1992;267(20):13964-13971. [PubMed] [Google Scholar]
- 9.Jennings ML, Anderson MP. Chemical modification and labeling of glutamate residues at the stilbenedisulfonate site of human red blood cell band 3 protein. J Biol Chem. 1987;262(4):1691-1697. [PubMed] [Google Scholar]
- 10.Chernova MN, Jiang L, Crest M, et al. . Electrogenic sulfate/chloride exchange in Xenopus oocytes mediated by murine AE1 E699Q. J Gen Physiol. 1997;109(3):345-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller-Berger S, Karbach D, Kang D, et al. . Roles of histidine 752 and glutamate 699 in the pH dependence of mouse band 3 protein-mediated anion transport. Biochemistry. 1995;34(29):9325-9332. [DOI] [PubMed] [Google Scholar]
- 12.Okawa Y, Li J, Basu A, Casey JR, Reithmeier RA. Differential roles of tryptophan residues in the functional expression of human anion exchanger 1 (AE1, Band 3, SLC4A1). Mol Membr Biol. 2014;31(7-8):211-227. [DOI] [PubMed] [Google Scholar]
- 13.Loiselle FB, Casey JR. Measurement of intracellular pH. Methods Mol Biol. 2010;637:311-331. [DOI] [PubMed] [Google Scholar]
- 14.Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18(11):2210-2218. [DOI] [PubMed] [Google Scholar]
- 15.Quilty JA, Li J, Reithmeier RA. Impaired trafficking of distal renal tubular acidosis mutants of the human kidney anion exchanger kAE1. Am J Physiol Renal Physiol. 2002;282(5):F810-F820. [DOI] [PubMed] [Google Scholar]
- 16.Quilty JA, Reithmeier RA. Trafficking and folding defects in hereditary spherocytosis mutants of the human red cell anion exchanger. Traffic. 2000;1(12):987-998. [DOI] [PubMed] [Google Scholar]
- 17.Sinha R, Agarwal I, Bawazir WM, Bruce LJ. Distal renal tubular acidosis with hereditary spherocytosis. Indian Pediatr. 2013;50(7):693-695. [DOI] [PubMed] [Google Scholar]
- 18.Shmukler BE, Kedar PS, Warang P, et al. . Hemolytic anemia and distal renal tubular acidosis in two Indian patients homozygous for SLC4A1/AE1 mutation A858D. Am J Hematol. 2010;85(10):824-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrotta S, Borriello A, Scaloni A, et al. . The N-terminal 11 amino acids of human erythrocyte band 3 are critical for aldolase binding and protein phosphorylation: implications for band 3 function. Blood. 2005;106(13):4359-4366. [DOI] [PubMed] [Google Scholar]
- 20.Hsu L, Morrison M. The interaction of human erythrocyte band 3 with cytoskeletal components. Arch Biochem Biophys. 1983;227(1):31-38. [DOI] [PubMed] [Google Scholar]
- 21.Inaba M, Yawata A, Koshino I, et al. . Defective anion transport and marked spherocytosis with membrane instability caused by hereditary total deficiency of red cell band 3 in cattle due to a nonsense mutation. J Clin Invest. 1996;97(8):1804-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters LL, Shivdasani RA, Liu SC, et al. . Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell. 1996;86(6):917-927. [DOI] [PubMed] [Google Scholar]
- 23.Stehberger PA, Shmukler BE, Stuart-Tilley AK, Peters LL, Alper SL, Wagner CA. Distal renal tubular acidosis in mice lacking the AE1 (band3) Cl-/HCO3- exchanger (slc4a1). J Am Soc Nephrol. 2007;18(5):1408-1418. [DOI] [PubMed] [Google Scholar]