Abstract
In this issue of Blood, Manček-Keber et al report that exosomes and microvesicles (MVs) produced by lymphoma B cells and carrying MyD88L265P reprogram the bone marrow environment (BME) by activating endogenous proinflammatory signaling pathways in recipient nonmalignant cells.1
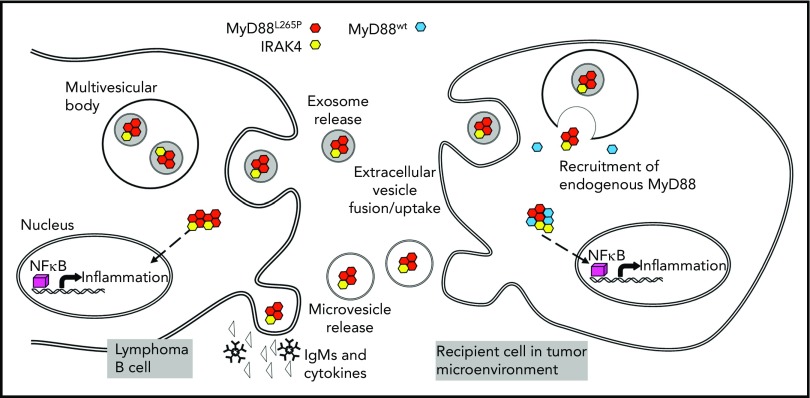
Lymphoma B-cell crosstalk with a recipient cell in the WM BM is mediated by MVs or exosomes carrying the MyD88265P complex (myddosome). In the parent B cell, the mutated MyD88 protein activates the NF-κB pathway inducing proinflammatory cytokine and immunoglobulin M (IgM) release. MyD88265P is also packaged into exosomes or is enclosed into MVs budding off the surface of the parent cell. These vesicles transport it to recipient cells such as mast cells or macrophages in the BME. Once taken up into the cytoplasm, the MyD88265P complex recruits endogenous MyD88wt, activates the NF-κB pathway, and reprograms the recipient cell to produce proinflammatory cytokines. See Figure 6 in the article by Manček-Keber et al that begins on page 1720.
The report by Manček-Keber et al highlights the key role of extracellular vesicles (EVs) derived from malignant B cells in the conversion of mast cells and macrophages into cells that promote lymphoma progression. In 90% of patients with Waldenström macroglobulinemia (WM), B cells harbor a mutation in the innate immune signaling adapter MyD88.2 MyD88L265P is a gain-of-function mutation encoding the membrane-associated protein with a propensity to aggregate into the myddosome. Manček-Keber and colleagues found that EVs produced by WM tumor cell lines or those isolated from bone marrow (BM) aspirates of WM patients carried the signaling-competent MyD88L265P and delivered the myddosome to mast cells and macrophages in coincubation experiments as well as in vivo in mice injected intravenously or intraperitoneally with PKH67-labeled EVs carrying MyD88L265P.
What are EVs and how do they manage to transfer signaling complexes from one cell to another? When EVs were first discovered in the 1980s,3 they were thought to be responsible for removing cellular waste. Today, EVs emerge as a universal communication system that conveys molecular and genetic signals to near or distant target cells and reprograms their functions.4 EVs are membrane-bound vesicles of diverse cellular origins present in all body fluids. EVs comprise exosomes, MVs, and apoptotic bodies.5 Exosomes are the smallest subset of EVs (30-150 nm) that originate from the endocytic compartment of parent cells. MVs are larger and are formed by budding of the parent cell membrane.6 EVs are coated with a wide variety of membrane-associated biologically active molecules, including cell adhesion molecules necessary for the uptake of EVs by recipient cells via phagocytosis, endocytosis, fusion with the membrane, or receptor-mediated uptake.7 The EV lumen contains various soluble molecules and nucleic acids (messenger RNA, microRNA [miRNA], and DNA) that EVs shuttle from parent to recipient cells. Small (femtomolar) quantities of cellular contents in EVs delivered to recipient cells need to be amplified to achieve physiological effects; hence, EVs carry their own amplification machinery in the form of enzymes, transcription factors, and cytokines.5 Isolation of EVs from cell line supernatants or body fluids by ultracentrifugation at >100 000g for 2 hours is still the most widely used collection method, although the recovered vesicles are mixtures of EV subsets. Methods for isolating EV from body fluids that discriminate between the EV subsets are being developed.8 Tumor-derived EVs are reported to be associated with cancer progression and downregulation of antitumor immune responses.9 EVs are also involved in chronic inflammation associated with the development of cancer.10 For these reasons, tumor-derived EVs have recently been of great interest.
The mechanisms EVs use for information transfer may vary, depending on the identity of a parent cell, the type of EV released, and the nature of recipient cells. Advantages of information transfer by EVs are that they circulate freely in body fluids, that intraluminal materials are protected from degradation en route, and that signaling molecules are delivered embedded in the membrane, which enhances their biological activity. Once EVs are delivered, they initiate signaling at the target cell membrane or reorganize transcription using miRNAs or both, and they initiate signaling cascades that activate endogenous molecular pathways. In some cases, EVs take advantage of pathways that already exist in recipient cells to induce phenotypic or functional alterations as described by Manček-Keber et al for EVs transferring the myddosome from malignant B cells to mast cells and macrophages in the BME. The molecular underpinnings of this transfer by EVs involve the constitutively activated protein complex assembled during toll-like receptor signaling in lymphoma B cells. The complex contains MyD88L265P, IRAK4, and IRAK1/2 kinases. It activates several transcription factors, including NF-κB, and promotes transcription of numerous proinflammatory genes resulting in secretion of interleukin-6 (IL-6), IL-10, interferon-β, and immunoglobulin M by lymphoma B cells. MyD88L265P supports lymphoma cell survival via NF-κB pathway activation, increased BcL-xL expression, and activation of Bruton tyrosine kinase. When the myddosome is transferred by EVs to the cytoplasm of mast cells or macrophages, it recruits endogenous MyD88wt, forming protein aggregates that activate NF-κB and induce production of proinflammatory cytokines and chemokines. The uptake of the myddosome into the cytoplasm is obligatory for downstream activation of NF-κB signaling in the recipient cells. In effect, Manček-Keber et al show that EVs reorganize endogenous signaling in recipient cells to create a proinflammatory niche in the BME (see figure).
How do EVs acquire the MyD88256P protein complex from lymphoma B cells? Manček-Keber et al suggest that both exosomes and MVs participate in the process. Although biogenesis of exosomes proceeds via the endocytic-multivesicular bodies (MVBs) pathway, MVs arise by budding off the cell surface.5 Immunocytochemistry localizes the MyD88 protein complex to the cytoplasm, mitochondrial membranes, and MVBs in lymphoma B cells. Thus, either the endocytic pathway or cell membrane budding could yield EVs carrying the complex. The packaging of the complex into exosomes in the parent cell is a complex process executed by the endosomal sorting complex responsible for transport.6 It is unclear whether EVs leaving the parent cell are addressed or directed to activated mast cells and/or macrophages in the BME. The mechanisms for transferring the EV cargo might differ in various malignancies. Manček-Keber et al show that lymphoma B cells modify the BME by an EV-based mechanism that involves MyD88L265P. Because the malignant B cells in most patients with WM express MyD88L265P and package it into EVs, this transfer mechanism is used in the BME of WM.
The purpose of the myddosome transfer from leukemic to other BM cells by EVs is to ensure the well-being of leukemia cells. MyD88265P is a gain-of-function mutation that enables lymphoma cells to thrive. Its transfer to stromal and/or immune cells in the BME endows these cells with proleukemia functions and the ability to propagate chronic inflammation and thus cancer progression. The clever use of EVs by the tumor to subvert nonmalignant cells in the BME into creating a proinflammatory milieu is but one example of many different mechanisms tumors evolve to escape from the host antitumor surveillance.
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Manček-Keber M, Lainšček D, Benčina M, et al. Extracellular vesicle–mediated transfer of constitutively active MyD88L265P engages MyD88wt and activates signaling. Blood. 2018;131(15):1720-1729. [DOI] [PubMed] [Google Scholar]
- 2.Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med. 2012;367(9):826-833. [DOI] [PubMed] [Google Scholar]
- 3.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101(3):942-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyiadzis M, Whiteside TL. The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia. 2017;31(6):1259-1268. [DOI] [PubMed] [Google Scholar]
- 5.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25(6):364-372. [DOI] [PubMed] [Google Scholar]
- 7.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3(1):24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma P, Ludwig S, Muller L, et al. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J Extracell Vesicles. 2018;7(1):1435138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiteside TL. Exosomes carrying immunoinhibitory proteins and their role in cancer. Clin Exp Immunol. 2017;189(3):259-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bui TM, Mascarenhas LA, Sumagin R. Extracellular vesicles regulate immune responses and cellular function in intestinal inflammation and repair [published online ahead of print 2 February 2018]. Tissue Barriers. doi:10.1080/21688370.2018.1431038. [DOI] [PMC free article] [PubMed] [Google Scholar]