Abstract
In celery (Apium graveolens L.), long-distance transport of reduced carbon occurs both in the form of sucrose (Suc) and mannitol. The presence of mannitol has been related to the resistance of celery to salt stress. To investigate the transport events occurring during salt stress, we have cloned the H+/Suc transporter of celery AgSUT1 (A. graveolens Suc uptake transport 1) from a mature leaf cDNA library. The function of the encoded protein was confirmed by expression in yeast. AgSUT1 is a H+/Suc transporter with a high affinity for Suc (Km of 139 μm). Another closely related cDNA (AgSUT2) was also identified. AgSUT1 is mainly expressed in mature leaves and phloem of petioles, but also in sink organs such as roots. When celery plants were subjected to salt stress conditions (30 d watering with 300 mm NaCl) favoring mannitol accumulation (J.D. Everard, R. Gucci, S.C. Kann, J.A. Flore, W.H. Loescher [1994] Plant Physiol 106: 281–292), AgSUT1 expression was decreased in all organs, but markedly in roots. The results are discussed in relation to the physiology of celery.
Plants are autotrophic organisms using the energy of light through photosynthesis to reduce ambient CO2 to carbohydrates and other compounds. Plant organs are specialized into source (producers and exporters of sugars) and sink (importers and users of sugars) organs. Sugars are generally transported in the form of Suc (see below) from source to sink organs by mass flow into specialized cells of the phloem called the sieve tubes. This so-called “long-distance” transport of sugar is therefore an important determinant of plant productivity (Gifford and Evans, 1981).
In many species, the high Suc concentration measured in the phloem sap (Winter et al., 1994) compared with surrounding cells has led to the model of an active transport of Suc into the sieve element/companion cell complex from the apoplasmic space. This model has received confirmation after the cloning of a cDNA encoding a H+/Suc carrier from spinach (SoSUT1: Spinacia oleracea Suc uptake transport 1; Riesmeier et al., 1992), potato (StSUT1: Solanum tuberosum SUT1; Riesmeier et al., 1993), and then many species (for review, see Lemoine, 2000). The localization of the carrier to plasma membranes of sieve elements (Kühn et al., 1997) and companion cells (Truernit et al., 1995) and the impairment of Suc export from leaves of plants where the expression of the Suc carrier was lowered (Riesmeier et al., 1994; Bürkle et al., 1998) demonstrated the involvement of SUT carriers in the loading of Suc in the sieve element/companion cell complex.
In a significant number of species, Suc is transported in parallel with other types of sugars such as oligosaccharides (e.g. raffinose and trehalose in cucurbits; Turgeon, 1996) and polyols (mainly mannitol and sorbitol). Polyols (or sugar alcohols) are the reduced form of α-ketoses and are found in more than 100 species (Stoop et al., 1996). The derivatives of Glc (sorbitol or dulcitol) and Man (mannitol) are the most frequently found polyols. It has been calculated that up to 30% of the carbon fixed by plants on earth is in the form of polyols (Bieleski, 1982). The exact function of the polyols is still unknown, but their presence has been related to different events. Mannitol has been implicated in the resistance to stress (mainly salt), because its synthesis is increased in response to stress in celery (Apium graveolens) (Everard et al., 1994). Mannitol, like other polyols, is considered to be a compatible solute, accumulating in stressed cells to increase their osmotic potential while protecting cellular structures and activities. Seeds from Arabidopsis plants engineered to produce mannitol showed an increased resistance to salt stress (Thomas et al., 1995). In planta, mannitol is synthesized in the cytoplasm of source leaves from photosynthesis-derived hexose phosphate and exported to sink organs, presumably in the same way as Suc. Several lines of evidence indicate that Suc and mannitol are transported by different carriers in celery (Daie, 1987; Salmon et al., 1995), but the transport events in polyol-synthesizing species received little attention except in Plantago major (Gahrtz et al., 1994), where sorbitol is also transported.
Salt stress has been studied extensively as soil salinity represents an increasing problem for agriculture in many countries. Several species are tolerant to salt and can still show some growth when cultivated on soils containing high NaCl levels. For example, celery plants develop new leaves when grown on a 300 mm NaCl solution, a salinity equal to one-half that of seawater. Under such conditions, both in plants (Everard et al., 1994) and cell cultures (Prata et al., 1997), mannitol accumulation is the result of an increase in mannitol synthesis at the expense of Suc and a decrease of mannitol catabolism. However, the flux of sugar from source and to sink organs has not been considered in such situations. Generally speaking, regulation of Suc transporter expression has been rarely studied and very few reports are available (see Delrot et al., 2000; Lalonde et al., 1999).
In this paper, we report the identification of Suc transporters from celery, AgSUT1 and AgSUT2 (A. graveolens Suc uptake transport 1 and 2) and the regulation of their expression during salt stress. We demonstrate that in response to salt stress, AgSUT1 expression is lowered in all organs but to different extents. Those results are discussed in relation to the physiology of celery.
MATERIALS AND METHODS
Plant Material
Celery plants (Apium graveolens L. var dulce cv Vert d'Elne) were grown under greenhouse conditions as described by Davis et al. (1988). Mature plants were approximately 4 months old. Prior to salt stress treatment, the open surfaces of 5.4-L pots containing the plants were covered with plastic film to minimize surface evaporation. Salt treatments were stepped up in 50 mm d−1 increments (25 mm in the morning, 25 mm in the afternoon) until the desired final concentration (300 mm) was achieved. NaCl was dissolved in either Peters 20:20:20 fertilizer (100 ppm nitrogen working strength) or tap water. Plants were watered alternatively with each solution for 4 weeks. At the end of the 300 mm NaCl treatment, half of the treated plants were watered for 1 week with tap water without salt (rehydrated plants).
Strains
The following strains were used in this study. Escherichia coli strains DH5α(supE44, ΔlacU169 [φ80, lacZM15], hsdR17, recA, endA1, gyrA96, thi-1, and relA1), XL1Blue MRF′ (Stratagene, La Jolla, CA), and SOLR (Stratagene) were cultured according to standard techniques (Sambrook et al., 1989). The yeast strain SUSY7 (suc2: :URA3, mal0, and trp1) expresses a potato Suc synthase gene (Riesmeier et al., 1992).
Screening of cDNA Library and DNA Manipulations
The source leaf cDNA library in λZAP II was a generous gift from Prof. W.H. Loescher (Everard et al., 1997). Recombinant phages (750,000) were screened using SoSUT1 as a radioactive probe according to the manufacturer's protocols (Stratagene). Hybond-N nylon filters (Amersham, Les Ulis, France) were hybridized overnight at 42°C according to standard conditions (Stratagene). Filters were washed 15 min at 42°C with 0.1% (w/v) SDS and 2× SSC (1× SSC = 0.15 m NaCl and 0.015 m sodium citrate), 15 min at 50°C with 0.1% (w/v) SDS and 2× SSC, and 30 min at 50°C with 0.1% (w/v) SDS and 1× SSC. In vivo excision was performed on the eight clones that gave positive signals during the three rounds of screening.
The cDNAs were sequenced using an automated sequencer (ABI 310, Perkin-Elmer, Foster City, CA). Sequence comparisons with other databases were performed through the National Center for Biotechnology Information via the BLAST site (Altschul et al., 1997). Transmembrane regions and protein orientation were predicted using the TMpred tool (Hofmann and Stoffel, 1993).
Expression of AgSUT1 in Saccharomyces cerevisiae
AgSUT1 cDNA was ligated into the EcoRI-XhoI site of the yeast shuttle vector YEP112A1XE (Riesmeier et al., 1992). This vector allows expression of full-length cDNAs under the control of the S. cerevisiae ADH1 promoter. Competent yeast cells were prepared and transformed according to the method of Dohmen et al. (1991). SUSY7 transformed with YEP 112A1XE was used for control experiments.
Uptake of Radiolabeled Sugars
Yeast cells were grown to the early logarithmic phase, washed with distilled water, and resuspended to 1% (w/v) in yeast nitrogen base (YNB) medium containing 25 mm 2-(N-morpholino)-ethanesulfonic acid (MES) buffer, pH 4.5. The pH dependence of Suc uptake was determined in YNB medium buffered at different pH values with 25 mm MES. A 100-μL aliquot of the cell suspension was incubated at 28°C for 0, 60, 120, 180, and 300 s in 100 μL of a solution containing [3H]Suc (final specific acitivity: 14.8 MBq mmol−1). The reaction was stopped by addition of 8 mL of ice-cold water and filtration on glass-fiber filters (13400–25–S, Sartorius, Palaiseau, France). The radioactivity incorporated in the cells was determined in a liquid scintillation counter (Packard Instruments, Meriden, CT). For inhibition and competition studies, reagents were added 30 s prior to Suc.
RNA Isolation and Northern-Blot Analyses
For each organ studied, samples from four independent plants treated under the same conditions were harvested after 4 h of photosynthesis, pooled, and frozen in liquid nitrogen. Total RNA was isolated as described by Kay et al. (1987). Twenty micrograms of total RNA was separated on 1.2% (w/v) agarose gels containing formaldehyde and transferred to nylon filters as described in Sambrook et al. (1989). Hybridization was at 65°C and the EcoRI/XhoI fragment of AgSUT1 was used as a probe. The final wash was 0.1% (w/v) SDS, 0.5× SSC at 68°C for 15 min. Radioactivity in the reacting bands was counted with an Instant Imager (Packard Instruments), and the background counts of the membrane were subtracted. The amount of RNA loaded in each lane was calibrated by hybridization with a 25S riboprobe and Instant Imager counting (Packard Instruments) as described above. The values obtained with the riboprobe were used to calculate the relative intensity of the signals for AgSUT1 in each lane.
RESULTS
Isolation of Celery Suc Transporters
Screening of the celery leaf cDNA library with the radiolabeled DNA of the spinach Suc transporter SoSUT1 (Riesmeier et al., 1992) resulted in eight positive clones (λ1–8). In vivo excision was performed with these clones, which were then partially sequenced. One of these clones did not show any similarity to the Suc transporters. The remaining seven clones were homologous to the SoSUT1 sequence. However, some of the clones differed in the length of their 5′ and 3′ non-coding regions. This phenomenon was already observed when the Man-6-P reductase of celery was cloned in the same library (Everard et al., 1997). Three different cDNAs were therefore identified: two (AgSUT2A, accession no. AF167415, and AgSUT2B, accession no. AF167416) had identical coding regions, whereas the third one (AgSUT1, accession no. AF063400) also showed slight differences in the coding region (nine different amino acids residues compared with AgSUT2A/B). Two 5′ noncoding sequences were identified: one starts 109 bp in front of the ATG, the other was 43 nucleotides shorter in the more distal 5′ region (Fig. 1A). All 3′-untranslated ends had a polyadenylation signal (AATAAA) upstream of the poly(A+) tail. These regions were different downstream of this motif (Fig. 1B). In addition, the 5′ and 3′ noncoding sequences share between 73% and 80% identical nucleotides with those of DcSUT2 (Daucus carota SUT2; Shakya and Sturm, 1998) cDNA.
Figure 1.
Comparison of 5′ (A) and 3′ (B) untranslated regions of AgSUT1, AgSUT2A, AgSUT2B, and DcSUT2 (Shakya and Sturm, 1998).
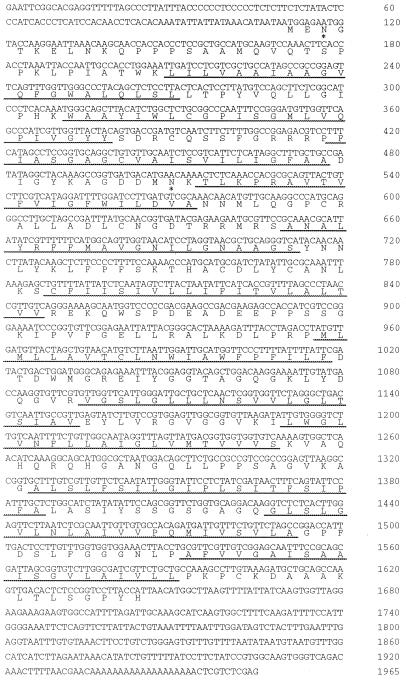
The sequence of the longest cDNA clone λ1, AgSUT1, is shown in Figure 2. The longest open reading frame for AgSUT1 is 1536 bp long, encoding a protein of 512 amino acid residues, a calculated molecular mass of 54.5 kD, and a pI of 9.27. The AgSUT1 protein sequence shows 12 hydrophobic regions (underlined in Fig. 2; Kyte and Doolittle, 1982; see Lemoine, 2000 for a detailed predictive model) representing potential transmembrane helices. As shown in Table I, the AgSUT1 protein shares between 63% and 70% identical amino acids with the Suc transporters from spinach (Riesmeier et al., 1992), from Arabidopsis (Sauer and Stolz, 1994) and from Plantago major (Gahrtz et al., 1994). However, a very high identity (92%) was found with DcSUT2, the Suc transporter from carrot, another member of the Apiaceae family. Interestingly, AgSUT1 was identified from a leaf cDNA library, whereas DcSUT2 was found in a root cDNA library (see below for a longer discussion of this point). Two Asn residues that are part of a consensus sequence for N-glycosylation (Asn-3 and Asn-134) are located on the cytoplasmic side and should therefore not be glycosylated. Potential phosphorylation sites are located at residues T136, T175, S252, and S263.
Figure 2.
Complete cDNA of AgSUT1. The translated protein sequence is also indicated. Predicted transmembrane regions (TmPred program) are underlined.
Table I.
Percentage of identical amino acid residues between AgSUT1 and Suc transporters from other species
Characterization of AgSUT1 in S. cerevisiae
To demonstrate that the identified cDNAs code for Suc carriers, they were heterologously expressed in yeast. AgSUT1, AgSUT2A, and AgSUT2B cDNAs were ligated into the EcoRI-XhoI sites of the S. cerevisiae expression plasmid YEP 112A1XE. Competent YSH SUSY7 cells (Riesmeier et al., 1992) were transformed with those plasmids and YEP 112A1XE was used as a control.
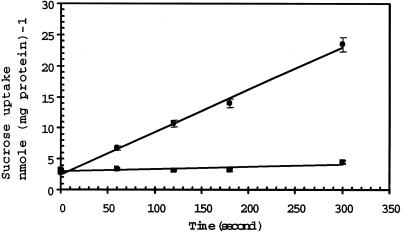
Figure 3 shows that yeast cells expressing AgSUT1 transported 3H-Suc at a much higher rate (4.1 nmol Suc mg−1 protein min−1) than control cells (0.2 nmol Suc mg−1 protein min−1). Although not presented, similar results were obtained with AgSUT2A and AgSUT2B. Due to the very high level of sequence identity, only AgSUT1 was used for the rest of the characterization of Suc uptake.
Figure 3.
Uptake of Suc into transgenic S. cerevisiae cells. The Suc concentration was 250 μm and the external pH 4.5. ▪, Uptake into cells transformed with AgSUT1 cDNA; ●, Suc uptake into control cells transformed with the plasmid YEP. The results are the means ± sd of three independent experiments (three replicates per experiment).
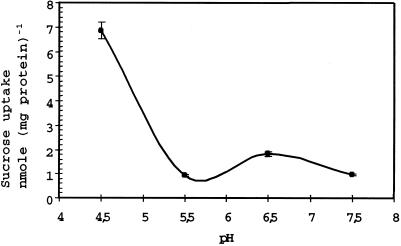
The pH dependence of Suc transport by AgSUT1 was determined in the pH range of 4.5 to 7.5 (Fig. 4). AgSUT1 is much more active at pH 4.5, and the relative activity decreased to 10% when the pH was increased to 5.5 (Fig. 4). Therefore, as far as pH dependence is concerned, AgSUT1 is more closely related to, e.g. SoSUT1 or AtSUC2 than AtSUC1, which displays a broader optimal pH range. These data are in agreement with a Suc/proton cotransport mechanism.
Figure 4.
pH dependence of Suc transport in transgenic yeast cells expressing AgSUT1. Measurements were performed at 250 μm Suc in YNB medium containing 25 mm MES buffered at the indicated pH. Incubation time was 2 min. The results are the means ± sd of three independent experiments (three replicates per experiment).
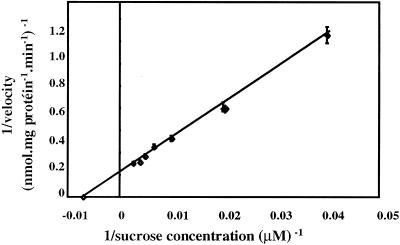
When the uptake of Suc by AgSUT1 in yeast cells was studied for 1 min at concentrations ranging from 25 to 750 μm, a clear saturation was visible (data not shown). The Lineweaver-Burk plot of the data (Fig. 5) gave an apparent affinity (Km) of 139 μm and a maximum velocity (Vmax) of 5.9 nmol mg−1 protein min−1. The Km value of AgSUT1 for Suc is in the same range as the Km value determined for this carrier in plasma membrane vesicles from phloem strands (Km = 280 μm; Vmax = 3 nmol mg−1 protein 30 s−1; Salmon et al., 1995).
Figure 5.
Lineweaver-Burk plot of Suc uptake as a function of concentration in transgenic yeast cells expressing AgSUT1. The results are the means ± sd of three independent experiments (three replicates per experiment).
A protonophore such as carbonyl cyanide m-chlorophenylhydrazone strongly inhibits Suc transport catalyzed by AgSUT1 protein (Table II). These data confirm the assumption that Suc uptake is mediated by a proton cotransport mechanism. Suc transport by AgSUT1 is also sensitive to thiol reagents such as N-ethylmaleimide and p-chloro-mercuriphenylsulfonic acid or to diethylpyrocarbonate (an imidazole group reagent). The histidyl residue involved in diethylpyrocarbonate sensitivity identified in AtSUC2 (Lu and Bush, 1998) is also conserved in AgSUT1 at position 66. Thiol groups and imidazole groups may therefore be implicated in substrate recognition or in the uptake reaction itself.
Table II.
Sensitivity of the AgSUT1 activity to inhibitors
| Inhibitor | Relative Transport Rate |
|---|---|
| % | |
| None | 100 |
| 50 μm CCCP | 9.0 ± 11.2 |
| 100 μm PCMBS | 29.1 ± 7.8 |
| 100 μm NEM | 24.9 ± 6.7 |
| 0.5 mm DEPC | 40.6 ± 5.9 |
Potential inhibitors were added 30 s prior to labeled Suc. All transport tests were performed at 250 μm Suc at pH 4.5. The results are the means ± sd of three independent experiments (three replicates per experiment).
To determine the substrate specificity of AgSUT1, transport of Suc was studied in the presence of various sugars and derivatives that might be potential substrates for a Suc transporter (Table III). Mannitol had no influence on the transport rates, which supports the assumption that Suc and mannitol uptakes are mediated by two independent transport systems in celery (Daie, 1986; Salmon et al., 1995). α-Phenylglucoside strongly inhibits Suc uptake, which agrees with published data on other Suc carrier specificity (AtSUC1/2, SoSUT1, and StSUT1). On the other hand, the inhibition by maltose is weak, in the same range as for StSUT1 (Riesmeier et al., 1993) and RcSCR1 (Weig and Komor, 1996). All other Suc carriers expressed in yeast are inhibited by maltose (see Lemoine, 2000). The addition of raffinose led to a higher inhibition than that noted for other Suc carriers expressed in yeast. This could be related to the fact that small amounts of raffinose are detected among the soluble sugars present in celery (N. Noiraud, L. Maurousset, S. Delrot, and R. Lemoine, unpublished data).
Table III.
Specificity of the AgSUT1 Suc carrier
| Carrier | Absorption |
|---|---|
| % | |
| None | 100 |
| 5 mm Suc | 65.4 ± 0.4 |
| 5 mm Mannitol | 90.0 ± 1.2 |
| 5 mm Maltose | 81.6 ± 1.2 |
| 5 mm Raffinose | 74.7 ± 1.6 |
| 2 mm α-Phenylglucoside | 32.0 ± 1.1 |
Sugars were added 30 s prior to labeled Suc. All transport tests were performed at 250 μm Suc, pH 4.5, and incubation time was 3 min. The results are the means ± sd of three independent experiments (three replicates per experiment).
Expression of AgSUT1 in Different Organs and Tissues
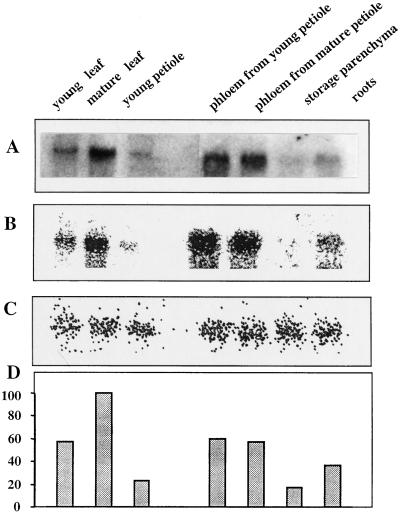
The results presented in Figure 6 have been obtained with AgSUT1 as a probe, and the quantity of RNA loaded per lane was calibrated with a riboprobe. The results are expressed as a percentage of expression in mature leaf, where AgSUT1 was found to be maximal. This is therefore in agreement with AgSUT1 being the ortholog to SUT1 in other species. AgSUT1 expression was also detected in young leaves, but only at low level in roots. As originally described by Daie (1986), phloem strands are easy to isolate from celery fleshy petioles. Those phloem strands are composed of sieve elements, companion cells, and phloem parenchyma cells, and can be used for plasma membrane isolation (Salmon et al., 1995) and RNA extraction. As can be seen in Figure 6, AgSUT1 expression was also high in phloem from both mature and young petioles, which corresponds to the same developmental stage as mature and young leaves. On the contrary, AgSUT1 expression in the storage parenchyma or total petiole was very weak.
Figure 6.
Expression of AgSUT1 in the different organs and tissues of celery. Organs from four different plants were pooled before RNA extraction. A, Film of the northern-blot analysis with AgSUT1 as a probe (20 μg RNA per lane). B, Scan of the image obtained after radioactivity counting with an Instant Imager (Packard Instruments). C, Same as B except that a 25S riboprobe from Arabidopsis was used to calibrate the amount of RNA loaded per lane. D, Quantification of AgSUT1 expression as percent of mature leaves (100%). Results derived from radioactivity counts obtained in B and C.
Expression of AgSUT1 during Salt Stress
The expression of the Suc carrier was investigated under salt stress conditions. Plants were watered during 4 weeks with 300 mm NaCl as described by Everard et al. (1994). Under these conditions, growth of young leaves still occurred, but the senescence of older leaves was accelerated and roots were shorter and larger (Everard et al., 1994; N. Noiraud, S. Delrot, and R. Lemoine, unpublished data). Half of the stressed plants were watered with tap water for 1 extra week (rehydrated plants).
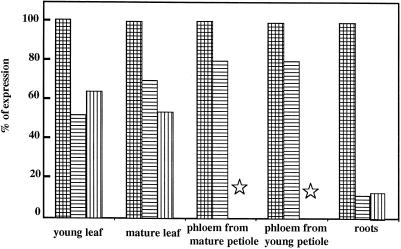
The results presented in Figure 7 show that under salt stress conditions, the expression of Suc carriers was lowered in all organs but to a different extent. In source leaves the expression was reduced by 30%. A strong reduction (50%) of AgSUT1 expression was observed in young leaves. In phloem tissues isolated from either young or mature petioles, AgSUT1 was only slightly reduced to 80% of control in both cases. The most striking alteration was noted in the roots: the expression was dramatically reduced in the case of salt stress, representing only 10% of expression in control roots. If AgSUT1 expression pattern in the different organs of control plants is taken into consideration (Fig. 6), the residual expression in young leaves and roots was very low following a 1-month salt stress. In all organs studied, a 1-week rehydration period was not sufficient to restore AgSUT1 expression to control levels (without salt stress). Work by Pardossi et al. (1998) indicated that a significant reduction in the salt content of stressed celery plants was only observed after a 4-month rehydration period. Therefore, the 1-week rehydration period used here was certainly not sufficient for a significant reduction in the salt content.
Figure 7.
Effect of salt stress on AgSUT1 expression in different organs of celery. Hatched bars, Control plants; horizontally striped bars, plants stressed for 4 weeks with 300 mm NaCl; vertically striped bars, plants rehydrated for 1 week after the salt stress. The results were obtained as described in Figure 6 (counting of radioactivity with an Instant Imager and calibration with a riboprobe), and the expression in control organs taken as 100%. Stars indicate non-determined conditions.
DISCUSSION
Suc Transporters in Celery
Three different cDNAs encoding Suc transporters were identified by hybridization screening. Two of these (AgSUT2A and AgSUT2B) have identical coding sequences and differ only in their 5′ and 3′ non-coding regions. AgSUT1 and AgSUT2A/AgSUT2B are closely sequence related, because they only differ by nine amino acids. Moreover, all the coding sequences have the same length and are all able to transport Suc when expressed in yeast. This situation is reminiscent of that in carrot, in which two cDNAs encoding Suc carriers also differ only in these regions (DcSUT1A and DcSUT1B; Shakya and Sturm, 1998). However, in that case, the third cDNA identified (DcSUT2) was not closely related to the other two. As expected from species of the same family, a high conservation level was found between AgSUTs and DcSUT2 (more than 90% identity at the amino acid level, and 80% at the nucleotide level). Surprisingly, the conservation extends to 5′ and 3′ untranslated regions with identity levels around 80%. Similar homologies were observed with other 5′ and 3′ flanking regions of cDNAs such as the AgCam (A. graveolens calmodulin, accession no. AF064552) and the DcCam-1 (D. carota calmodulin, accession no. X59751) (data not shown). This indicates that gene sequences are highly conserved between those two members of the Apiaceae family.
According to its kinetic characteristics (pH dependence, sensitivity to protonophores), AgSUT1 works as an H+/Suc transporter functionally related to the other SUT1 transporters identified so far. The Km value for AgSUT1 (139 μm) represents the highest affinity determined so far for Suc transporters expressed in yeast (Riesmeier et al., 1992, 1993; Gahrtz et al., 1994; Sauer and Stolz, 1994). However, due to different protocols and/or yeast strains used for uptake experiments, the differences in affinity may not be significant. Mannitol had no influence on the activity of AgSUT1, confirming the existence of independent transport systems for both substrates (Daie, 1986; Salmon et al., 1995).
Localization of AgSUT1 Expression
AgSUT1 was used as a probe for northern analysis. However, due to the very high level of sequence identity with AgSUT2A/AgSUT2B, cross-hybridization is more than probable and it was not possible to discriminate the expression of either of these carriers. For the rest of the discussion, we will refer to AgSUT to indicate that the signals observed in northern experiments were either due to AgSUT1 or AgSUT2. All of the transport interpretations are based on results obtained at the mRNA level and, therefore, post-transcriptional regulation has not been considered here. Some recent data indicate that phosphorylation could regulate the Suc carrier activity (Roblin et al., 1998), but transcriptional regulation is also important. This was demonstrated by the rapid turnover of the Suc carrier mRNA and protein measured in potato leaves (Kühn et al., 1997), and by the parallel decrease of Suc transporter mRNA levels and Suc transport activity in transgenic potato leaves (Lemoine et al., 1996) and sugar beet leaves (Chiou and Bush, 1998). Moreover, results showing higher AgSUT expression in phloem than in storage parenchyma in mature petioles (Fig. 6) are in perfect agreement with the higher Suc transport activity reported in plasma membrane vesicles from phloem than from storage parenchyma (Salmon et al., 1995).
AgSUT expression is higher in source leaves than in any other organ, and higher in phloem than in surrounding tissues in mature petioles, in agreement with results obtained with SUT1 carriers in other species. In spite of the direct access to phloem in petioles of celery, AgSUT expression is higher in leaves (all tissues of the leaves) than in phloem strands, which are highly enriched in conducting cells. This indicates that the minor veins network of mature leaves is the site where expression of Suc carriers is the highest.
Expression of AgSUT in mature petioles of celery may indicate a different role for the Suc transporter as, for example, in potato stem. In potato, it was proposed that the Suc carrier retrieves Suc leaking from the phloem (Riesmeier et al., 1993). Daie (1986) suggested that in mature petiole of celery, loading of Suc and mannitol mimics phloem loading in leaf minor veins, because sugars stored in the fleshy parenchyma of petioles are reloaded in the phloem to feed sink organs (new leaves and reproductive organs at the time of flowering). High expression of AgSUT in the phloem and very low expression in the storage parenchyma (Fig. 6) are in agreement with the model proposed by Daie. AgSUT expression in phloem from mature petioles confirms results from Salmon et al. (1995) on Suc uptake in plasma membrane vesicles indicating that, although hexoses are stored in storage parenchyma (Keller and Matile, 1989), Suc is reloaded into the phloem. Attempts to isolate a hexose carrier by reverse transcriptase-PCR from phloem RNA were not successful (data not shown), confirming the absence of hexose uptake in phloem.
During their development, petioles undergo a sink to source transition similar to the one existing in leaves. Recent results favor symplasmic unloading in young sink organs (Schmaltig and Geiger, 1985; Roberts et al., 1997; Imlau et al., 1999). However, data from northern analysis (Fig. 6) clearly indicate that AgSUT expression was detected in young leaves and in the phloem of young petioles. At the present time no information is available on the unloading pathway of Suc in sink petioles. One cannot rule out the possibility that young leaves and petioles used for RNA extraction (one-fifth of their final length) were already engaged in the sink to source transition. In fact several studies conducted in celery indicate that Suc synthesizing activities are detected quite early in sink leaves (Davis and Loescher, 1990) and that Suc export starts at a very early stage. This could explain why AgSUT was detected in young leaves. Similar levels of AgSUT expression were found in phloem from source and sink petioles. Depending on the stage of development, AgSUT could be involved either in Suc unloading from the phloem (sink situation) or Suc loading into the phloem (source situation). Obviously, more experiments are needed to precisely describe the function of the carriers during petiole and leaf development. Expression in root would also indicate that unloading occurs through AgSUT as suggested by several studies (Riesmeier et al., 1993; Truernit and Sauer, 1995).
Despite high sequence homologies between AgSUT and DcSUT2, their expression pattern is quite different. DcSUT2 was identified from a root cDNA library and detected in reproductive organs, and has therefore been classified as a sink-specific Suc transporter. However, some level of expression was also detected in source leaves. Nevertheless, AgSUT is more closely sequence-related to DcSUT2 than to DcSUT1. Therefore, there is no relationship between sequence and site of expression. We performed reverse transcriptase-PCR on RNA extracted from celery roots, but the partial cDNA obtained matched perfectly AgSUT1 (data not shown). More conclusive information will be obtained only after promoter analysis of the different genes.
Changes in AgSUT1 Expression in Response to Salt Stress
There are many situations where plants have to adapt their carbon allocation to sink organs and define new priorities among sinks. It is well known that under limiting nutrient supply, plants can flower and set seeds much faster than under normal conditions. Salt stress can be regarded as a situation in which plants have to cope both with decreased water availability and ion toxicity (Levitt, 1980). Carbon metabolism under stress has been investigated mainly in plants able to synthesize compatible solutes such as Pro, Gly betaine, and, as in celery, polyols. Polyols are unique because, like Suc, they are primary photosynthetic products and are thus different from nitrogen compounds. However, in many cases the source-to-sink transport of these compounds has not been taken into consideration. In celery, mannitol and Suc are synthesized in source leaves and then transported to sink organs. A shift in photosynthetic carbon allocation from Suc towards mannitol has been reported (4-fold increase in the mannitol/Suc ratio compared with controls; Everard et al., 1994) in the same stress conditions as the ones used in this study.
To maintain a high level of mannitol (an osmoprotectant), mannitol dehydrogenase is repressed, and it has been suggested that Suc is then metabolized to meet the need for energy of stressed tissues (Everard et al., 1994), just as in flowering plants and sink tissues (Fellman and Loescher, 1987). A high level of Suc (hexose) was shown to repress mannitol dehydrogenase expression in celery cells (Prata et al., 1997). This would indicate that Suc transport has to be maintained, which is in agreement with the rather low reduction of AgSUT expression reported here in source-exporting organs (leaves and petioles). The general down-regulation of AgSUT expression could be the result of a decreased demand for energy as growth is reduced. A more dramatic reduction of AgSUT expression is observed in roots. Expression of Suc carriers in roots has been related to Suc unloading (Riesmeier et al., 1993; Truernit and Sauer, 1995). If this is true, this would indicate that, in the case of salt stress, Suc unloading in roots is extremely reduced. As roots are the primary site of contact with the salt solution, mannitol accumulation may be of major importance in increasing the osmotic potential and allowing water uptake. Root growth is dramatically reduced in the case of salt stress (Everard et al., 1994; our own observations). Therefore, the reduced expression of AgSUT in roots could be the result of a decreased metabolic demand. Residual expression of AgSUT might be sufficient to account for the reduced flux of Suc to the roots. However, the possibility of salt-induced expression of a root-specific Suc carrier not related to AgSUT1 or AgSUT2 cannot be ruled out at the present time.
The salt stress results presented here certainly represent a new steady state in celery plants adapted to growth on high-salt conditions. Other studies on sugar carrier regulation have been conducted on much shorter periods of time (Weber et al., 1997; Chiou and Bush, 1998), because they were run on detached organs. The only transporter that has been studied in planta under salt stress conditions is the Pro transporter ProT2 from Arabidopsis (Rentsch et al., 1996), the expression of which is highly increased in response to 200 mm NaCl. In that case, an increase in ProT2 expression was already detected after 4 h. Interestingly, this increase in ProT2 expression is paralleled by a decrease in a general amino acid permease. In celery, this could mean that mannitol transporter expression is increased during salt stress.
Growing plants on high soil salinity represents a challenge for the future and it is therefore important to understand the strategies used by plants to cope with such stress. Up to now, most of the research devoted to polyols concerned their metabolism, both at the fundamental level and in transgenic plants engineered for salt resistance. However, transport events of osmolytes may be of significant importance because they are synthesized only in leaves. Plants such as celery may represent an excellent model for the study of polyol and sugar transport in response to stress because of a variety of responses (Stoop and Pharr, 1994; Pardossi et al., 1998). This work demonstrates down-regulation of a Suc transporter expression in roots in response to an abiotic stress highly relevant to culture conditions. Although mannitol biosynthesis has been considered as a response to salt stress, our results indicate that Suc transport is certainly not suppressed under such conditions. Further work is now under way to relate the changes in Suc metabolism and, more precisely, to describe the transporters involved by identifying their respective promoters.
ACKNOWLEDGMENTS
The authors wish to thank Prof. W. Loescher and Dr. J.D. Everard (Michigan State University, East Lansing, MI) for the generous gift of the excellent celery leaf cDNA library.
Footnotes
This work was supported by the French Ministry for Research and Higher Education, the Centre National de la Recherche Scientifique, and the Region Poitou-Charentes.
LITERATURE CITED
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acid Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieleski RL. Sugar alcohols. In: Loewus F, Tanner W, editors. Encyclopedia of Plant Physiology. Vol. 13. Berlin: Springer-Verlag; 1982. pp. 158–192. [Google Scholar]
- Bürkle L, Hibberd JM, Quick WP, Kühn C, Hirner B, Frommer WB. The H+-sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves. Plant Physiol. 1998;118:59–68. doi: 10.1104/pp.118.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR. Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA. 1998;95:4784–4788. doi: 10.1073/pnas.95.8.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daie J. Kinetics of sugar transport in isolated vascular bundles and phloem tissues of celery. J Am Soc Hortic Sci. 1986;111:216–220. [Google Scholar]
- Daie J. Sucrose uptake in isolated phloem of celery is a single saturable transport system. Planta. 1987;171:474–482. doi: 10.1007/BF00392294. [DOI] [PubMed] [Google Scholar]
- Davis JM, Fellman JK, Loescher WH. Biosynthesis of sucrose and mannitol as a function of leaf age in celery (Apium graveolens L.) Plant Physiol. 1988;86:129–133. doi: 10.1104/pp.86.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Loescher WH. [14C]-Assimilate translocation in the light and dark in celery (Apium graveolens) leaves of different ages. Physiol Plant. 1990;79:656–662. doi: 10.1111/j.1399-3054.1990.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Delrot S, Atanassova R, Maurousset L (2000) Regulation of sucrose, amino acid and peptide plasma membrane transporters. Biochim Biophys Acta (in press) [DOI] [PubMed]
- Dohmen RJ, Strasser AWM, Höner CB, Hollenberg CP. An efficient transformation procedure enabling long term storage of competent cells from various genera. Yeast. 1991;7:691–692. doi: 10.1002/yea.320070704. [DOI] [PubMed] [Google Scholar]
- Everard JD, Cantini C, Grumet R, Plummer J, Loescher WH. Molecular cloning of mannose-6-phosphate reductase and its developmental expression in celery. Plant Physiol. 1997;113:1427–1435. doi: 10.1104/pp.113.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard JD, Gucci R, Kann SC, Flore JA, Loescher WH. Gas exchange and carbon partitioning in the leaves of celery (Apium graveolens L.) at various levels of root zone salinity. Plant Physiol. 1994;106:281–292. doi: 10.1104/pp.106.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellman JK, Loescher WH. Comparative studies on sucrose and mannitol utilization in celery (Apium graveolens L.) Physiol Plant. 1987;69:337–341. doi: 10.1104/pp.86.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahrtz M, Stolz J, Sauer N. A phloem-specific sucrose-H+ symporter from Plantago major L. supports the model of apoplastic phloem loading. Plant J. 1994;6:697–706. doi: 10.1046/j.1365-313x.1994.6050697.x. [DOI] [PubMed] [Google Scholar]
- Gifford RM, Evans LT. Photosynthesis, carbon partitioning and yield. Annu Rev Plant Physiol. 1981;32:485–509. [Google Scholar]
- Hofmann K, Stoffel W. Tmbase: a database of membrane spanning protein segments. Biol Chem. 1993;347:166. [Google Scholar]
- Imlau A, Truernit E, Sauer N. Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplasmic unloading of the protein in sink tissues. Plant Cell. 1999;11:309–322. doi: 10.1105/tpc.11.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller F, Matile P. Storage of sugars and mannitol in petioles of celery leaves. New Phytol. 1989;113:291–299. doi: 10.1111/j.1469-8137.1989.tb02406.x. [DOI] [PubMed] [Google Scholar]
- Kühn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB. Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science. 1997;275:1298–1300. doi: 10.1126/science.275.5304.1298. [DOI] [PubMed] [Google Scholar]
- Kühn C, Quick WP, Schulz A, Sonnewald U, Frommer WB. Companion cell-specific inhibition of the potato sucrose transporter SUT1. Plant Cell Environ. 1996;19:1115–1123. [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lalonde S, Boles E, Hellmann H, Barker L, Patrick JW, Frommer WB, Ward JM. The dual function of sugar carriers: transport and sugar sensing. Plant Cell. 1999;11:707–726. doi: 10.1105/tpc.11.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine R (2000) Sucrose transporters in plants: update on function and structure. Biochim Biophys Acta (in press) [DOI] [PubMed]
- Lemoine R, Gallet O, Gaillard C, Frommer W, Delrot S. Plasma membrane vesicles from source and sink leaves: changes in solute transport and polypeptide composition. Plant Physiol. 1992;100:1150–1156. doi: 10.1104/pp.100.3.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine R, Kühn C, Thiele N, Delrot S, Frommer WB. Antisense inhibition of the sucrose transporter in potato: effects on amount and activity. Plant Cell Environ. 1996;19:1124–1131. [Google Scholar]
- Levitt J. Responses of Plants to Environmental Stresses. II: Water, Radiation, Salt and Other Stresses. New York: Academic Press; 1980. Salt and ion stresses; pp. 365–488. [Google Scholar]
- Lu JMY, Bush DR. His-65 in the proton-sucrose symporter is an essential amino acid whose modification with site-directed mutagenesis increases transport activity. Proc Natl Acad Sci USA. 1998;95:9025–9030. doi: 10.1073/pnas.95.15.9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardossi A, Malorgio F, Oriolo D, Gucci R, Serra G, Tognoni F. Water relations and osmotic adjustment in Apium graveolens during long-term NaCl stress and subsequent relief. Physiol Plant. 1998;102:369–376. [Google Scholar]
- Prata RTN, Williamson JD, Conkling MA, Pharr DM. Sugar repression of mannnitol dehydrogenase activity in celery cells. Plant Physiol. 1997;114:307–314. doi: 10.1104/pp.114.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentsch D, Hirner B, Schmelzer E, Frommer WB. Salt stress-induced proline transporters and salt stress-repressed broad specificity amino acid permease identified by suppression of a yeast amino acid permease-targeting mutant. Plant Cell. 1996;8:1437–1446. doi: 10.1105/tpc.8.8.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Hirner B, Frommer WB. Potato sucrose transporter expression in minor veins indicate a role in phloem loading. Plant Cell. 1993;5:1591–1598. doi: 10.1105/tpc.5.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB. Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 1992;11:4705–4713. doi: 10.1002/j.1460-2075.1992.tb05575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AG, Santa Cruz S, Roberts IM, Prior DAM, Turgeon R, Oparka KJ. Phloem unloading in sink leaves of Nicotiana benthamiana: comparison of a fluorescent solute with a fluorescent virus. Plant Cell. 1997;9:1381–1396. doi: 10.1105/tpc.9.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblin G, Sakr S, Bonmort J, Delrot S. Regulation of a plant plasma membrane sucrose transporter by phosphorylation. FEBS Lett. 1998;424:165–168. doi: 10.1016/s0014-5793(98)00165-3. [DOI] [PubMed] [Google Scholar]
- Rumpho ME, Edwards GE, Loescher WH. A pathway for photosynthetic carbon flow to mannitol in celery leaves. Plant Physiol. 1983;73:869–873. doi: 10.1104/pp.73.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon S, Lemoine R, Jamaï A, Bouche-Pilon S, Fromont JC. Study of sucrose and mannitol transport in plasma-membrane vesicles from phloem and non-phloem tissues of celery (Apium graveolens L.) petioles. Planta. 1995;197:76–83. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sauer N, Stolz J. SUC1 and SUC2: two sucrose transporters from Arabidopsis thaliana; expression and characterization in baker's yeast and identification of the histidine-tagged protein. Plant J. 1994;6:67–77. doi: 10.1046/j.1365-313x.1994.6010067.x. [DOI] [PubMed] [Google Scholar]
- Schmalstig JG, Geiger DR. Phloem unloading in developing leaves of sugar beet: I. Evidence for pathway through the symplasm. Plant Physiol. 1985;79:237–241. doi: 10.1104/pp.79.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya R, Sturm A. Characterization of source- and sink-specific sucrose/H+ symporters from carrot. Plant Physiol. 1998;118:1473–1480. doi: 10.1104/pp.118.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop JMH, Pharr DM. Mannitol metabolism in celery stressed by excess macronutrients. Plant Physiol. 1994;106:503–511. doi: 10.1104/pp.106.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop JMH, Williamson JD, Pharr DM. Mannitol metabolism in plants: a method for coping with stress. Trends Plant Sci. 1996;1:139–144. [Google Scholar]
- Thomas JC, Sepahi M, Arendall B, Bohnert HJ. Enhancement of seed germination in high salinity by engineering mannitol expression in Arabidopsis thaliana. Plant Cell Environ. 1995;18:801–806. [Google Scholar]
- Truernit E, Sauer N. The promoter of the Arabidopsis thaliana SUC2 sucrose-H+ symporter gene directs expression of β-glucuronidase to the phloem: evidence for phloem loading and unloading by SUC2. Planta. 1995;196:564–570. doi: 10.1007/BF00203657. [DOI] [PubMed] [Google Scholar]
- Turgeon R. Phloem unloading and plasmodesmata. Trends Plant Sci. 1996;1:418–423. [Google Scholar]
- Weber H, Borisjuk L, Heim U, Sauer N, Wobus U. A role for sucrose transporters during seed development: molecular characterization of a hexose and a sucrose carriers in faba bean seeds. Plant Cell. 1997;9:895–908. doi: 10.1105/tpc.9.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weig A, Komor E. An active sucrose carrier (Scr1) that is predominantly expressed in the seedlings of Ricinus communis L. J Plant Physiol. 1996;147:685–690. [Google Scholar]
- Winter H, Robinson DG, Heldt HW. Subcellular volumes and metabolites concentrations in spinach leaves. Planta. 1994;193:530–535. [Google Scholar]