Abstract
Knowledge of the properties that govern the effectiveness of transcranial magnetic stimulation (TMS) interventions is critical to clinical application. Extrapolation to clinical populations has been limited by high inter-subject variability and a focus on intrinsic muscles of the hand in healthy populations. Therefore, the current study assessed variability of continuous theta burst stimulation (cTBS), a patterned TMS protocol, across an agonist–antagonist pair of extrinsic muscles of the hand. Secondarily, we assessed whether concurrent agonist contraction could enhance the efficacy of cTBS. Motor evoked potentials (MEP) were simultaneously recorded from the agonist flexor (FCR) and antagonist extensor (ECR) carpi radialis before and after cTBS over the FCR hotspot. cTBS was delivered with the FCR relaxed (cTBS-Relax) or during isometric wrist flexion (cTBS-Contract). cTBS-Relax suppressed FCR MEPs evoked from the FCR hotspot. However, the extent of FCR MEP suppression was strongly correlated with the relative difference between FCR and ECR resting motor thresholds. cTBS-Contract decreased FCR suppression but increased suppression of ECR MEPs elicited from the FCR hotspot. The magnitude of ECR MEP suppression following cTBS-Contract was independent of the threshold-amplitude relationships observed with cTBS-Relax. Contraction alone had no effect confirming the effect of cTBS-Contract was driven by the interaction between neuromuscular activity and cTBS. Interactions across muscle representations should be taken into account when predicting cTBS outcomes in healthy and clinical populations. Contraction during cTBS may be a useful means of focusing aftereffects when differences in baseline excitability across overlapping agonist–antagonist cortical representations may mitigate the inhibitory effect of cTBS.
Keywords: theta burst stimulation, transcranial magnetic stimulation, plasticity, motor cortex, flexor carpi radialis, contraction
INTRODUCTION
The motor cortex is capable of rapid and persistent activity dependent reorganization, known as “neural plasticity”. The reorganization results from the strengthening of relevant synaptic efficacy via long-term potentiation and/or the weakening in efficacy of task-irrelevant synapses via long-term depression (Sanes and Donoghue, 2000; Cardenas-Morales et al., 2010). Long-term potentiation and long-term depression are critical neural processes in the acquisition and retention of motor skills in healthy individuals as well as the recovery of functional motor ability (Hadj Tahar et al., 2004). However, the changes induced by experience alone require extensive, time intensive interventions (Birkenmeier et al., 2010). As a result, methods to increase neural plastic response have been an area of interest.
Non-invasive brain stimulation applied to the motor cortex can alter neurophysiology and motor performance (Muellbacher et al., 2002). The plastic changes induced by brain stimulation are mechanistically similar to the long-term potentiation and long-term depression that underlie motor learning (Censor and Cohen, 2011). One variant of non-invasive transcranial magnetic stimulation, theta burst stimulation, has been of particular interest given its relative efficiency and long-lasting aftereffects (Huang et al., 2005). However, the benefits of magnetic stimulation in the recovery of motor deficits are moderate (Hsu et al., 2012) and characterized by variability within and across studies (Di Pino et al., 2014).
For intrinsic muscles of the hand the efficacy of both intermittent (iTBS) and continuous (cTBS) theta burst stimulation has been linked to the differential recruitment of intracortical networks rather than inherent differences in the potential for plasticity across individuals (Hamada et al., 2013). However, relatively little research has studied variability of transcranial magnetic stimulation induced aftereffects in the extrinsic muscles of the hand, such as the wrist flexors and extensors. Relative differences in corticospinal control (Fetz and Cheney, 1980; Palmer and Ashby, 1992; Park et al., 2004) and/or stimulation specific transcranial magnetic parameters (Mirdamadi et al., 2015) may determine which intracortical networks are most readily recruited from overlapping cortical representations at the site of stimulation.
The current study sought to assess the effect of cTBS over the FCR cortical hotspot upon the overlapping cortical representations of the FCR and ECR muscles. Consistent with a common underlying mechanism mediating both forms of TBS-induced plasticity (Huang et al., 2011; Hamada et al., 2013) and our previous work using iTBS (Mirdamadi et al., 2015) we hypothesized that individuals with FCR thresholds that were lower or in close proximity to their ECR threshold would demonstrate stronger suppression of FCR MEPs post-cTBS. Similarly, we hypothesized that individuals with FCR thresholds that were increasingly greater than their ECR threshold would demonstrate progressively stronger suppression of ECR MEPs. Finally, we sought to determine whether intrinsic depolarization associated with isometric FCR contraction could selectively bias cTBS aftereffects to either the agonist or antagonist muscle regardless of relative resting thresholds. In intrinsic hand muscles, the suppressive effect of cTBS over a muscle’s motor cortical representation is mitigated by concurrent contraction of that muscle (Huang et al., 2008). Given reciprocal changes in input–output curves of the FCR and ECR during motor skill learning (Suzuki et al., 2012) we hypothesized that concurrent contraction of the FCR (10% of maximum voluntary force) during cTBS would interfere with the buildup of long-term depression-like effects in the FCR muscle and favor suppression of ECR MEP amplitude.
EXPERIMENTAL PROCEDURE
Participants
Fifteen healthy individuals (six males, nine females, 22±4.7 years) participated in Experiment 1. An independent sample of thirteen healthy individuals (three males, 10 females, 21±1.5 years) were recruited to participate in a separate control condition (Experiment 2). All participants provided informed consent; the Institutional Review Board of the University of Michigan Medical School (IRBMED) approved the study protocol.
Experimental design and procedure
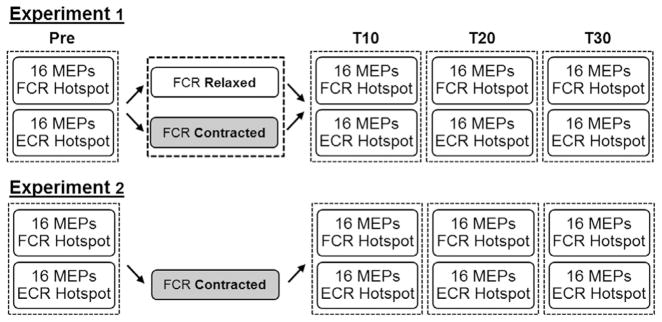
For Experiment 1, the same participants completed two testing sessions separated by three days. At each session MEPs were simultaneously recorded from the FCR and ECR muscles in response to single pulses of transcranial magnetic stimulation before and 10, 20 and 30 min after application of cTBS over the FCR cortical hotspot (Fig. 1). Sixteen single pulses were delivered over both the FCR (120% of FCR resting motor threshold) and ECR (120% of ECR resting motor threshold) cortical hotspots. The two testing sessions only differed by the state of the FCR muscle during cTBS. For Session 1, the FCR was relaxed. For Session 2, subjects maintained an isometric contraction of the FCR with the wrist in a flexed position. Isometric contraction was set to 10% of maximum voluntary force. Visual feedback regarding force was provided on a computer screen in front of the participant. Session and order of hotspot stimulation were counterbalanced across participants. Experiment 2 was similar to Experiment 1, except that an independent sample of participants was recruited to complete a session involving isometric wrist flexion in the absence of cTBS (Fig. 1). The effect of isometric wrist flexion in this independent sample was subsequently compared to that of cTBS paired with isometric wrist flexion from the original cohort in Experiment 1.
Fig. 1.
Protocol for Experiments 1 and 2. For Experiment 1 participants completed two sessions. The only difference between sessions was whether participants were instructed to keep their forearm in a relaxed, neutral position for the duration of cTBS or were instructed to maintain an isometric wrist flexion corresponding to 10% of maximum force production in the FCR muscle. The protocol for Experiment 2 was similar except participants completed only one session during which the FCR was contracted for 40 s in the absence of cTBS.
Stimulation and recording
Transcranial magnetic stimulation was delivered using a MagVenture MagPro X100 with option stimulator (MagVenture Inc., Atlanta, GA) and a statically cooled figure-8 coil (MCF-B70). The coil was oriented tangentially to the scalp over the left motor cortex with the handle at 45° to the midline in a posterior lateral orientation. Surface electromyography was recorded using LabChart 7 software in conjunction with a Dual BioAmp and PowerLab 8/30 acquisition system (AD Instruments, Colorado Springs, CO). Surface electromyography recording was triggered using a 5 V TTL pulse with an epoch of −0.3 to 0.5 s. During acquisition, data were amplified (×1000), digitized (×40,000 Hz) and filtered (band pass filtered 5–1000 Hz, notch filter – 60 Hz). Surface electromyography data were subsequently down-sampled to 5000 Hz during offline analysis.
The FCR and ECR motor cortical hotspots were localized separately. The hotspot for each muscle was defined as the position that elicited the largest MEP in the targeted contralateral muscle. The position of the coil on the scalp for each motor cortical hotspot was recorded using the BrainSight™ stereotactic system (Rogue Research, Montreal, QC). Resting motor threshold was defined for both the FCR and ECR hotspots as the percentage of stimulator output that elicited an MEP of ≥50 μV peak to peak on five out of 10 trials in the relevant muscle. Active motor threshold for the FCR at the FCR hotspot was defined as the percentage of stimulator output that elicited an FCR MEP of ≥200 μV peak to peak on five out of 10 trials during tonic wrist flexion of 20% of the maximum force production.
cTBS consisted of three pulses presented at 50 Hz, repeated at 5 Hz for 40 s (600 magnetic stimuli total). Intensity was set to 80% of the active motor threshold for the FCR (Huang et al., 2005).
Data analysis
For both experiments the root mean square error for each MEP was calculated 50 ms prior to stimulus onset. Any trials in which root mean square error of either the targeted or non-targeted muscle exceeded 15 μV were excluded from subsequent analysis (Ackerley et al., 2011; Mirdamadi et al., 2015). The mean peak-to-peak amplitude of the MEP was then derived for each combination of Time (pre, T10, T20, T30), Muscle (Targeted, Non-targeted), Hotspot (FCR, ECR) and Session (cTBS-Relax, cTBS-Contract, cTBS-Alone). The targeted muscle was defined by hotspot. For example, FCR was the targeted muscle when single pulses were delivered over the FCR motor cortical hotspot at 120% of FCR resting motor threshold.
For Experiment 1, separate paired t-tests were first run to compare pre-cTBS MEP amplitudes across Session for FCR and ECR MEPs elicited from the FCR hotspot. A Session (cTBS-Relax, cTBS-Contract) × Muscle (FCR, ECR) × Time (Pre, T10, T20, T30) repeated measures ANOVA was used to assess the efficacy of cTBS-Relax and cTBS-Contract upon MEPs evoked from the FCR hotspot. The significant three-way interaction was decomposed using the simple effect of Time for each Session and Muscle followed by contrasts where applicable. Greenhouse-Geisser epsilon and Bonferroni corrections were employed where applicable (Keppel and Wickens, 2004). Separate Pearson Product Moment Correlations (PPMC) were used to determine the relationship between cTBS aftereffect and measures of resting cortical excitability. For each correlation the dependent variable was the percent change in MEP amplitude, relative to pre-stimulation, at the FCR hotspot averaged across T10, T20 and T30. Independent variables included FCR resting motor threshold, ECR resting motor threshold and FCR-ECR resting motor threshold difference. Separate correlations were performed for MEPs recorded from each muscle. Significance was set at p<0.05. The same analytical approach was repeated for FCR and ECR MEPs simultaneously recorded from the ECR hotspot.
The analyses for Experiment 2 were similar to those outlined for Experiment 1. The only difference was that the repeated measure Session was replaced by the between-subjects factor Group (Contract-Alone, cTBS-Contract) as the Contract-Alone and cTBS-Contract sessions were completed by mutually exclusive groups.
RESULTS
Experiment 1
The FCR motor cortical hotspot was located 46.3± 4.6 mm lateral and 11.5±7.3 mm anterior to the vertex. The ECR motor cortical hotspot was 44.7± 5.7 mm lateral and 13.5±7.0 mm anterior to the vertex. Consistent with our previous work (Mirdamadi et al., 2015) and others (Suzuki et al., 2012) FCR resting motor threshold was significantly greater than ECR resting motor threshold regardless of session [Main EffectMuscle: F1,14=32.05, p<0.0001, FCR=42±8 (mean±standard deviation), ECR=39±8]. Resting motor thresholds for each muscle were not different across session [InteractionSession×Muscle: F1,14=0.10, p=0.76; Main EffectSession: F1,14=1.00, p=0.33]. Average FCR active motor threshold was 34±5% for Session 1 and 33±5% for Session 2 [t14=−0.50, p=0.62].
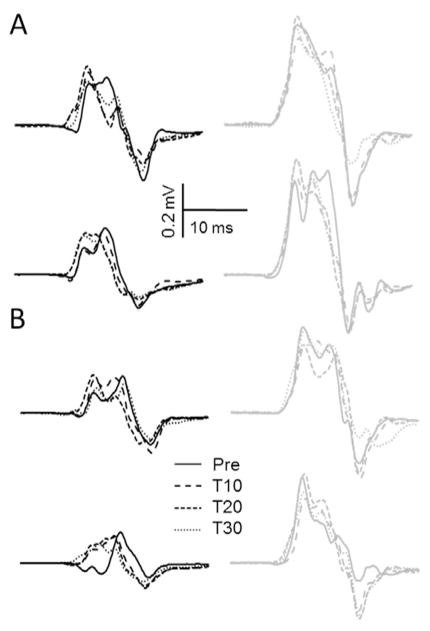
Fig. 2 illustrates example MEPs recorded from the FCR and ECR muscles evoked from the FCR and ECR hotspots across Session and Time for one subject. There were no differences in pre-cTBS FCR [t14=0.89, p=0.39] or ECR [t14=1.33, p=0.20] MEP amplitude across the cTBS-Relax and cTBS-Contract sessions.
Fig. 2.
Average FCR (black lines) and ECR (grey lines) MEPs elicited from the (A) FCR hotspot and (B) ECR hotspot during the cTBS-Relax (top row) and cTBS-Contract (bottom row) sessions for a single participant. Consistent with the relationship between FCR/ECR thresholds and post-cTBS MEP amplitude this individual demonstrated FCR suppression and ECR enhancement following cTBS-Relax.
MEPs elicited from FCR hotspot
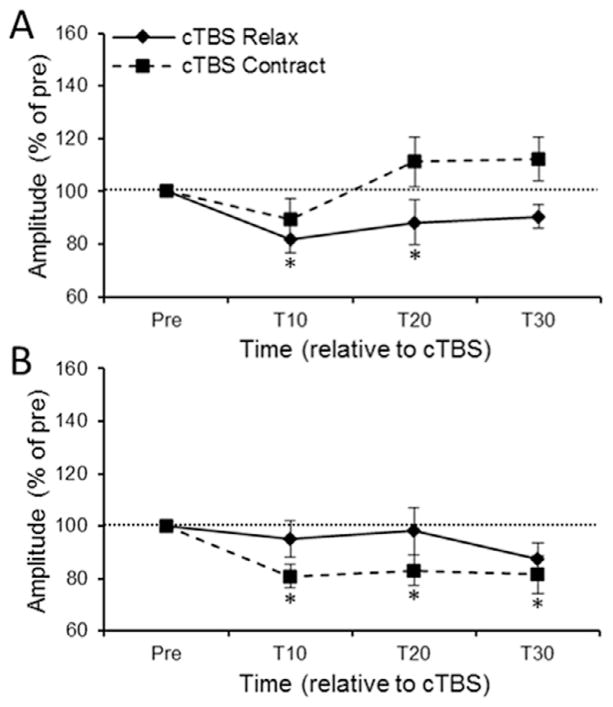
The Session × Muscle × Time repeated measures ANOVA upon MEP amplitude evoked from the FCR hotspot revealed a significant three-way interaction [F3,42=3.56, ε=0.75, p=0.036]. Decomposition of the three-way interaction using the simple main effect of Time revealed different effects of cTBS-Relax and cTBS-Contract across muscle. cTBS-Relax significantly reduced FCR MEP amplitude [F3,42=3.63, ε=0.65, p=0.04] at T10 (p=0.01) and T20 (p=0.03) but not T30 (p=0.20) compared to pre-cTBS (Fig. 3A – solid line). In contrast, cTBS-Relax had no effect upon the amplitude of ECR MEPs evoked from the FCR hotspot [F3,42=0.68, ε=0.55, p=0.49] (Fig. 3B – solid line). The opposite pattern was observed following cTBS-Contract. cTBS-Contract had no effect upon FCR MEP amplitude across time [F3,42=2.01, ε=0.69, p=0.15] (Fig. 3A – dashed line) whereas ECR MEPs evoked from the FCR hotspot were significantly reduced [F3,42=4.67, ε=0.56, p=0.02] at T10 (p=0.01), T20 (p=0.03) and T30 (p=0.03) compared to precTBS (Fig. 3B – dashed line).
Fig. 3.
(A) FCR and (B) ECR MEP amplitude evoked from the FCR hotspot at each time point across the session. For both figures MEP amplitude is expressed as a percentage of pre-cTBS MEP amplitude. The horizontal dashed line represents pre-cTBS baseline amplitude. Error bars represent standard error of the mean. * denotes corrected p<0.05 when comparing raw MEP amplitude at a time point relative to raw pre-cTBS MEP amplitude.
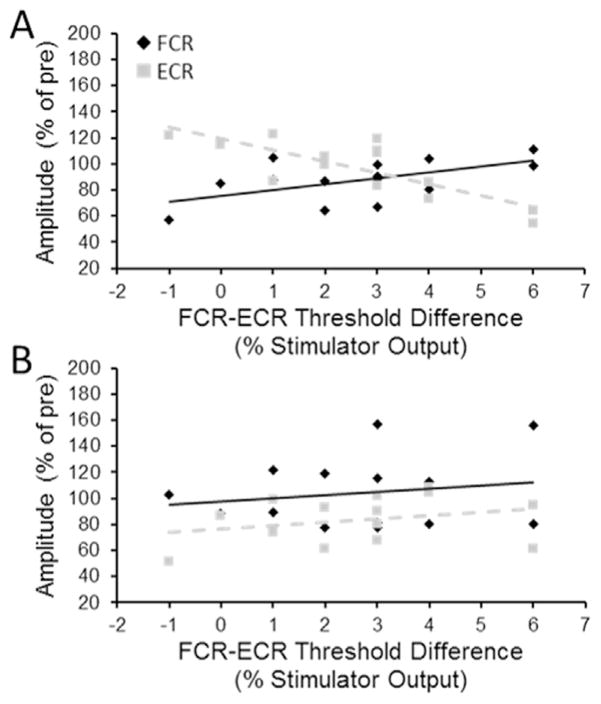
Table 1 shows the results of the PPMCs for both FCR and ECR MEPs elicited from the FCR hotspots and resting motor thresholds. For cTBS-Relax there was a significant moderate positive correlation between FCR-ECR resting motor threshold difference and average FCR MEP suppression post-cTBS-Relax. FCR MEP suppression was greatest in individuals who had lower FCR-ECR threshold differences (Fig. 4A – solid line). A significant moderate negative correlation was observed between FCR-ECR threshold difference and average ECR MEP suppression post-cTBS-Relax. Individuals with relatively lower FCR-ECR threshold difference demonstrated weaker suppression and even facilitation of ECR MEP amplitude post-cTBS (Fig. 4A – dashed line). There were no significant correlations involving the FCR resting motor threshold or ECR resting motor threshold in isolation. For cTBS-contract none of the PPMCs, including those between cTBS-contract aftereffect and FCR-ECR resting motor threshold difference, were significant (Fig. 4B). A test of equality (Lee and Preacher, 2013) revealed that the correlation coefficient between MEP amplitude change and FCR-ECR resting motor threshold difference for cTBS-Relax was significantly greater than that for cTBS-Contract for both FCR [z=2.10, p=0.035] and ECR [z=2.17, p=0.030] MEPs elicited from the FCR hotspot.
Table 1.
Correlation coefficients for Pearson product moment correlations between MEP suppression at the FCR hotspot and resting motor thresholds
| cTBS-Relax
|
cTBS-Contract
|
||||
|---|---|---|---|---|---|
| r-Statistic | p-Value | r-Statistic | p-Value | ||
| MEP | Threshold | ||||
| FCR | FCR RMT | −0.28 | 0.34 | 0.04 | 0.89 |
| ECR RMT | −0.42 | 0.14 | 0.09 | 0.75 | |
| FCR-ECR RMT difference | 0.55 | 0.04* | −0.19 | 0.51 | |
| ECR | FCR RMT | −0.02 | 0.94 | 0.07 | 0.81 |
| ECR RMT | 0.17 | 0.55 | 0.02 | 0.91 | |
| FCR-ECR RMT difference | −0.79 | 0.001* | 0.16 | 0.58 | |
Denotes significant correlation.
Fig. 4.
Correlation between FCR-ECR resting motor threshold (RMT) difference and FCR (diamond) and ECR (square) motor evoked potentials following (A) cTBS-Relax and (B) cTBS-Contract. All MEPs were evoked from the FCR hotspot.
MEPs elicited from ECR hotspot
The Session × Muscle × Time repeated measures ANOVA upon MEP amplitude evoked from the ECR hotspot failed to reveal a significant three-way interaction [F3,42=0.30, ε=0.56, p=0.71]. The only significant effect within the omnibus ANOVA model was a significant main effect of Muscle [F1,14=8.78, p=0.01] driven by larger ECR MEPs (713±154 μV) compared to FCR MEPs (250 ±62 μV) evoked from the ECR hotspot. The absence of any effects involving Session or Time indicates that cTBS applied over the FCR hotspot only had an effect on MEPs elicited from the FCR hotspot.
Experiment 2
For the Contract-Alone group the FCR motor cortical hotspot was 42.0±5.2 mm lateral and 14.7±6.8 mm anterior to the vertex. The ECR motor cortical hotspot was 40.6±5.7 mm lateral and 14.6±7.0 mm anterior to the vertex. Similar to Experiment 1, the Contract- Alone group demonstrated significantly greater FCR compared to ECR resting motor threshold [t12=3.87, p=0.002, FCR=41±7%, ECR=38±6% (mean± standard deviation)]. Active motor threshold for the Contract-Alone group was 33±4%. There were no differences in baseline FCR [t27=−0.36, p=0.72] or ECR [t27=1.44, p=0.16] MEP amplitude across the Contract-Alone and Contract-cTBS groups.
MEPs elicited from FCR hotspot
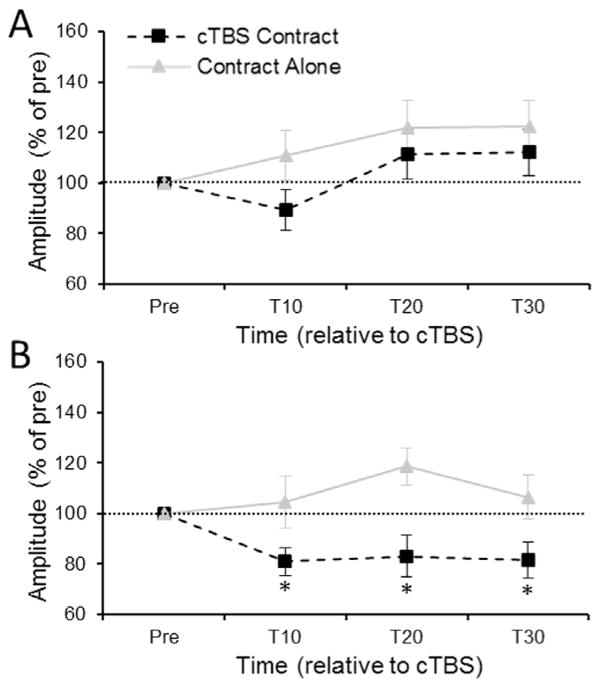
The Group × Muscle × Time mixed measures ANOVA revealed a significant three-way interaction [F3,81=2.81, ε=0.91, p=0.049]. Decomposition of the three-way interaction using the simple effect of Time revealed that Contract- Alone did not have any sustained effect upon FCR [F3,39=0.55, ε=0.88, p=0.63] or ECR [F3,39=0.72, ε=0.67, p=0.50] MEP amplitude across time (Fig. 5A, B – grey lines). cTBS-Contract significantly suppressed ECR MEPs [F3,42=4.67, ε=0.56, p=0.02] at T10 (p=0.01), T20 (p=0.03) and T30 (p=0.03) compared to pre-cTBS whereas cTBS-Contract failed to produce an effect upon FCR amplitude [F3,42=2.01, ε=0.69, p=0.15]. PPMCs failed to reveal any significant correlations between post-intervention MEP amplitudes and FCR-ECR resting motor threshold difference for the FCR or ECR muscles. Further, there were no significant correlations between MEP amplitudes and FCR resting motor threshold or ECR resting motor threshold.
Fig. 5.
(A) FCR and (B) ECR MEP amplitude evoked from the FCR hotspot at 10-, 20- and 30-min post-intervention for the cTBS-Contract and Contract-Alone sessions. The horizontal dashed line represents pre-cTBS baseline amplitude. Error bars represent standard error of the mean. * denotes corrected p<0.05 when comparing raw MEP amplitude at a time point relative to raw pre-cTBS MEP amplitude.
MEPs elicited from ECR hotspot
The Group × Muscle × Time repeated measures ANOVA upon MEP amplitude evoked from the ECR hotspot failed to reveal a significant three-way interaction [F3,81=2.12, ε=0.89, p=0.11]. However, within the omnibus ANOVA model there was a significant main effect of Muscle [F1,27=13.72, p=0.001]. Again, the main effect was driven by larger ECR MEPs (656 ±78 μV) compared to FCR MEPs (318±55 μV) evoked from the ECR hotspot. Contract-Alone had no persistent effect upon motor cortical excitability of either muscle at the ECR hotspot.
DISCUSSION
The current study demonstrated that cTBS suppressed motor cortical excitability of the targeted muscle across a cohort of young healthy adults. However, the magnitude of suppression within each individual was dependent upon the relative thresholds of overlapping cortical representations. The current study also provides evidence that isometric wrist flexion enhanced the suppressive effect of cTBS upon the overlapping cortical representation of an antagonist muscle. Enhanced antagonist suppression occurred independent of relative threshold differences between the agonist and antagonist muscles, indicating that concurrent contraction is a useful method of enhancing the efficacy of cTBS in extrinsic muscles of the hand.
The primary finding of the current study was the relationship between cTBS aftereffect and the relative difference between the FCR and ECR resting motor thresholds. At the FCR hotspot, the magnitude of FCR suppression decreased as FCR threshold became increasingly greater than that of the ECR despite stimulation parameters optimized for the FCR. Relatively little research has focused on the efficacy of TBS protocols upon the cortical excitability of the extrinsic muscles of the hand (for a summary see Table 1, Wischnewski and Schutter, 2015). For intrinsic muscles of the hand variation in cTBS-induced aftereffect has been linked to the propensity of repetitive stimulation to recruit specific interneuron networks within motor cortex (Hamada et al., 2013). Our results suggest that it may not be the propensity to recruit specific networks of the target muscle per se that determines cTBS aftereffect. Instead, our results suggest that cTBS aftereffect is governed by the relative recruitment of intracortical networks, both targeted and non-targeted, in the stimulated region. For extrinsic muscles of the hand interactions across muscle representation may not be as evident as the resting motor threshold of the first dorsal interosseous and abductor pollicus brevis muscles tend to be the lowest within motor cortex. Further, the primary antagonists for the intrinsic muscles are rarely recorded from in transcranial magnetic stimulation studies.
The relationship between FCR-ECR threshold difference and cTBS aftereffect can be accounted for within the framework of indirect(I)-wave models of theta burst stimulation physiology (Di Lazzaro et al., 2012). The inhibitory nature of cTBS is strongest for individuals in whom cTBS readily recruits the later I-wave known as I3 (Hamada et al., 2013). The relative contribution of later I-waves, like I3, to the TMS evoked response increases as stimulation intensity increases (Di Lazzaro et al., 1998). In the current study, the relative stimulation intensity for the FCR during cTBS was consistent across all participants regardless of the actual FCR-ECR threshold difference as stimulation intensity was set relative to the FCR threshold. However, stimulus intensity during cTBS was not fixed relative to ECR resting threshold and varied across individual. Therefore, individuals with relatively bigger FCR-ECR threshold differences would be predicted to elicit relatively greater recruitment of later I-waves in the ECR cortical representation compared to those with smaller FCR-ECR threshold differences. This pattern of later I-wave recruitment across muscle cortical representation would predict enhanced suppressive effects of cTBS upon the ECR and decreased suppressive effects upon the FCR, consistent with our results.
An interesting result from the current study in light of our previous work is that both the facilitatory effect of iTBS (Mirdamadi et al., 2015) and the suppressive effect of cTBS upon the FCR are stronger in individuals with lower FCR-ECR threshold differences. The similar direction of the relationship is consistent with a common underlying mechanism mediating TBS-induced plasticity. In the first dorsal interosseous muscle, greater contribution of I3 wave to MEP generation was associated with increased MEP enhancement post-iTBS as well as increased MEP suppression post-cTBS (Hamada et al., 2013). Theoretical models of theta burst protocols also posit a common NMDA receptor dependence (Huang et al., 2011). Therefore, it is not surprising that individuals who demonstrate the greatest FCR MEP enhancement post-iTBS or those that demonstrate the greatest FCR MEP suppression post-cTBS both have smaller FCR-ECR threshold differences. That the efficacy of each transcranial magnetic stimulation protocol upon the FCR cortical representation is enhanced in individuals with lower FCR-ECR threshold difference does suggest that, for the FCR at least, stimulation at rest is less a function of the resting state of the FCR (Artola et al., 1990) and instead reflects the relative accumulation of facilitation/inhibition across the overlapping muscle representations.
Concurrent contraction reduced the suppressive effect of cTBS upon the FCR cortical representation but enhanced the suppressive effect upon the overlapping ECR cortical representation. This effect is attributable to the interaction between concurrent contraction and cTBS as contraction alone for 40 s (Experiment 2) had no effect upon motor cortical excitability. This is consistent with previous work demonstrating that voluntary contraction of either the agonist (Huang et al., 2008) or antagonist (Fang et al., 2014) muscle alone had no effect on cortical excitability of the transcranial magnetic stimulation target. It should be noted that there were qualitative differences in the relative locations of the FCR and ECR hotspots along the anterior/posterior axis across experiments. While the FCR was ~2 mm more lateral and posterior to the ECR hotspot in Experiment 1, consistent with a previous report (Suzuki et al., 2012), the difference in the anterior/posterior axis was not observed in the independent sample recruited for Experiment 2. The difference in the relative locations of the FCR and ECR hotspots may explain the absence of a contraction alone effect in Experiment 2. However, given each hotspot was defined based upon physiological response (MEP amplitudes) and that there were no differences in resting motor thresholds or baseline MEP amplitudes across the independent samples it is more likely that the differential effects of cTBS paired with contraction and contraction alone are specific to the interaction between cTBS and contraction rather than being driven by relative location of each muscle’s hotspot.
The reduction in FCR MEP suppression following cTBS during FCR contraction is consistent with previous work in the intrinsic muscles of the hand (Huang et al., 2008). Two hypotheses have been proposed to explain the abolishment of MEP suppression in intrinsic muscles of the hand. The first hypothesis is that the LTD-like aftereffects induced by cTBS are sensitive to the physiological state of the neurons impacted by stimulation (Huang et al., 2008). Animal models suggest that depolarization of the postsynaptic membrane favors LTP whereas hyperpolarization favors LTD (Artola et al., 1990). Depolarization of the post-synaptic neuron in support of the volitional contraction likely raised the threshold for LTD and attenuated the inhibitory effect of cTBS in the FCR (Ziemann and Siebner, 2008). In contrast, those projecting to the antagonist muscle were likely hyperpolarized favoring the LTD-like effect of cTBS in the ECR. The second hypothesis posits that FCR contraction engaged the same synaptic pathways targeted by cTBS creating a “busy signal” (Huang et al., 2008). Under this hypothesis recruitment of NMDA-receptors in support of the voluntary isometric FCR contraction meant that cTBS could not recruit these same receptors to modulate ionic flow and post-synaptic second messenger dynamics (Huang et al., 2011). Conversely, in the lengthened antagonist ECR, NMDA-receptors would not be engaged since descending drive to the muscle would be minimal and available for recruitment by cTBS providing the potential to modulate ionic flow and second messenger activity. Regardless of the mechanism, the weakened relationship between cTBS aftereffect and FCR-ECR threshold difference suggest that contraction paired with concurrent TBS can overcome the factors that dominate variability in the efficacy of resting state cTBS.
The effects of cTBS at rest and cTBS during contraction were largely localized to the neuronal populations at FCR hotspot that elicited MEPs in each muscle. cTBS at rest did not have any effect upon FCR or ECR amplitude evoked from neuronal populations for which the stimulating coil was not optimally oriented (i.e. those at the ECR hotspot) during cTBS despite the close proximity of the FCR and ECR hotspot and the significant overlap between the cortical representations of these muscles (Suzuki et al., 2012). Further, unlike at the FCR hotspot, FCR-ECR threshold difference, FCR resting motor threshold or ECR resting motor threshold explained minimal variance in after effect upon the ECR hotspot of either the ECR or FCR across individuals. Concurrent contraction of the antagonist ECR has been shown to enhance FCR MEP suppression following cTBS over the FCR hotspot (Fang et al., 2014). Unfortunately, the authors did not assess concurrent changes in both FCR and ECR MEP amplitude. Taken together with our results, cTBS appears to enhance suppression of the antagonist muscle only at the site of theta burst stimulation. In contrast, pairing isometric wrist flexion with iTBS produced effects specific to the contracted muscle in neuronal populations not optimally oriented to the stimulating coil during iTBS (Mirdamadi et al., 2015). Thus pairing contraction with cTBS may be effective in increasing muscle differentiation in motor cortex whereas pairing iTBS with contraction may be most effective in increasing descending drive by recruiting additional neuronal populations. The latter is consistent with selective modulation of intracortical networks following 5 Hz repetitive transcranial magnetic stimulation paired with contraction post-stroke (Massie et al., 2013).
Overall, the current work highlights variability in cTBS aftereffects driven by differences in resting thresholds across overlapping agonist–antagonist cortical representations. Variability in cTBS induced aftereffect as a result of differential resting excitability could mitigate the apparent benefits of pairing brain stimulation with subsequent functional practice/rehabilitation. Resting motor thresholds of both the target muscle(s) and other overlapping cortical representations should be assessed to predict cTBS outcomes. Tonic contraction should also be explored as a method by which to enhance the specificity of cTBS aftereffect considered especially in clinical populations where threshold differences might be exacerbated.
Acknowledgments
The present work was supported by the Claude D. Pepper Older Americans Independence Center at the University of Michigan (P30AG024824). The authors would like to thank Everlin Gutierrez for their help during data collection. Jasmine Mirdamadi is currently a doctoral student in the Department of Kinesiology at Indiana University-Bloomington.
Footnotes
DISCLOSURES
The authors declare no competing financial interests.
References
- Ackerley SJ, Stinear CM, Byblow WD. Promoting use-dependent plasticity with externally-paced training. Clin Neurophysiol. 2011;122:2462–2468. doi: 10.1016/j.clinph.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Artola A, Brocher S, Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990;347:69–72. doi: 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: a proof-of-concept study. Neurorehab Neural Repair. 2010;24:620–635. doi: 10.1177/1545968310361957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas-Morales L, Nowak DA, Kammer T, Wolf RC, Schonfeldt-Lecuona C. Mechanisms and applications of theta-burst rTMS on the human motor cortex. Brain Topogr. 2010;22:294–306. doi: 10.1007/s10548-009-0084-7. [DOI] [PubMed] [Google Scholar]
- Censor N, Cohen LG. Using repetitive transcranial magnetic stimulation to study the underlying neural mechanisms of human motor learning and memory. J Physiol. 2011;589:21–28. doi: 10.1113/jphysiol.2010.198077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr Clin Neurophysiol. 1998;109:397–401. doi: 10.1016/s0924-980x(98)00038-1. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Profice P, Ranieri F, Capone F, Dileone M, Oliviero A, Pilato F. I-wave origin and modulation. Brain Stimul. 2012;5:512–525. doi: 10.1016/j.brs.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, Ranieri F, Tombini M, Ziemann U, Rothwell JC, Di Lazzaro V. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014;10:597–608. doi: 10.1038/nrneurol.2014.162. [DOI] [PubMed] [Google Scholar]
- Fang JH, Huang YZ, Hwang IS, Chen JJ. Selective modulation of motor cortical plasticity during voluntary contraction of the antagonist muscle. Eur J Neurosci. 2014;39:2083–2088. doi: 10.1111/ejn.12565. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Postspike facilitation of forelimb muscle-activity by primate corticomotoneuronal cells. J Neurophysiol. 1980;44:751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- Hadj Tahar A, Blanchet PJ, Doyon J. Motor-learning impairment by amantadine in healthy volunteers. Neuropsychopharmacology. 2004;29:187–194. doi: 10.1038/sj.npp.1300317. [DOI] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex. 2013;23:1593–1605. doi: 10.1093/cercor/bhs147. [DOI] [PubMed] [Google Scholar]
- Hsu WY, Cheng CH, Liao KK, Lee IH, Lin YY. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke. 2012;43:1849–1857. doi: 10.1161/STROKEAHA.111.649756. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 2008;18:563–570. doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Chen RS, Lu CS, Chuang WL. The theoretical model of theta burst form of repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2011;122:1011–1018. doi: 10.1016/j.clinph.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and analysis: a researcher’s handbook. Upper Saddle River, N.J: Pearson Prentice Hall; 2004. [Google Scholar]
- Lee IA, Preacher KJ. Calculation for the test of the difference between two dependent correlations with one variable in common [Computer Software] 2013 http://quantpsy.org.
- Massie CL, Tracy BL, Malcolm MP. Functional repetitive transcranial magnetic stimulation increases motor cortex excitability in survivors of stroke. Clin Neurophysiol. 2013;124:371–378. doi: 10.1016/j.clinph.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Mirdamadi JL, Suzuki LY, Meehan SK. Agonist contraction during intermittent theta burst stimulation enhances motor cortical plasticity of the wrist flexors. Neurosci Lett. 2015;30:69–74. doi: 10.1016/j.neulet.2015.02.020. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. J Physiol. 1992;448:397–412. doi: 10.1113/jphysiol.1992.sp019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MC, Belhaj-Saif A, Cheney PD. Properties of primary motor cortex output to forelimb muscles in rhesus macaques. J Neurophysiol. 2004;92:2968–2984. doi: 10.1152/jn.00649.2003. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Ann Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kirimoto H, Onishi H, Yamada S, Tamaki H, Maruyama A, Yamamoto J. Reciprocal changes in input-output curves of motor evoked potentials while learning motor skills. Brain Res. 2012;1473:114–123. doi: 10.1016/j.brainres.2012.07.043. [DOI] [PubMed] [Google Scholar]
- Wischnewski M, Schutter DJ. Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimul. 2015;8:685–692. doi: 10.1016/j.brs.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Siebner HR. Modifying motor learning through gating and homeostatic metaplasticity. Brain Stimul. 2008;1:60–66. doi: 10.1016/j.brs.2007.08.003. [DOI] [PubMed] [Google Scholar]