Abstract
Objective
To compare overall survival of patients who underwent radical prostatectomy or radiotherapy versus non-cancer controls in order to discern if there is a survival advantage according to prostate cancer treatment and the impact of selection bias on these results.
Patients and Methods
A matched cohort study was performed using the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. We identified 34,473 patients age 66 to 75 years without significant comorbidity from who were diagnosed with localized prostate cancer treated with surgery or radiotherapy between 2004 and 2011. These patients were matched to a non-cancer control cohort. We compared the rates of all-cause mortality that occurred within the study period. We used Cox Proportional Hazards Regression analysis to identify determinants associated with overall survival.
Results
Of the total 34,473 patients who were included in the analysis, 21,740 (63%) received radiation therapy and 12,733 (37%) received surgery. There was improved survival in patients treated with surgery (hazard ratio [HR], 0.35; 95% CI, 0.32-0.38) as well as with radiotherapy (HR, 0.72; 95% CI, 0.68-0.75) when compared to non-cancer controls. There was significantly improved overall survival among both treatment groups with most benefit observed among patients who underwent surgery (log rank p<0.001).
Conclusions
Using population based data, treatment with either surgery or radiotherapy demonstrated improved overall survival when compared to a cohort of matched non-cancer controls. Treatment with surgery resulted in longer survival compared to those receiving radiation therapy. These results suggest inherent selection-bias due to unmeasured confounding variables.
Keywords: prostatectomy, prostate cancer, treatments, survival, utilization, outcomes
Introduction
Prostate cancer remains the most commonly diagnosed solid organ tumor among U.S. men with an estimated 220,800 new cases and 27,540 deaths in 2015. 1 Curative treatment options for prostate cancer include surgery and radiation. 2, 3 Driven by intensive PSA screening over the last quarter century, prostate cancer has witnessed a marked stage migration,4 toward a more indolent course in the majority of newly diagnosed cases. 5 It has therefore been suggested that active surveillance may be the most appropriate treatment strategy for most newly diagnosed patients with low risk disease (clinical stage T1-2a, Gleason score ≤6, PSA<10 ng/ml). 6 Despite this recommendation, a significant proportion of men eligible for active surveillance undergo curative therapy with either surgery or radiation. 7
With increased concern regarding the over-diagnosis and overtreatment of prostate cancer, treatment decisions regarding primary therapy are understandably complex. Prior studies have questioned the perceived survival benefit in patients treated for prostate cancer. 8 In a recent randomized clinical trial assessing men with clinically localized prostate cancer, radical prostatectomy did not reduce prostate cancer specific or overall mortality as compared to observation. 9 In an attempt to ameliorate overtreatment and select patients most likely to benefit from treatment, guidelines have now incorporated life expectancy into the prostate cancer treatment decision-making process. 6
Despite recent level 1 evidence concluding no significant difference in prostate cancer specific-mortality among men with localized disease treated versus those that underwent active monitoring10, physicians have had to use observational studies to answer clinical questions. Giordano et al. examined men treated from 1992 to 1999 with and without androgen deprivation for locally advanced prostate cancer to explore the effect of selection biases in observational studies. 11 They found men who underwent androgen deprivation had higher prostate cancer mortality despite clinical trial evidence that this treatment improves cancer mortality thus suggesting outcomes derived from observational studies should be used with caution. 11 Limitations in that study include results derived from historical data (i.e. prior to year 2000) where results may not be applicable to modern cohort and relatively heterogeneous cohort of patients with advanced disease. In an attempt to further explore the impact of selection bias using contemporary observational data in the treatment of prostate cancer, we conducted a population-based matched cohort study comparing overall survival in men undergoing radical prostatectomy or primary prostate radiotherapy for localized prostate cancer to non-cancer controls. We hypothesize that selection for treatment of localized prostate cancer would lend to improved survival outcomes over non-cancer controls suggesting selection bias for men undergoing those particular treatments.
Patients and Methods
Data Sources
We used Surveillance, Epidemiology, and End Results (SEER)–Medicare data for analysis, which are composed of a linkage of population-based cancer registry data from 18 SEER areas with Medicare administrative data. The SEER program covers approximately 30% of the U.S. population, and the Medicare program provides benefits to 97% of Americans aged ≥65 years 12.
Study Population
Due to baseline differences between patient populations undergoing radiotherapy and surgery, we limited our analysis to only include patients expected to be candidates for either surgery or radiotherapy based on age and limited comorbid medical conditions. From the SEER-Medicare linked database, we identified 34,473 patients who met the following criteria: age 65–75 years, Charlson Comorbidity Index (CCI) scores of 0 or 1, localized prostate cancer (clinical stage T1/T2), diagnosed with prostate cancer between 2004 and 2011, and treated with radical prostatectomy or radiotherapy. To ensure data completeness and to allow enough follow-up time to evaluate treatment and hospitalization, we included only patients who had full medical insurance coverage provided by Medicare Part A and Part B during the 12 months before and after treatment and who were not Health Maintenance Organization members. Patients with a diagnosis of any other cancer prior or post to prostate cancer were excluded.
Control Group
Patients characteristics differ between surgery and radiotherapy patients with men treated with radiotherapy often older with increased comorbidities, therefore we matched each prostate cancer treatment group (surgery and radiotherapy) to non-cancer controls, by age, race/ethnicity, state, and Charlson Comorbidity Index13. Non-cancer controls were selected from a 5% random sample of Medicare beneficiaries aged ≥66 years and only included men without a prior cancer diagnosis at time of matching 14.
Study Variables
Patient demographics, tumor characteristics, and treatments
Patient demographics and tumor characteristics at the time of diagnosis, including age, race/ethnicity, geographic region, census variables (urban/rural, education, poverty level), diagnosis year, grade and stage (T1/T2), were extracted from the PEDSF file. Tumor grade is dichotomized into low (well differentiated and moderately differentiated) and high grade (poorly differentiated and undifferentiated). Treatment variables including surgery and radiotherapy were determined from Medicare claims. Comorbidity was assessed using the Klabunde modification of the CCI during the year before diagnosis.15 The Klabunde modification uses comorbid conditions identified by the CCI and incorporates the diagnostic and procedure data contained in Medicare physician (Part B) claims. Variables were categorized as in Table 1.
Table 1.
Demographics of patients diagnosed with prostate cancer
| Characteristic | Category | Total | Prostate Cancer Patients | Non-Cancer Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Surgery | % | RT | % | p-value* | Total | % | Total | % | p-value** | |||
| Year of diagnosis | <0.001 | 0.973 | ||||||||||
| 2004 | 9045 | 1520 | 11.9 | 3003 | 13.8 | 4523 | 13.1 | 4522 | 13.1 | |||
| 2005 | 8732 | 1475 | 11.6 | 2884 | 13.3 | 4359 | 12.6 | 4373 | 12.7 | |||
| 2006 | 9442 | 1609 | 12.6 | 3097 | 14.3 | 4706 | 13.7 | 4736 | 13.7 | |||
| 2007 | 9848 | 1832 | 14.4 | 3094 | 14.2 | 4926 | 14.3 | 4922 | 14.3 | |||
| 2008 | 9014 | 1709 | 13.4 | 2771 | 12.8 | 4480 | 13.0 | 4534 | 13.2 | |||
| 2009 | 8417 | 1647 | 12.9 | 2550 | 11.7 | 4197 | 12.2 | 4220 | 12.2 | |||
| 2010 | 7860 | 1592 | 12.5 | 2353 | 10.8 | 3945 | 11.4 | 3915 | 11.4 | |||
| 2011 | 6588 | 1349 | 10.6 | 1988 | 9.1 | 3337 | 9.7 | 3251 | 9.4 | |||
| Race/ethnicity | <0.001 | <0.001 | ||||||||||
| Non-Hispanic White | 58339 | 10985 | 86.3 | 17974 | 82.7 | 28959 | 84.0 | 29380 | 85.2 | |||
| Non-Hispanic Black | 5622 | 773 | 6.1 | 2172 | 10.0 | 2945 | 8.5 | 2677 | 7.8 | |||
| Hispanics | 1624 | 309 | 2.4 | 503 | 2.3 | 812 | 2.4 | 812 | 2.4 | |||
| Other | 3361 | 666 | 5.2 | 1091 | 5.0 | 1757 | 5.1 | 1604 | 4.7 | |||
| State | <0.001 | 0.892 | ||||||||||
| California | 19047 | 4427 | 34.8 | 5064 | 23.3 | 9491 | 27.5 | 9556 | 27.7 | |||
| Connecticut | 3632 | 536 | 4.2 | 1282 | 5.9 | 1818 | 5.3 | 1814 | 5.3 | |||
| Georgia | 9621 | 1199 | 9.4 | 3651 | 16.8 | 4850 | 14.1 | 4771 | 13.8 | |||
| Hawaii | 831 | 197 | 1.6 | 235 | 1.1 | 432 | 1.3 | 399 | 1.2 | |||
| Iowa | 4208 | 947 | 7.4 | 1168 | 5.4 | 2115 | 6.1 | 2093 | 6.1 | |||
| Kentucky | 4923 | 921 | 7.2 | 1498 | 6.9 | 2419 | 7.0 | 2504 | 7.3 | |||
| Louisiana | 4691 | 820 | 6.4 | 1546 | 7.1 | 2366 | 6.9 | 2325 | 6.7 | |||
| Michigan | 4243 | 738 | 5.8 | 1391 | 6.4 | 2129 | 6.2 | 2114 | 6.1 | |||
| New Jersey | 9364 | 1138 | 8.9 | 3532 | 16.3 | 4670 | 13.6 | 4694 | 13.6 | |||
| New Mexico | 1571 | 318 | 2.5 | 469 | 2.2 | 787 | 2.3 | 784 | 2.3 | |||
| Utah | 2244 | 507 | 4.0 | 634 | 2.9 | 1141 | 3.3 | 1103 | 3.2 | |||
| Washington | 4571 | 985 | 7.7 | 1270 | 5.8 | 2255 | 6.5 | 2316 | 6.7 | |||
| Charlson Comorbidity Score | <0.001 | 0.781 | ||||||||||
| 0 | 52671 | 10306 | 80.9 | 16014 | 73.7 | 26320 | 76.4 | 26351 | 76.4 | |||
| 1 | 16275 | 2427 | 19.1 | 5726 | 26.3 | 8153 | 23.7 | 8122 | 23.6 | |||
| Clinical Stage | <0.001 | - | ||||||||||
| T1 | - | 7406 | 58.2 | 13865 | 63.8 | 21271 | - | - | - | |||
| T2 | - | 5327 | 41.8 | 7875 | 36.2 | 13202 | - | - | - | |||
| Tumor Grade | <0.001 | |||||||||||
| Low | - | 4340 | 34.1 | 10817 | 49.8 | 15157 | - | - | - | |||
| High | - | 8393 | 65.9 | 10923 | 50.2 | 19316 | - | - | - | |||
Note:
P-value from the Chi-square between the surgery group and RT group;
P-value from the Chi-square between the Prostate Cancer Patients and Non-Cancer Control.
The primary exposure was the treatment received within 6 months after diagnosis, identified in the claims data using International Classification of Diseases 9th edition (ICD-9) procedure codes and Current Procedural Terminology (CPT) codes in Supplemental material 1. The primary outcome of interest was overall survival.
For descriptive purposes, patients were classified into two, mutually exclusive categories based on the treatment received within this initial period: radical prostatectomy (open, minimally invasive or perineal) and radiotherapy (external beam, brachytherapy or both) (see Supplemental material 1). Patients who received both radical prostatectomy and radiotherapy were excluded from analysis. CPT-4 code 55899 (unspecified male genitourinary procedure) may sometimes be used with an open radical prostatectomy administrative code to specify minimally invasive radical prostatectomy with robotic assistance for private health plans, but Medicare does not recognize this coding schema, and very few men had this combination of codes; therefore, this was not used to identify minimally invasive radical prostatectomy.
Statistical Analysis
For all prostate cancer groups, follow-up began at the date of diagnosis. The non-cancer control group’s follow-up began at the pseudo-diagnosis date, which is the date of diagnosis of their matched prostate cancer cases. The primary outcome measure overall survival was calculated from the start of follow-up until the date of death (from the Medicare files) or the last follow-up. Overall survival for each prostate cancer treatment was compared with non-cancer controls.
Chi-square test was used to evaluate whether differences existed between cases and the non-cancer control group. The Kaplan-Meier method was used to calculate overall survival estimates. Differences were calculated using a log-rank test. Risk stratification into low and high risk disease was estimated based upon clinical stage and tumor grade. Patients were classified as having low-risk cancer if they had a T1 tumor and low histologic grade with high-risk disease including T1 or 2 tumor with high grade histology. Additionally, a multivariable Cox proportional hazard model was used to assess the influence of treatment type on outcome between the cases and control groups. To minimize potential selection bias, we used propensity score-based 1:1 matching algorithm. In this algorithm, a logistic regression model was performed controlling for all demographic and clinical variables to generate the predicted probability that is used for matching. The purpose of this matching is to create, based on existing covariates, a similar case and control cohort that will be used for further analysis. Although our greedy propensity score matching algorithm matched patients on several key variables, the proportion of case and control patients by race/ethnicity variable is still significant after matching and that may influence survival outcome. Also previous studies have reported racial disparities in prostate cancer care, therefore we further stratified our Cox proportional hazard model base on four race/ethnicity groups. P values less than .05 were considered statistically significant. The SAS software program version 9.4 (SAS Institute, Cary, NC) was used to perform all data management and statistical analyses. This study was deemed exempt by the Institutional Review Boards at the University of Texas MD Anderson Center as well as the University of Texas Medical Branch.
RESULTS
Of the total 34,473 patients (median age: 66; range: 66-75) who were included in the analysis, 21,740 (63%) received radiation therapy (median age: 66; range: 66-75) and 12,733 (37%) received surgery (median age: 66; range: 66-75). The demographics of our prostate cancer study population are summarized in Table 1. The median follow-up time is 63 months (min, 1 month; max, 120 months) for study cohort, 71 months for low D’Amico risk patients and 62 months for high D’Amico risk patients.
When compared to the non-cancer control (median age: 66), there was no significant difference between the prostate cancer cohort and the non-cancer control group with exception of race/ethnicity (p<0.001). The prostate cancer cohort had a significantly higher percentage of non-Hispanic blacks (52.4% vs. 47.6%) and race/ethnicity defined as other (52.3% vs. 47.7%), respectively (Table 1).
In multivariable analysis, there was improved survival in patients treated with surgery (hazard ratio [HR], 0.35; 95% CI, 0.32-0.38) as well as with radiotherapy (HR, 0.72; 95% CI, 0.68-0.75) when compared to non-cancer controls (Table 2). When stratified by race/ethnicity, improved survival persisted among patients regardless of race/ethnicity who received surgery or radiotherapy when compared to non-cancer control (all p<0.01).
Table 2.
Multivariable Cox Proportional Hazards Regression: original cohort and stratified analysis by race/ethnicity
| HR | 95% CI | P-value | ||
|---|---|---|---|---|
| Original cohort | ||||
|
| ||||
| Treatment | ||||
| Non-cancer Control | 1.00 | |||
| Surgery | 0.35 | 0.32 | 0.38 | <.001 |
| Radiation Therapy | 0.72 | 0.68 | 0.75 | <.001 |
| Race | ||||
| Non-Hispanic White | 1.00 | |||
| Non-Hispanic Black | 1.66 | 1.55 | 1.78 | <.001 |
| Hispanics | 0.99 | 0.84 | 1.17 | 0.935 |
| Other | 0.79 | 0.70 | 0.89 | 0.002 |
|
| ||||
| Stratification: | ||||
|
| ||||
| Non-Hispanic White | ||||
| Non-cancer Control | 1.00 | |||
| Surgery | 0.34 | 0.31 | 0.37 | <.001 |
| Radiation Therapy | 0.74 | 0.70 | 0.78 | <.001 |
| Non-Hispanic Black | ||||
| Non-cancer Control | 1.00 | |||
| Surgery | 0.37 | 0.29 | 0.48 | <.001 |
| Radiation Therapy | 0.61 | 0.53 | 0.70 | <.001 |
| Hispanics | ||||
| Non-cancer Control | 1.00 | |||
| Surgery | 0.42 | 0.24 | 0.72 | 0.002 |
| Radiation Therapy | 0.61 | 0.42 | 0.88 | 0.008 |
| Other | ||||
| Non-cancer Control | 1.00 | |||
| Surgery | 0.36 | 0.24 | 0.55 | <.001 |
| Radiation Therapy | 0.62 | 0.47 | 0.81 | 0.001 |
There was significantly improved overall survival among both treatment groups with most benefit seen among patients who underwent surgery (log rank p<0.001) as seen in Figure 1. These findings persisted when prostate cancer patients were stratified according to by low and high-risk stratification as shown in Figure 2. Therefore, we would expect patients who received prostate cancer treatment would have a longer life expectancy. When comparing the rate of other cause mortality and overall mortality between prostate cancer and non-cancer control patients, respectively, we found a significantly increased cumulative incidence of overall deaths in the non-cancer control cohort (p<0.001) (see Supplemental material 2).
Figure 1.
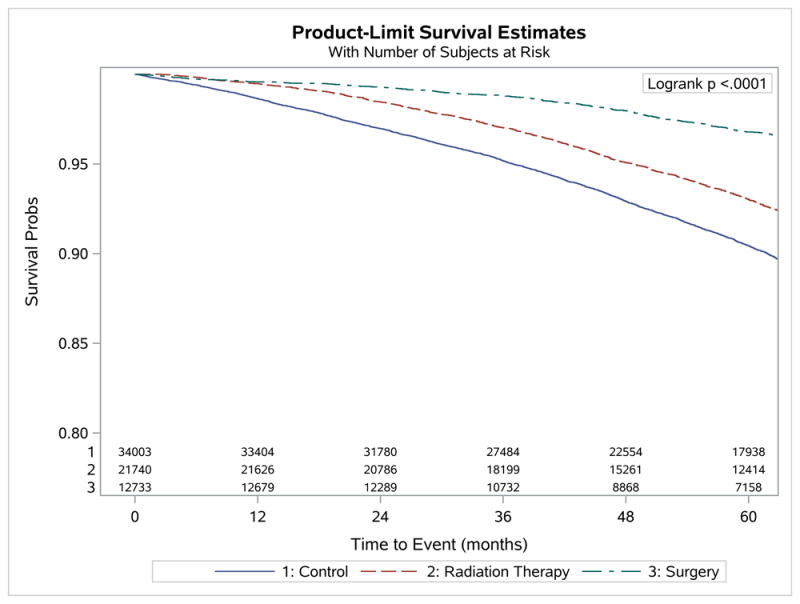
Plots of Kaplan-Meier product limit estimates of survival
Figure 2.
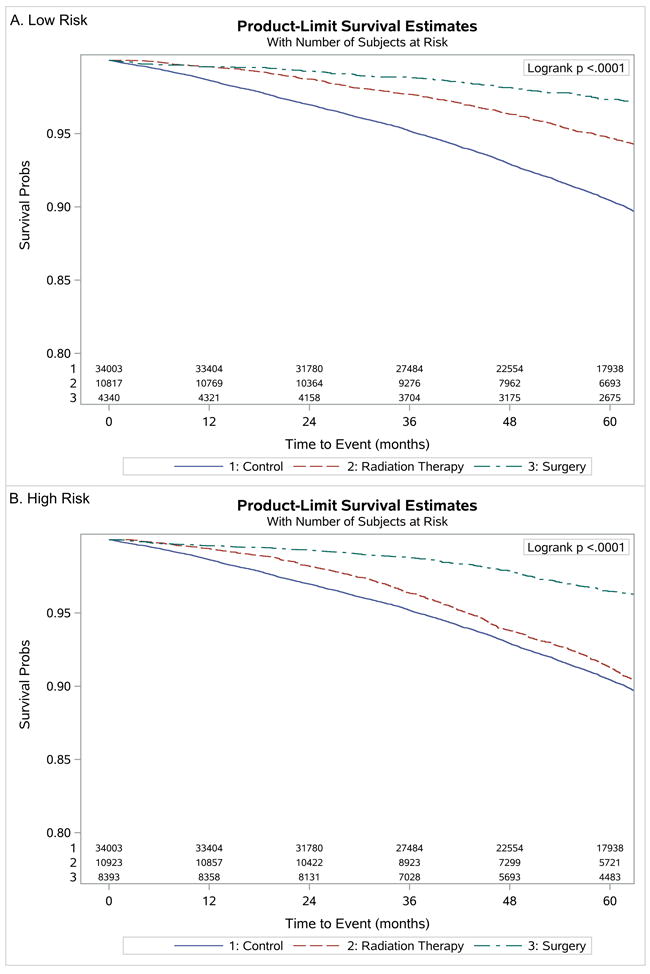
Plots of Kaplan-Meier product limit estimates of survival by risk. A, low risk. B, high risk.
DISCUSSION
In a cohort of men aged 66-75 identified from SEER-Medicare claims, while treatment with either surgery or radiotherapy was associated with improved overall survival, men treated with surgery had the longest survival when compared to men without cancer. Given the matching adjustments, these results suggest that some of the improved observed benefit is likely related to inherent selection-bias among men who are treated for prostate cancer which are most pronounced among men who underwent surgery due to unmeasured confounding variables.
Despite the difficulty in performing a randomized study between surgery and radiation for localized prostate cancer, due to patient choice and physician bias, a recent trial concluded low prostate cancer-specific mortality with no significant difference among men treated (surgery or radiotherapy) versus those who underwent active monitoring 10. Prior to this landmark study, patients with localized prostate cancer decided on treatment of their primary tumor with either surgery or radiotherapy based on retrospective and observational data. While much observational data suggests either a slight advantage, with surgical excision or at least similar oncologic benefit, it is important to understand the limitations to this type of data. Given the benefit of treatment observed in those receiving treatment for prostate cancer compared with non-cancer controls, this study suggests potential limitations of using cancer registry data to compare survival outcomes in otherwise healthy men with prostate cancer.
Our study has several important findings. First, in a cohort of men who would theoretically be candidates for either surgery or radiotherapy because of age and good overall health, we found men who underwent surgery had the greatest overall survival benefit over radiation and the non-cancer control cohort. Studies using retrospective population-based cancer registry have noted similar selection bias in treating other malignancies 14. Surgery and radiation for prostate cancer have come under scrutiny as many of these men have competing risks which may have a greater impact on their overall survival than their underlying prostate cancer 8. Given the existence of these competing risks and the potential for their impact on physician recommendations, decisions regarding therapy is at risk of selection bias. These unmeasured confounding variables, inherent to using cancer registry data, likely account for a portion of the perceived survival benefit.
Second, we found an improved overall survival benefit independent of race/ethnicity when compared to a non-cancer control cohort. These results persisted for both men who underwent either surgery or radiotherapy with men who underwent surgery to have the greatest overall survival benefit. Racial disparities in prostate cancer care has been previously published, however, this is the first report to our knowledge of improved overall survival when compared to non-cancer controls regardless of treatment and independent of race/ethnicity. These findings are relevant given the uncertainty regarding inferior oncologic outcomes which may be due to increased cancer risk and/or socioeconomic determinants such as lesser availability and access to primary health care facilities in among black patients previously implicated with decreased survival 16, 17. It appears the use of big data such as SEER-Medicare introduces unmeasured confounders which impacts survival outcomes reporting regardless of race/ethnicity.
Third, we found men who underwent surgery to have the greatest overall survival benefit when compared to men who underwent radiotherapy or non-cancer controls. Men who undergo surgery are often younger and healthier as depicted in our study. While we attempted to control for this using a roughly homogeneous group of men who would theoretically be fit to undergo either treatment, we cannot control for inherent selection bias which likely contributes to this observation. Moreover, this unmeasured selection bias more often explains our observation of improved survival benefit among men who underwent surgery to non-cancer controls. Prior randomized data suggest improved overall survival benefit among men treated with radiotherapy or surgery for prostate cancer 18-20. While clinical trials overcome concerns of internal validity, there are often concerns regarding external validity and generalizability— clinical trial enrollees tend to be younger and healthier than most cancer patients and often times represent highly selected patient subgroups 21-23. We caution against ignoring the level one evidence suggesting benefit to treatment for prostate cancer and do not condone abandoning surgery as a treatment option. However, our data does suggest that some of the observed survival benefit to surgery seen in observational studies may be contributed by selection bias. Furthermore, use of overall survival as a study endpoint and use of such data in guideline-based recommendations should be further scrutinized prior to making treatment recommendations.
It is not clear how this selection bias can be overcome, particularly when using population based data. Extensive modeling and statistical adjustments do not seem capable of overcoming physician judgment or limit these inherent biases. While randomized control trials are not plausible in this population, there are other potential options for effective comparisons. One option would be to prospectively enroll patients in observational studies of prostate cancer local therapy by creating a narrow inclusion criteria, required multi-specialty consultation, followed by patient choice for therapy. This would generate a more homogeneous population of men better fit for comparison of both oncologic and quality of life outcomes. In summary and as previously shown using older observational data, we also conclude results of observational studies which compare outcomes of different therapies should be viewed with some skepticism due to inherent selection bias.11
While our findings are policy relevant, they must be interpreted in the context of the study design. First, SEER-Medicare is limited to men aged 65 years of age and older and our results may not be generalizable to younger men diagnosed with prostate cancer. Moreover, this study primarily analyzed healthy men with prostate cancer aged 66-70 years old (only 0.7% were >70 years) and further excluded patients treated with both prostatectomy and radiation who are clearly at increased risk of death. The combination of these two factors is likely to contribute significantly to the results in the survival analyses and account for some of the observed selection bias 24. Second, we excluded PSA values in the present study, due to preliminary evaluation of SEER data uncovered problems with the quality and interpretation of the PSA value 25. While this questions the validity of large datasets, prior studies have suggested the limited impact PSA may have on disease risk stratification with patients having similar tumor characteristics as those with complete data 26. Lastly, while we attempted to control for known predictors for survival, the findings are hypothesis-generating and there may be omitted variable bias. While we used the Charlson comorbidity index there may have been differences in health between surgery and radiotherapy groups that were not reflected in the Charlson comorbidity scores. However, observational studies reflect practice patterns and when compared with results from well-conducted randomized controlled trials they do not appear to overestimate treatment effects nor differ qualitatively 22, 27.
Conclusions
Using a large population based registry we demonstrated treatment of localized prostate cancer, with either surgery or radiotherapy, was associated with improved overall survival benefit compared to non-cancer controls. Although the cohorts were matched, men treated with surgery appeared to have the greatest overall survival benefit. These results suggest inherent selection-bias due to unmeasured confounding variables.
Supplementary Material
Acknowledgments
This work was supported by The University of Texas MD Anderson Center for Radiation Oncology Research (CROR) seed-grant awarded to Stephen B. Williams, M.D. and the Duncan Family Institute. Dr. Williams is a Comparative Effectiveness Research on Cancer in Texas (CERCIT) Scholar. Dr. Giordano is supported by CPRIT RP140020 and Komen SAC150061. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Conflict of interests: All authors have no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Mohler JL, Kantoff PW, Armstrong AJ, et al. Prostate cancer, version 2.2014. J Natl Compr Canc Netw. 2014;12:686–718. doi: 10.6004/jnccn.2014.0072. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich A, Aus G, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol. 2008;53:68–80. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Makarov DV, Trock BJ, Humphreys EB, et al. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology. 2007;69:1095–1101. doi: 10.1016/j.urology.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302:1202–1209. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 7.Chamie K, Williams SB, Hu JC. Population-Based Assessment of Determining Treatments for Prostate Cancer. JAMA Oncol. 2015;1:60–67. doi: 10.1001/jamaoncol.2014.192. [DOI] [PubMed] [Google Scholar]
- 8.Daskivich TJ, Kwan L, Dash A, Saigal C, Litwin MS. An Age Adjusted Comorbidity Index to Predict Long-Term, Other Cause Mortality in Men with Prostate Cancer. J Urol. 2015;194:73–78. doi: 10.1016/j.juro.2015.01.081. [DOI] [PubMed] [Google Scholar]
- 9.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–213. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375:1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 11.Giordano SH, Kuo YF, Duan Z, Hortobagyi GN, Freeman J, Goodwin JS. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112:2456–2466. doi: 10.1002/cncr.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 13.Alibhai SM, Leach M, Warde P. Major 30-day complications after radical radiotherapy: a population-based analysis and comparison with surgery. Cancer. 2009;115:293–302. doi: 10.1002/cncr.24008. [DOI] [PubMed] [Google Scholar]
- 14.Shuch B, Hanley J, Lai J, et al. Overall survival advantage with partial nephrectomy: a bias of observational data? Cancer. 2013;119:2981–2989. doi: 10.1002/cncr.28141. [DOI] [PubMed] [Google Scholar]
- 15.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 16.Blair IV, Havranek EP, Price DW, et al. Assessment of biases against Latinos and African Americans among primary care providers and community members. Am J Public Health. 2013;103:92–98. doi: 10.2105/AJPH.2012.300812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hargraves JL, Cunningham PJ, Hughes RG. Racial and ethnic differences in access to medical care in managed care plans. Health Serv Res. 2001;36:853–868. [PMC free article] [PubMed] [Google Scholar]
- 18.Bolla M, Maingon P, Carrie C, et al. Short Androgen Suppression and Radiation Dose Escalation for Intermediate- and High-Risk Localized Prostate Cancer: Results of EORTC Trial 22991. J Clin Oncol. 2016;34:1748–1756. doi: 10.1200/JCO.2015.64.8055. [DOI] [PubMed] [Google Scholar]
- 19.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 20.Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932–942. doi: 10.1056/NEJMoa1311593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. Journal of Clinical Oncology. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 23.Gross CP, Mallory R, Heiat A, Krumholz HM. Reporting the recruitment process in clinical trials: who are these patients and how did they get there? Ann Intern Med. 2002;137:10–16. doi: 10.7326/0003-4819-137-1-200207020-00007. [DOI] [PubMed] [Google Scholar]
- 24.Eifler JB, Humphreys EB, Agro M, Partin AW, Trock BJ, Han M. Causes of death after radical prostatectomy at a large tertiary center. J Urol. 2012;188:798–801. doi: 10.1016/j.juro.2012.04.109. [DOI] [PubMed] [Google Scholar]
- 25.Sun M, Trinh QD. A seer database malfunction: perceptions, pitfalls and verities. BJU Int. 2015 doi: 10.1111/bju.13226. [DOI] [PubMed] [Google Scholar]
- 26.Elliott SP, Johnson DP, Jarosek SL, Konety BR, Adejoro OO, Virnig BA. Bias due to missing SEER data in D’Amico risk stratification of prostate cancer. J Urol. 2012;187:2026–2031. doi: 10.1016/j.juro.2012.01.070. [DOI] [PubMed] [Google Scholar]
- 27.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


